Isoflavones, weak oestrogenic compounds found in soya beans, have been investigated as protective agents against prostate and other cancers(Reference Adlercreutz1). Although their oestrogenic and anti-oestrogenic effects have been the focus of most research, alternate pathways have also been explored(Reference Ford2). Based on genistein's ability to down-regulate cytokine-induced signal transduction in immune cells and to suppress cell-mediated inflammatory responses and atherosclerosis in experimental models, isoflavones have been proposed as possible anti-inflammatory agents(Reference Verdrengh, Jonsson and Holmdahl3, Reference Register, Cann and Kaplan4). A number of small trials in human subjects, primarily with supplements, suggested possible effects on the immune system(Reference Ryan-Borchers, Park and Chew5, Reference Nasca, Zhou and Welty6). The idea that nutritional factors influence immune responses is supported by evidence that adipose tissue produces the cytokines leptin and IL-6 which contribute to a state of chronic inflammation and increased hepatic production of C-reactive protein (CRP). The role of inflammatory pathways in carcinogenesis(Reference Coussens and Werb7) and the link to certain nutrients, for example, n-3 fatty acids(Reference James, Gibson and Cleland8), have only recently been elucidated. The present analysis utilised previously collected serum samples from a randomised cross-over trial(Reference Maskarinec, Morimoto and Hebshi9) to explore the hypothesis that soya may reduce markers of chronic inflammation. The focus of the original study was the effect of soya on markers of prostate cancer risk(Reference Maskarinec, Morimoto and Hebshi9). The objectives of the present report are to examine the association of a soya diet with serum levels of six inflammatory markers, leptin, adiponectin, monocyte attractant protein 1 (MCP-1), macrophage inflammatory protein-1b (MIP-1b), IL-6 and CRP, and to explore their relationship to BMI and lifetime soya intake. Leptin may mediate the effect of obesity on cancer risk(Reference Garofalo and Surmacz10) and adiponectin is the most abundant protein in adipocytes with a strong anti-inflammatory function(Reference Tilg and Wolf11). IL-6 is an important mediator of acute inflammatory responses produced by adipocytes and macrophages, while CRP represents a non-specific indicator of inflammation produced in the liver as a result of stimulation by IL-6(Reference Ishihara and Hirano12, Reference Albert, Glynn and Buring13). MIP-1b and MCP-1 are chemokines secreted by adipose tissue and induced by inflammatory cytokines(Reference Gerhardt, Romero and Cancello14) with possible effects on metabolic functions and pathologies associated with obesity, insulin resistance and atherosclerosis(Reference Sartipy and Loskutoff15).
Materials and methods
Study design and procedures
The goal of the original randomised, cross-over study was to evaluate the feasibility of a soya trial among men and to examine serum prostate-specific antigen and testosterone levels(Reference Maskarinec, Morimoto and Hebshi9). In short, men aged 44–69 years were recruited from Kaiser Permanente Hawaii. Of the ninety men who expressed interest in the study, twenty-five eligible men were invited for a screening visit(Reference Maskarinec, Morimoto and Hebshi9). We excluded men who travelled off-island extensively, had ever been diagnosed with cancer, took finasteride or sex steroids, had dietary restrictions, or consumed six or more servings of soya foods per week. Interested men completed a one-page soya FFQ to report soya intake during the past 12 months and a lifetime soya FFQ for different phases in life(Reference Maskarinec, Aylward and Erber16). The nutritional intervention consisted of two 3-month study periods separated by a 1-month washout period (Table 1). We randomised twenty-four subjects to group A (started with the low-soya diet) or group B (started with the high-soya diet), but one man dropped out of the study during the first diet period. During the high soya period, the men consumed approximately two daily servings of soya foods, predominantly tofu, soya milk and soya nuts with an isoflavone content of 30–35 mg per serving, while they maintained their regular diet during the low soya period. According to several measures of compliance, the men closely adhered to the study regimen; the mean isoflavone intakes during the high and low soya periods were 69 and < 5 mg, respectively(Reference Maskarinec, Morimoto and Hebshi9). The University of Hawaii Committee on Human Studies and the Kaiser Permanente Institutional Review Board approved the study protocol. All subjects signed an informed consent form.
Table 1 Study design for the cross-over soya intervention among men
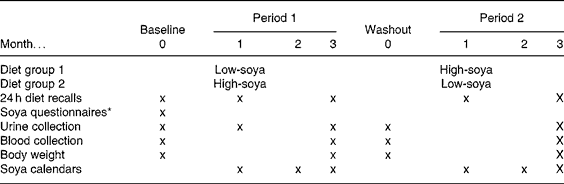
* One-year and lifetime soya consumption.
Sample collection and analysis
Fasting blood samples were collected at baseline, and after 3, 4 and 7 months (Table 1). The blood draw at month 4 was scheduled after a 1-month washout period (no soya foods) in order to re-establish a baseline for the second diet period. The frozen serum was stored at − 80°C and shipped on dry ice to Minnesota. Using two plates of multiplex bead immunoassays, fifty-four of the ninety-three samples and six blinded quality-control samples were analysed in duplicate; the remaining thirty-nine samples were analysed as single samples. The concentrations of cytokines were measured simultaneously by Luminex, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA) using Base Kit A (MIP-1b and MCP-1) or Obesity Panel (adiponectin, CRP, IL-6, leptin, TNF-α). Briefly, 50 μl of standard or a 1:3 dilution of the test serum sample was combined with the beads (1:1 ratio). After 2 h incubation at room temperature, the plates were washed and 50 μl of biotinylated detection antibody was added to each well. After a further 2 h incubation, the plates were washed and 50 μl of streptavidin-phycoerythrin was added to each well followed by a 30 min incubation. The beads were then re-suspended in 100 μl wash buffer and analysed on a Luminex 200 instrument (Luminex, Austin, TX, USA) using Bioplex Manager Software 4.1 (Bio-Rad, Hercules, CA, USA). A minimum of fifty beads per region were analysed; a five parameter logistic curve was applied to each standard curve and cytokine concentrations were interpolated from these curves. The detection limits in the multiplex assay were 2·54 pg/ml for MCP-1, 2·50 pg/ml for MIP-1b, 19·8 pg/ml for adiponectin, 16 pg/ml for CRP, 3·8 pg/ml for IL-6, 20·2 pg/ml for leptin and 3·5 pg/ml for TNF-α. Based on the blinded quality-control samples, the inter-assay variability was 7·1 % for leptin, 5·5 % for MIP-1b and 2·4 % for MCP-1. Due to problems with manufacturing, only one plate was available for adiponectin (baseline and 3-month samples); the intra-assay CV was 4·4 %.
A high-sensitivity IL-6 ELISA kit (purchased from R&D Systems) was used to measure IL-6 that had not been detectable by the Luminex assays. Serum CRP was also measured by ELISA assay (R&D Systems) as its serum concentration was too high to be assessed by multiplex assay. Since there was not enough serum left to perform three ELISA assays, we decided not to measure TNF-α, the marker with relatively high within-subject variability(Reference Gonzalez, Cava and Ayllon17). The IL-6 and CRP assay detection limits were 0·01 pg/ml and 0·01 ng/ml, respectively. The CV was 4·4 % for IL-6 and 5·8 % for CRP. Because some serum samples had been depleted, we were only able to analyse seventy-six of the ninety-two specimens (twenty-two baseline, fifteen 3-month, sixteen 4-month and twenty-three 7-month samples) by ELISA. TNF-α levels were also mostly undetectable in the multiplex assay, but could not be repeated by ELISA due to depletion of serum.
Statistical analysis
The statistical analysis was performed using the SAS statistical software package (version 9.1; SAS Institute, Inc., Cary, NC, USA). Based on the soya FFQ, we estimated soya food consumption before age 20 years and since age 20 years in servings per year. Due to the non-normal distributions, we categorised soya intake into low and high and performed log transformations of the analytes when necessary. We conducted two-sample t tests to compare measured analytes between the two groups after randomisation and after the washout period. Unadjusted means and standard deviations were calculated for the inflammatory markers by group and treatment. In each treatment group, differences in analyte levels from the randomisation and washout periods were calculated after months 3 and 7, respectively. To account for the repeated-measurement design, we applied mixed models to evaluate the effect of the soya intervention on the markers. The association of BMI, body weight and soya intake during early life and during adulthood was also evaluated in mixed models while adjusting for age and ethnicity.
Results
At baseline, the mean age of the participants was 58·7 (sd 7·2) years and the mean BMI was 28·4 (sd 4·9) kg/m2 with a range of 21·5–44·0 kg/m2. The two groups did not differ significantly by age or BMI. Half of group A and one-third of group B were of Asian ancestry. The baseline levels of all markers were similar by group; the respective P values were 0·32, 0·29, 0·10, 0·39, 0·24 and 0·79 for leptin, MCP-I, MIP-1b, adiponectin, IL-6 and CRP. We observed no intervention effect of the soya diet on any of the six markers; none of the P values for the diet effect in the mixed models was significant (Table 2). Inclusion of covariates did not change the lack of an intervention effect.
Table 2 Serum levels of inflammatory markers by soya treatment
(Unadjusted mean values and standard deviations)
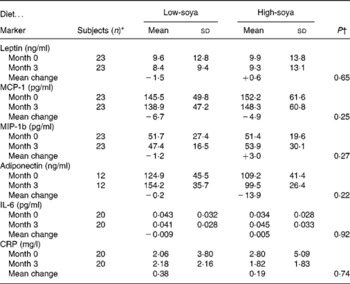
MCP-1, monocyte attractant protein 1; MIP-1b, macrophage inflammatory protein-1b; CRP, C-reactive protein.
* Same number of subjects for both diets.
† The effect of diet based on mixed models.
After adjustment for age and ethnicity, highly significant associations of BMI and body weight with leptin and MCP-1 were observed (Table 3). The levels of leptin and MCP-1 were positively related to BMI (P < 0·001 for both) and body weight (P = 0·006 and P < 0·001). Men with high soya intake early in life also had significantly higher levels of leptin (P < 0·001) and borderline higher MCP-1 levels (P = 0·06), whereas no association was seen for soya intake during adulthood. After including BMI and early-life soya intake into the same mixed model, both variables remained significantly associated with leptin (P = 0·0005 and P = 0·008). However, for MCP-1, only BMI remained significant (P = 0·05). MIP-1b, adiponectin, IL-6 and CRP were not related to BMI, body weight or soya intake at any time in life.
Table 3 Relationship of serum markers to anthropometric variables and soya intake*
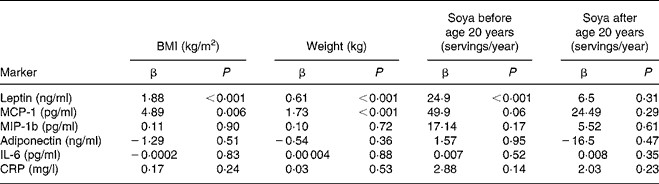
MCP-1, monocyte attractant protein 1; MIP-1b, macrophage inflammatory protein-1b; CRP, C-reactive protein.
* Based on mixed models and controlling for age and ethnicity.
Discussion
Contrary to our hypothesis, a high-soya diet for 3 months did not modify the levels of six cytokines measured in serum. However, leptin and MCP-1 were strong predictors of body weight and BMI. Interestingly, both markers were also associated with soya intake early in life but not soya intake during adulthood. This observation is new and difficult to explain although the importance of timing for the chemopreventive effects of isoflavones has been described in animals(Reference Lamartiniere, Cotroneo and Fritz18).
Despite some support for an effect of soya on immune responses in animals(Reference Verdrengh, Jonsson and Holmdahl3, Reference Register, Cann and Kaplan4), our findings agree with human soya interventions that did not observe a change in leptin(Reference Phipps, Wangen and Duncan19) or MCP-1(Reference Hall, Vafeiadou and Hallund20). On the other hand, CRP decreased in some studies(Reference Nasca, Zhou and Welty6, Reference Hall, Vafeiadou and Hallund20, Reference Yildiz, Kumru and Godekmerdan21) and IL-6 was modified in two interventions(Reference Jenkins, Kendall and Jackson22, Reference Campbell, Brown and Dufner23). The association of adiposity with leptin and MCP-1 levels is in agreement with published reports; in particular visceral fat appears to be an important determinant of MCP-1(Reference Considine, Sinha and Heiman24, Reference Malavazos, Cereda and Morricone25) and weight loss lowers leptin and MCP-1(Reference Yamashita, Sasahara and Pomeroy26, Reference Troseid, Lappegard and Claudi27). Circulating monocytes in obese individuals are in a pro-inflammatory state accompanied by an increased expression of pro-inflammatory cytokines and chemokines(Reference Ghanim, Aljada and Hofmeyer28) which control fat accumulation and leptin expression in human adipocytes(Reference Gerhardt, Romero and Cancello14). Since the elevated circulatory levels of MCP-1 in obese subjects are associated with a number of obesity parameters, it appears that serum levels reflect concentrations in adipose tissue(Reference Kim, Park and Kawada29). MCP-1 and MIP-1a appear to be central in monocyte recruitment into the arterial wall and developing atherosclerotic lesions(Reference Reape and Groot30) and they show a markedly increased expression on both gene and protein level in adipocytes and adipose tissue of obese animals(Reference Gerhardt, Romero and Cancello14, Reference Sartipy and Loskutoff15, Reference Xu, Barnes and Yang31). Of these, MCP-1 has been previously studied, but MIP-1b was included as a new inflammatory marker that may be affected by soya.
Our small study had a number of limitations in addition to the small sample size. The original goal had been to measure seven markers in one multiplex assay, but this was impossible due to the wide range in concentrations. Therefore, we had to turn to ELISA assays for IL-6 and CRP. Due to depletion of our serum samples, TNF-α could not be measured at all. Furthermore, an issue with one of the plates only allowed us to measure adiponectin in half of the samples. The strengths of our intervention lie in the use of soya foods instead of supplements and the excellent compliance of the men to the high-soya diet(Reference Maskarinec, Morimoto and Hebshi9). Despite the inclusion of several inflammatory markers, it would take an even larger number of markers to assess the overall effect of soya on inflammation. The influence of BMI on cytokine levels as shown here and elsewhere may reduce the ability to detect small changes due to soya foods. Results may be different in a population with subjects within a normal range of BMI. Because soya beans are a good source of n-3 fatty acids, which are known for their anti-inflammatory effect(Reference James, Gibson and Cleland8), the hypothesis that dietary patterns rich in soya food may have a beneficial effect on chronic inflammation remains plausible and deserves further investigation.
Acknowledgements
We are very grateful to the dedicated study participants. This research was supported by a grant from the Friends of the Cancer Research Center. We also appreciate the support through food donations from Aloha Tofu Factory, DrSoy Nutrition and The Solae Company. G. M. conceived the original study, obtained funding, supervised the data analysis and finalised the manuscript. R. O. performed the statistical analysis, drafted parts of the manuscript and reviewed the final draft. A. K. C. conducted the laboratory analyses and contributed to the writing of the paper. S. O. contributed the expertise on inflammatory markers, oversaw the laboratory analysis, drafted parts of the manuscript and reviewed the final paper.
None of the authors had a conflict of interest related to this project.





