Necrotising enterocolitis (NEC) is one of the most common and often fatal intestinal disorders that affects nearly 10 % of all very-low-birth weight (VLBW) infants( Reference Neu and Walker 1 ). About a quarter of the affected infants die from NEC, and the survivors are often faced with long-term neurological complications( Reference Rees, Pierro and Eaton 2 ). Breast-fed infants are at a 6–10-fold lower risk of developing NEC than formula-fed (FF) infants( Reference Lucas and Cole 3 , Reference Meinzen-Derr, Poindexter and Wrage 4 ). However, the reasons for the benefits of breast milk remain elusive.
Human milk is an abundant source of oligosaccharides that are currently not present in infant formula( Reference Bode 5 ). We have recently shown that these human milk oligosaccharides (HMO) improve survival and reduce pathology scores in a preclinical NEC model in neonatal rats( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ). HMO are a group of more than 150 structurally distinct complex sugars, and we were able to identify one specific oligosaccharide named disialyllacto-N-tetraose (DSLNT) that is most effective in preventing NEC in the rat model( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ). However, DSLNT is unique to human milk and, similar to most other HMO, currently not commercially available to supplement infant formula. Today, several infant formulas contain prebiotic oligosaccharides such as galacto-oligosaccharides (GOS) to mimic some of the beneficial effects of HMO( Reference Macfarlane, Steed and Macfarlane 7 ). In infants, consumption of formula enriched with GOS showed a significant increase in bifidobacteria and lactobacilli in the infant gut microbiota, which resembles that of breast-fed infants( Reference Fanaro, Marten and Bagna 8 – Reference Vandenplas, Zakharova and Dmitrieva 10 ). However, GOS are structurally different from HMO and do not contain sialic acid, which is an essential component for DSLNT to be effective in preventing NEC. Removal of just one sialic acid from DSLNT abrogates its beneficial effects( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ). Although we have shown previously that GOS is ineffective in preventing disease in the neonatal rat model of NEC( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ), we now use the same model to assess whether or not modified GOS that is decorated with sialic acid (N-acetyl-neuraminic acid (Neu5Ac))( Reference Wilbrink, ten Kate and Sanders 11 ) reduces NEC pathology.
In previous studies, the neutral HMO fraction, which contains high amounts of the HMO 2′-fucosyllactose (2′FL), also showed a significant reduction in pathology scores in the rat NEC model (P<0·05)( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ). Although not significant, 2′FL tended to decrease the incidence and severity of NEC in a preterm piglet model( Reference Cilieborg, Jensen and Bering 12 ). Therefore, we tested whether or not 2′FL reduces NEC pathology in the neonatal rat model.
Methods
Source of galacto-oligosaccharides
Vivinal® GOS (FrieslandCampina), a mixture of GOS, is synthesised by the enzymatic transgalactosylation of cows’ milk-derived lactose using a β-galactosidase enzyme from Bacillus circulans. The syrup contains approximately 59 % GOS, 21 % lactose, 19 % glucose and 1 % galactose on DM (75 %); in terms of the degree of polymerisation (DP), it contains approximately 20 % DP1, 42 % DP2, 24 % DP3, 10 % DP4; 3 % DP5 and <0·5 % DP6-DP9( Reference Van Leeuwen, Kuipers and Dijkhuizen 13 ). Recently, the structures of >40 components up to DP5 have been reported( Reference Van Leeuwen, Kuipers and Dijkhuizen 13 ). A purified GOS material (97 % GOS, 0·7 % galactose, 1·6 % glucose and 0·7 % lactose) was prepared from Vivinal® GOS by means of an enzymatic treatment with lactase to hydrolyse the lactose into glucose and galactose, after which the monosaccharides were removed by nanofiltration.
Source and generation of sialylated galacto-oligosaccharides
Sia-GOS (provided by Microbial Physiology, Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen) was synthesised by Sia(α2-3) transfer from donor κ-casein-derived glycomacropeptide (GMP; Sia≥99 % Neu5Ac) (provided by FrieslandCampina Innovation Centre) to acceptor Vivinal® GOS, using trans-sialidase from Trypanosoma cruzi as biocatalyst. The enzyme transfers Sia(α2-3) from a Sia(α2-3)Gal(β1-x) sequence on a donor to a β-linked galactose on an acceptor( Reference Wilbrink, ten Kate and Sanders 11 ).
A solution of 5 mm (α2-3)-linked Neu5Ac (2·5 g GMP), 2 mm Vivinal® GOS (average DP of 3; 10 % NaCl was partially removed via a 3 kDa cut-off filter) and 3·3 mU/ml recombinant T. cruzi trans-sialidase (TcTS) in 500 ml of 50 mm-sodium citrate pH 5 was incubated for 48 h at 25°C( Reference Wilbrink, ten Kate and Sanders 11 ). Then, the reaction mixture was heated for 20 min at 60°C, it was centrifuged for 15 min at 4500 g and filtered over a 3-kDa cut-off filter using a DiaFlow system (removal of (partially desialylated) GMP and TcTS). The obtained filtrate was lyophilised and separated (Sia-GOS, GOS, salt) on a Bio-Gel P-2 column (60×5 cm) using Milli-Q water as eluent and a refractive-index detection system. The Sia-GOS-containing fractions, as traced by high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD; Dionex ICS-3000 workstation) on a CarboPac PA-1 column (250×4 mm; gradient of 30–600 mm-sodium acetate in 0·1 m-NaOH)( Reference Wilbrink, ten Kate and van Leeuwen 14 ), were pooled and lyophilised. A further purification was carried out on a Dowex 1×8 column (formate form, 50×1·5 cm), eluted with Milli-Q water, followed by 50 mm-ammonium bicarbonate. The Sia-GOS-containing fractions, eluted with ammonium bicarbonate, were pooled and lyophilised. The purity of Sia-GOS (as a colourless glass) was calculated to be >95 %. Charge analysis on Resource Q with UV detection revealed mono-Sia-GOS–di-Sia-GOS in a molar ratio of 97:3( Reference Wilbrink, ten Kate and Sanders 11 ). Fig. 1(a) presents the HPAEC-PAD profile of Sia-GOS and Fig. 1(b) shows its 1H NMR spectrum.

Fig. 1 Characterisation of sialylated galacto-oligosaccharides (Sia-GOS). (a) High-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) profile on CarboPac PA-1. Mono-Sia-GOS elutes between 7 and 15 min and di-Sia-GOS elutes between 15 and 20 min( Reference Wilbrink, ten Kate and Sanders 11 ). (b) 1H NMR spectrum of Sia-GOS. Neu5Ac, N-acetyl-neuraminic acid; ppm, parts per million.
Source of 2′-fucosyllactose
2′FL was produced by fermentation technology, and it was provided by FrieslandCampina Innovation Centre. The 2′FL product contained approximately 93 % 2′FL, 3 % glucose, 3 % lactose and 1 % sucrose.
Source of human milk oligosaccharides
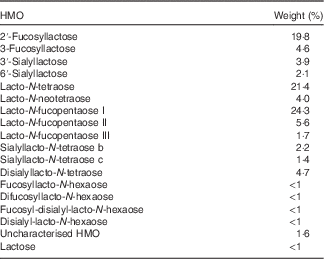
Human milk was obtained from thirty-seven healthy volunteers of preterm infants recruited at the University of California, San Diego Medical Center, San Diego, CA, USA, after approval by the university’s institutional review board. After centrifugation, the lipid layer was removed and proteins were precipitated from the aqueous phase by addition of two volumes of ice-cold ethanol (100 %) and subsequent centrifugation. Ethanol was removed from the HMO-containing supernatant by roto-evaporation. Lactose and salts were removed by gel filtration chromatography over a Bio-Gel P-2 column (100 cm length, 16 mm ID; Bio-Rad) using a semi-automated fast protein liquid chromatography system. The HMO composition (Table 1) was determined by HPLC and fluorescence detection after 2-aminobenzamide labelling. Peak annotation was based on standard retention times and MS analysis on a Thermo LCQ Duo Ion trap MS equipped with a Nano-ESI-source.
Table 1 Composition of pooled human milk oligosaccharides (HMO)
Efficacy study in neonatal rat necrotising enterocolitis model
As there are no in vitro models on NEC available, and human intervention studies are not feasible because of limited availability of HMO and other newly developed oligosaccharides, we used an NEC model in neonatal rats that was originally described by Barlow et al. and later modified and used to test HMO efficacy in the previous study( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ). In brief, pregnant time-dated Sprague–Dawley rats were induced at term using Pitocin (1–2 U/animal). Immediately after birth, neonatal rats were randomised into one of the following six study groups: dam-fed as healthy control (DF; n 18), formula-fed as disease control (FF; n 22), FF containing pooled HMO at 10 mg/ml as positive intervention control (HMO; n 15), FF containing GOS at 8 mg/ml as negative intervention control (GOS; n 15), FF containing Sia-GOS at 500 µm (approximately 0·4 mg/ml) as investigative intervention (Sia-GOS; n 11) and FF containing 2′FL at 2 mg/ml as a second investigative intervention (2′FL; n 14). The dosage of HMO and 2′FL was based on the average content of these compounds in mature human milk. The dosage of Sia-GOS was based on the amount of DSLNT in human milk at 2–3 weeks postpartum, and on the dosage that was previously shown to be effective in improving survival and reducing pathology scores in the rat NEC model. The 8 mg GOS/ml was based on the maximum amount of GOS, which is currently being used in infant formula.
Animals in the DF group remained with the dam. All other animals were separated from the dam, housed in a temperature- and humidity-controlled incubator and orally gavaged with a special rodent formula (0·2 ml; without and with different oligosaccharides) twice daily. The formula approximates the protein and caloric content of rat breast milk (Table 2) and consists of 15 g of Similac 60/40 (Ross Pediatrics) in 75 ml of Esbilac canine milk replacer (Pet-Ag). All animals, DF and gavaged, were exposed to 10 min of hypoxia (5 % O2, 95 % N2) three times daily in a modular chamber. All animals were killed 96 h postpartum; their intestines were collected and inspected for the presence of gross necrotic changes or pneumatosis intestinalis. A 0·5-cm section of the terminal ileum was prepared for H&E staining per standard protocols and scored blindly by two investigators based on morphological changes that included epithelial sloughing, villus oedema, infiltration of neutrophils, apoptosis of villus enterocytes, crypt hyperplasia and misaligned nuclei in the epithelium. If at least one pathology sign was observed, a score of 0·5–1·5 was assigned depending on severity. Two or three signs together resulted in a score of 2–3. The maximum score of 4 was given in case of complete obliteration of the epithelium with or without intestinal perforation. Studies were performed in two independent feeding experiments. The animal protocol was approved by The University of California San Diego Institutional Animal Care and Use Committee (IACUC), and complied with the Guide for the Care and Use of Laboratory Animals.
Table 2 Composition of the study formula compared with rat milk

* On day of life 5 and depending on the diet fed to the dam( Reference Nicholas and Hartmann 15 ).
Statistical analysis
Pathology scores were plotted for each animal and the mean calculated per group. Each intervention was tested in at least two independent sets of experiments with a total of 11–22 animals per intervention group. Differences between all groups were calculated by one-way ANOVA with the Kruskal–Wallis test and Dunn’s multiple comparison test. Differences between two groups were calculated with an unpaired, nonparametric Mann–Whitney test. Significance was defined as P<0·05.
Results
Feeding formula without oligosaccharides increases necrotising enterocolitis pathology scores, but addition of human milk oligosaccharides prevents the increase in pathology scores
Consistent with previous studies( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ), most animals in the DF group showed no or very little sign of pathology with scores of 0, 0·5 or 1·0, and the group’s mean pathology score was 0·67 (sd 0·34) (Fig. 2). Compared with the DF group, animals in the FF group that received formula without the addition of oligosaccharides had significantly higher pathology scores of 2·02 (sd 0·63) (P<0·0001). Animals that received HMO with their formula had an average pathology score of 0·90 (sd 0·47), which was significantly lower than the FF group (P<0·0001) and not significantly different from the DF group (P=0·1267).

Fig. 2 Sialylated galacto-oligosaccharides (Sia-GOS) and 2′-fucosyllactose (2′FL) reduce pathology scores in rat necrotising enterocolitis (NEC) model. Rat ileum pathology scores at 96 h postpartum (0: healthy; 4: complete destruction). Rat pups were either fed by the dam (DF) or separated from the dam and fed formula (FF) that was supplemented with human milk oligosaccharides (HMO, 10 mg/ml), galacto-oligosaccharides (GOS, 8 mg/ml), Sia-GOS (500 μm (approximately 0·4 mg/ml)) or 2′FL (2 mg/ml). Each intervention was tested in a total of eleven to twenty-two animals in two independent experiments. ●, The pathology score for an individual animal. Values are mean pathology scores, and standard deviations are represented by bars and whiskers plots. ** P<0·01, *** P<0·001.
Addition of unmodified galacto-oligosaccharides has no effect on necrotising enterocolitis pathology scores, but sialylation of galacto-oligosaccharides reduces pathology scores significantly
As previously shown( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ), the addition of GOS to formula did not lower NEC pathology scores (2·00 (sd 0·63)) when compared with the FF group that received formula without the addition of oligosaccharides (P=0·9895). However, when formula was supplemented with Sia-GOS, pathology scores were reduced to 1·32 (sd 0·56), which was significantly lower compared with the FF group (P<0·01) and the GOS group (P<0·01), but still significantly higher compared with the DF group (P<0·001) and the HMO group (P<0·05).
Addition of 2′-fucosyllactose reduces necrotising enterocolitis pathology scores
HMO are a heterogeneous group of oligosaccharides, and we have recently shown that an oligosaccharide that contained two sialic acids was most effective in reducing NEC pathology scores. Removal of just one sialic acid abrogated the protective effect( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ). However, we also showed that a neutral HMO fraction that contains >30 % 2′FL also reduces NEC pathology scores. Thus, we added 2′FL alone as an intervention, leading to an average pathology score of 1·43 (sd 0·51), which was significantly lower than the scores in the FF group (P<0·01) and the GOS group (P<0·05), but again still significantly higher compared with the DF group (P<0·0001) and the HMO group (P<0·05), confirming that oligosaccharides other than 2′FL contribute to the protective effects of HMO.
Discussion
Sia-GOS and 2′FL significantly reduced pathology scores in the neonatal rat model of NEC. However, the two interventions were less effective than the intervention with pooled HMO, and pathology scores in the Sia-GOS and 2′FL groups were also still significantly higher than in the DF group.
As previously shown( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ), the FF group had elevated pathology scores, whereas the addition of unmodified GOS to the formula had no effect. Animals receiving Sia-GOS had significantly lower pathology scores than the FF and GOS-fed animals. This is in accordance with previous results showing that sialic acid is an essential component for the protective effect of DSLNT. Removal of both sialic acids, as well as removal of only the sialic acid from the terminal galactose from DSLNT, abrogated its protective effect( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ). Sia-GOS as tested in this study is a mixture of mono- and di-sialylated GOS( Reference Wilbrink, ten Kate and Sanders 11 ). As the potentially active disialylated GOS comprised only a very minor fraction of the Sia-GOS material (approximately 3 %), it may be active at very low concentrations. Future purification and structure elucidation is required to identify the active compound(s) within the Sia-GOS mixture and establish their dose–response relationships. As Sia-GOS is not present in human milk, and infants are not exposed to it naturally, full safety and efficacy studies will be required.
2′FL, which also significantly reduced NEC pathology scores in the rat model, is in most cases a major constituent of human milk, and breast-fed infants are exposed to it naturally. However, there is currently no evidence to suggest that infants are more susceptible to develop NEC when they receive breast milk from non-secretor women who have an inactive fucosyltransferase 2 and do not secrete 2′FL with their milk. In a study in preterm piglets, addition of 5 g/l 2′FL to infant formula tended to decrease incidence and severity of NEC, although bacterial colonisation and intestinal structural and functional parameters were not significantly affected( Reference Cilieborg, Jensen and Bering 12 ). Although these results from preclinical models are encouraging, neither the rat nor the piglet model fully represents NEC etiology and pathogenesis in human preterm infants. Clinical intervention studies will be required to assess whether or not 2′FL reduces NEC in preterm infants.
Until now, clinical intervention studies on the effects of other prebiotic oligosaccharides on NEC are also very limited and not yet unambiguous. An example is a clinical trial on the effects of a prebiotic (inulin), a probiotic (Bifidobacterium lactis) and the combination of the prebiotic and probiotic (synbiotic) added to human milk or formula on the prevention of NEC in VLBW infants( Reference Dilli, Aydin and Fettah 16 ). The probiotic and synbiotic decreased NEC, whereas the prebiotic inulin alone, which is not sialylated and structurally different from 2′FL, did not show an effect. In another study, a prebiotic mixture of GOS and long chain fructo-oligosaccharides (ratio of 9:1) did reduce the incidence of NEC in VLBW infants who were exclusively breast-fed( Reference Armanian, Sadeghnia and Hoseinzadeh 17 ).
The underlying mechanisms for the beneficial effect of DSLNT, Sia-GOS and 2′FL on NEC remain unclear. Several potential mechanisms seem plausible. (1) Recent studies describe associations between gut microbiome and NEC onset( Reference Neu 18 , Reference Zhou, Shan and Sodergren 19 ), and one can hypothesise that specific HMO such as DSLNT and 2′FL, as well as other oligosaccharides such as Sia-GOS, serve as structure-specific prebiotics and help shape a desirable gut microbiome or prevent NEC-associated dysbiosis. (2) HMO act as soluble decoy receptors that block the attachment of potential pathogens to the intestinal epithelial cell surface, preventing pathogen proliferation and invasion( Reference Bode 5 ). However, there is currently no clear evidence that specific pathogens or combinations of pathogens are associated with NEC( Reference Zhou, Shan and Sodergren 19 , Reference Smith, Bode and Petersen 20 ). (3) Mucosal neutrophil infiltration is a hallmark during NEC pathogenesis, and HMO reduce selectin-mediated neutrophil infiltration and activation in vitro ( Reference Bode, Kunz and Muhly-Reinholz 21 , Reference Bode, Rudloff and Kunz 22 ). However, these effects are highly structure-specific and require that the effective oligosaccharides be both sialylated and fucosylated( Reference Varki 23 ). DSLNT and Sia-GOS are sialylated, but not fucosylated. 2′FL is fucosylated, but not sialylated. (4) HMO alter epithelial and immune cell responses( Reference Kuntz, Kunz and Rudloff 24 – Reference He, Liu and Leone 26 ), which might affect NEC pathogenesis or improve tissue healing. These effects are highly structure-specific and likely receptor-mediated. We had previously shown that the effects of DSLNT are highly structure-specific, as the removal of just one sialic acid abrogates the protective effect( Reference Jantscher-Krenn, Zherebtsov and Nissan 6 ). We have also shown that specific synthetic DSLNT derivatives reduce NEC in the neonatal rat model of NEC while other derivatives show no effect( Reference Yu, Lau and Thon 27 ), emphasising defined structural requirements. One can hypothesise that one or more active compounds in the Sia-GOS mixture fulfill these structural requirements and entertain the same receptor on epithelial or immune cells as DSLNT. However, 2′FL is structurally very different from DSLNT or Sia-GOS. 2′FL is not sialylated, but fucosylated. One can hypothesise that 2′FL acts through a mechanism different from DSLNT or Sia-GOS, either by using a different receptor on epithelial or immune cells or by using an entirely different route – for example serving as a structure-specific prebiotic.
As the administration of pooled HMO is consistently most effective in reducing pathology scores, providing human milk remains to be the best way to prevent premature infants from developing NEC. Available amounts of products such as Sia-GOS and 2′FL are currently limited for use in infants, but new technologies to up-scale oligosaccharide production advance rapidly, which offers the possibility to use them in the future for preterm infants, who do not have access to human milk. Just recently, 2′FL safety was established in preclinical and clinical studies( Reference Coulet, Phothirath and Allais 28 , Reference Marriage, Buck and Goehring 29 ), and has been registered as a novel ingredient for infant and toddler formula in Europe (Novel Food Application) and the USA (Generally Recognized As Safe registration). Further research is required to investigate the underlying mechanisms of how HMO and other oligosaccharides protect from NEC in preclinical models and to assess safety and efficacy of newly developed oligosaccharides in infants.
Acknowledgements
The authors thank M. H. Wilbrink, G. A. ten Kate and P. Sanders (Microbial Physiology, Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen) for making available Sia-GOS (project jointly financed by the European Union, European Regional Development Fund and the Dutch Ministry of Economic Affairs, Agriculture and Innovation, Peaks in the Delta, the Municipality of Groningen, and the Provinces of Groningen, Fryslan and Drenthe, as well as the Dutch Carbohydrate Competence Center).
Funding for this study was received from FrieslandCampina, Amersfoort, The Netherlands.
M. H. C. S., J. P. K. and L. B. designed the research; C. A. A., E. J.-K. and L. B. conducted the research; C. A. A., M. H. C. S. and L. B. analysed the data; M. H. C. S. and L. B. wrote the paper; M. H. C. S. and L. B. critically reviewed the paper; L. B. had primary responsibility for final content. All authors have read and approved the final manuscript.
M. H. C. S. is an employee of FrieslandCampina. FrieslandCampina donated the GOS and 2′FL used in this study. All other authors declare no conflicts of interest.







