Dementia affected 44·4 million people worldwide in 2013, a figure expected to rise to 135·5 million by 2050( Reference Prince, Guerchet and Prina 1 ). African countries are not spared by this phenomenon( Reference Prince, Guerchet and Prina 1 ); however, the literature on dementia is still sparse. Neurodegenerative dementia is preceded by a period of cognitive decline often termed mild cognitive impairment (MCI). Elderly individuals with MCI constitute a high-risk population for developing dementia( Reference Petersen, Doody and Kurz 2 ).
In Western industrialised countries, weight loss( Reference Renvall, Spindler and Nichols 3 , Reference Berlinger and Potter 4 ) and low late-life BMI( Reference Fitzpatrick, Kuller and Lopez 5 – Reference Luchsinger, Patel and Tang 7 ) have been associated with increased risk of dementia. Weight loss started before the onset of dementia( Reference Knopman, Edland and Cha 8 ) and continued thereafter( Reference Soto, Secher and Gillette-Guyonnet 9 ). Several mechanisms that differ depending on the progress of the disease could explain the weight loss( Reference Sergi, De Rui and Coin 10 ). In the early stages, weight loss may be related to a hypermetabolic state and/or an increase in physical activity linked to abnormal motor behaviour, a tendency to agitation and aggression( Reference Sergi, De Rui and Coin 10 ). At more advanced stages, weight loss could be linked to a reduction in food intake due to forgetting to eat, decreased appetite subsequent to brain dementia-related changes, the presence of concomitant chronic diseases or depression( Reference Sergi, De Rui and Coin 10 ). Weight loss in older people affects both lean and fat mass( Reference Baumgartner, Heymsfield and Roche 11 ). Loss of lean mass (but not fat mass) emerges in the early stages of dementia( Reference Burns, Johnson and Watts 12 ), while fat mass has been associated with more severe stages of dementia( Reference Abellan van Kan, Cesari and Gillette-Guyonnet 13 ).
In low- and middle-income countries, studies on the relationship between nutritional status and cognitive disorders are scarce. A multicentre, cross-sectional study conducted in seven countries (China, Cuba, Mexico, Venezuela, Peru, Dominican Republic and India) has reported that undernutrition, defined by mid-upper arm circumference (MUAC) below 21 cm, had been associated with a higher probability of dementia among people aged 65 years and older and its prevalence increased with dementia severity( Reference Prince, Acosta and Ferri 14 ). In the same population, Taylor and colleagues( Reference Taylor, Albanese and Stewart 15 ) showed that dementia and its severity were associated with both lower MUAC, used as proxy of lean mass, and lower waist circumference as a marker of fat mass. Albanese et al. ( Reference Albanese, Taylor and Siervo 16 ) showed that dementia severity had been independently associated with reported weight loss in older adults and the association strengthened through the stages of dementia. In a case–control study carried out in Chinese people aged 55 years or above, a decrease in BMI and waist circumference has been associated with amnesic MCI and Alzheimer's disease( Reference Chu, Tam and Lee 17 ).
In Africa, a multicentre study conducted in Central Africa( Reference Guerchet, Mouanga and M'belesso 18 ) and a study in Nigeria( Reference Ochayi 19 ) have observed that a BMI < 18·5 kg/m2 has been positively associated with dementia in older adults. A longitudinal study in Nigeria has found that BMI decline has been associated with incident MCI and dementia in older people followed up over 10 years( Reference Ogunniyi, Gao and Unverzagt 20 ).
Most studies used BMI as an anthropometric indicator of nutritional status. However, its interpretation in older people requires caution due to the decrease in height with age and because BMI cannot distinguish between different body compartments. MUAC and arm muscular circumference (AMC) are anthropometric indicators used as proxies of lean mass( Reference Taylor, Albanese and Stewart 15 , Reference Gurney and Jelliffe 21 ). A MUAC < 24 cm and an AMC < 5th percentile of reference population are also used to define undernutrition( 22 ).
We therefore hypothesised that cognitive disorders are associated with undernutrition. The objective of the present study was to evaluate the association between MCI and dementia and undernutrition in elderly people in the Central African Republic (CAR) and Republic of Congo (ROC) in Central Africa.
Materials and methods
Ethics statement
Approvals were obtained from the Ethics Committee of the Ministry of Public Health in CAR, the CERSSA (Comité d' Ethique de la Recherche en Sciences de Santé) in ROC, and the Comité de Protection des Personnes du Sud-Ouest et d'Outre-Mer 4 in France. All the participants and/or their families gave informed consent before being included in the study.
Study population
The present study population consisted of participants in the Epidemiology of Dementia in Central Africa (EPIDEMCA) programme – a multicentre, community-based, cross-sectional study conducted in rural and urban areas in CAR and ROC between November 2011 and December 2012. The detailed methodology is published elsewhere in an open-access publication( Reference Guerchet, Mbelesso and Ndamba-Bandzouzi 23 ). In brief, subjects aged 65 years and above who had lived in the study area for at least 6 months were eligible. The study areas consisted of the capitals of CAR (Bangui) and ROC (Brazzaville), and two rural regions (Nola in CAR and Gamboma in ROC). The sample size was calculated a priori with the aim of estimating dementia prevalence, and was then estimated at 500 in each study site. In urban areas, the selection was carried out using random sampling proportional to the main city subdivision size. In rural areas, exhaustive sampling using a door-to-door approach was preferred due to logistic and financial constraints.
Measures
Data were collected in two phases. The first phase was conducted in the general population at participants' homes. Interviews were carried out by ten specifically trained interviewers who were students in medical school (at least in their 6th year of curriculum), students of biology or nurses from Bangui and Brazzaville Universities using a questionnaire including cognitive, physical, demographic and risk factor assessment. Neurologists conducted the second phase in participants with probable cognitive disorders at the closest hospital or health centre between 3 and 14 weeks later.
Anthropometric measurements
Weight, height, knee height, MUAC and triceps skinfold thickness were measured during the first phase in every consenting participant. The detailed methodology is presented in Table 1.
Table 1 Anthropometric measurement and undernutrition markers

MUAC, mid-upper arm circumference; AMC, arm muscular circumference.
Mild cognitive impairment and dementia diagnoses
During the first phase, cognitive testing was performed using the Community Screening Interview for Dementia (CSI-D)( Reference Hall, Hendrie and Brittain 24 ) adapted, back-translated and pretested in local languages (Sango in CAR; Lari, Lingala and Kituba in ROC). A relative of each old person included was interviewed at the same time using the CSI-D informant section to assess daily activities. Every subject with a poor performance in the CSI-D cognitive tests (cognitive score ≤24·5; sensitivity = 93 % and specificity = 82 % in a previous study, personal data) was suspected to have cognitive impairment and invited for further clinical assessment with a neurologist (phase 2).
Further psychometric tests were conducted, including the Free and Cued Selective Reminding Test( Reference Grober, Buschke and Crystal 25 ), Zazzo's cancellation task( Reference Zazzo 26 ) and Isaac's Set Test of verbal fluency( Reference Isaacs and Kennie 27 ). Neurologists performed examinations during which histories of stroke and depressive disorders were sought. Orientation skills and daily activities were also investigated in order to evaluate the level of dependence. They diagnosed dementia and MCI according to the Diagnostic and Statistical Manual-IV criteria( 28 ) and MCI Petersen criteria( Reference Petersen 29 ), respectively. In case of uncertain cases, an experienced neurologist reviewed all medical records and test performances in order to reach a consensus.
Covariates
All covariates were collected during the first phase. Sociodemographic data included age, sex (male/female), marital status (living as a couple/living alone, including widow, single and separated), formal education (yes/no), country (CAR/ROC) and area (urban/rural). Age was ascertained by official documents, from an informant or through a local event calendar. Two historical landmarks were also used in each country according to the validated method of age estimation using historical events( Reference Ogunniyi and Osuntokun 30 , Reference Paraïso, Houinato and Guerchet 31 ). Health-related covariates included frailty (defined as Study of Osteoporotic Fractures (SOF) index>2( Reference Ensrud, Ewing and Taylor 32 )) and history of stroke (yes/no). Lifestyle covariates included smoking status (current non-smoker (including former smoker); current smoker), physical activity (defined as having walked or cycled at least 150 min the past week and categorised by yes/no)( 33 ) and excessive alcohol consumption (defined as having consumed at least 21 alcohol units for males and 14 alcohol units for females the past week). Depressive and anxiety symptoms were assessed with the Geriatric Mental State version B3( Reference Copeland, Dewey and Griffiths-Jones 34 ). Dietary factors included difficulties in eating an individual's fill (yes/no) and the daily frequency of consumption of dairy products, fruits, vegetables, legumes, starches, oleaginous food, meat/fish/egg and sweet food. Psychosocial factors consisted of death of spouse (yes/no) and death of a child, these two events occurring after the participant reached 65 years of age (yes/no).
Data management and analysis
All data collected were computerised directly during the interview using an interface specially created with Epidata version 3·1 (EpiData Association). Undernutrition was assessed using three different markers used as dependent variables: BMI < 18·5 kg/m2( 22 ), MUAC < 24 cm( 22 ) and AMC < 5th sex-specific percentile based on Frisancho chart for the age of 65–74·9 years( Reference Frisancho 35 ). Cognitive disorders, used as independent variables consisted of three categories: cognitively normal, MCI and dementia. All covariates were used as categorical variables, except for age, which was used as a continuous variable since linearity hypothesis could not be rejected.
Sample characteristics described by country of residence included age, area, sex, marital status, education level, health-related factors, lifestyle factors, psychological factors, dietary factors, psychosocial factors, cognitive disorders, anthropometric measures (weight, height, BMI, MUAC and AMC) and markers of undernutrition. Means with their standard deviation were used as summary statistics for age and continuous anthropometric measures. Percentages were calculated for all categorical variables. Characteristics were compared between countries using χ2 test for categorical variables or t test and ANOVA for continuous variables.
The prevalence of undernutrition as defined by each of the three markers was calculated according to cognitive disorders for each country.
To test the relationship between cognitive disorders and undernutrition, we used binary logistic regression models using a complete case analysis method. We started with unadjusted binary logistic regression model (model 0). The sociodemographic variables were entered first (model 1), then health-related covariates (model 2), lifestyle covariates (model 3), psychological covariates (model 4), dietary covariates (model 5) and finally psychosocial covariates (model 6). In the final models, we tested for interaction between cognitive disorders and sex, country of residence and area (rural/urban) using overall Wald χ2 test with a level of significance fixed at 0·05. Significant interaction was found between cognitive disorders and country of residence when AMC was considered as dependent variable. All results were thus presented stratified by country. Because of the lack of validated thresholds to define undernutrition in older African people, we modelled the effects of cognitive disorders on BMI, MUAC and AMC as continuous variable separately for each country using linear regression adjusted for sociodemographic, health-related, lifestyle, psychological, dietary and psychosocial covariates. In each model, a potential cluster effect of districts due to the study design was taken into account and entered into the model. The statistical analysis was carried out using Stata version 10.1 for Windows (StataCorp).
Results
Sample characteristics
In total, 2002 participants were included. The prevalence of dementia was 8·4 % in CAR and 6·9 % in ROC and the prevalence of MCI was 7·2 and 6·1 %, respectively (details on the prevalence of dementia are the purpose of another specific paper). Table 2 presents a comparison of the prevalence of undernutrition as defined by the three markers used in addition to other characteristics between CAR and ROC. Only undernutrition defined as BMI < 18·5 kg/m2 was significantly different between the countries, with a lower prevalence in ROC (P= 0·002).
Table 2 Characteristics of study population according to their residence country, EPIDEMCA (Epidemiology of Dementia in Central Africa), 2011–12 (Number of participants; percentages; mean values and standard deviations)

CAR, Central African Republic; ROC, Republic of Congo; MCI, mild cognitive impairment; MUAC, mid-upper arm circumference; AMC, arm muscular circumference.
Fig. 1 shows the prevalence of undernutrition according to cognitive disorders. Among MCI subjects, the prevalence of undernutrition ranged from 10·0 to 30·5 % in CAR and from 32·0 to 42·0 % in ROC, according to the marker considered. Among demented subjects, the figures varied from 30·8 to 65·6 % in CAR and from 28·9 to 52·3 % in ROC.

Fig. 1 The prevalence of undernutrition according to cognitive disorders in Central African Republic (![]() ) and Republic of Congo (
) and Republic of Congo (![]() ), EPIDEMCA (Epidemiology of Dementia in Central Africa), 2011–12. (a) BMI < 18·5 kg/m2, (b) mid-upper arm circumference < 24 cm, (c) arm muscular circumference < 5th percentile. MCI, mild cognitive impairment.
), EPIDEMCA (Epidemiology of Dementia in Central Africa), 2011–12. (a) BMI < 18·5 kg/m2, (b) mid-upper arm circumference < 24 cm, (c) arm muscular circumference < 5th percentile. MCI, mild cognitive impairment.
Association between cognitive disorders and undernutrition
Separate binary logistic regression models were used to test the association between cognitive disorders and undernutrition on a sample with no missing values for any undernutrition markers (n 1847 subjects; Table 3). In CAR, MCI was only associated with a lower probability of having MUAC < 24 cm in unadjusted and adjusted models. However, dementia was associated with a higher probability of being undernourished as defined by the three markers in unadjusted models but significantly with BMI < 18·5 kg/m2 and MUAC < 24 cm. Adjustment for sociodemographic covariates reduced the strength of the associations between dementia and undernutrition defined by BMI < 18·5 kg/m2 and MUAC < 24 cm; however, subsequent adjustments did not have notable effects on the relationships. While it did not reach significance in the unadjusted model, the association between dementia and AMC < 5th percentile was significant after adjustment for sociodemographic covariates and in subsequent models. In ROC, in unadjusted and fully adjusted models, all OR suggested an increased probability of undernutrition in individuals with MCI or dementia; however, the associations became non-significant after several adjustments. Only the association between AMC < 5th percentile and MCI remained highly significant in fully adjusted models.
Table 3 Unadjusted and adjusted OR from separate binary logistic regression measuring the association between cognitive disorders and undernutrition, EPIDEMCA (Epidemiology of Dementia in Central Africa), 2011–12 (Odds ratios and 95 % confidence intervals)

CAR, Central African Republic; ROC, Republic of Congo; AMC, arm muscular circumference; MUAC, mid-upper arm circumference; MCI, mild cognitive impairment.
* Unadjusted model.
† Model 0+age, sex, formal education, marital status and area.
‡ Model 1+health-related factors (frailty and stroke).
§ Model 2+lifestyle factors (excessive alcohol consumption, current smoking and physical activity).
∥ Model 3+psychological factors (depressive and anxious symptoms).
¶ Model 4+dietary factors (difficulties in eating an individual's fill, frequency of consumption of dairy products, fruits, vegetables, legumes, starches, oleaginous food, meat/fish/egg and sweet food).
** Model 5+psychosocial factors (death of spouse, death of a child after the age of 65 years).
Sensitivity analysis
Because of a lack of validated thresholds to define undernutrition in older African people, we examined the relationship of BMI, MUAC and AMC as continuous dependent variables with cognitive disorders in separate linear regression models (Table 4). In CAR, MCI was associated with none of the indicators; however, dementia was associated with all of them with coefficients indicating a lower mean in demented participants than normal ones. In ROC, β-coefficients indicated a lower BMI, MUAC and AMC mean in both MCI and demented subjects.
Table 4 β-Coefficients from separate multivariate linear regression* measuring the association between cognitive disorders and BMI, mid-upper arm circumference (MUAC) and arm muscular circumference (AMC), EPIDEMCA (Epidemiology of Dementia in Central Africa), 2011–12 (β-Coefficients and 95 % confidence intervals)
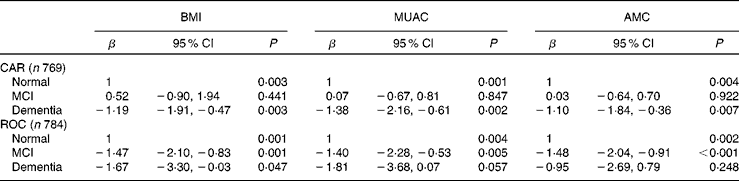
CAR, Central African Republic; MCI, mild cognitive impairment; ROC, Republic of Congo.
* Adjusted for age, sex, formal education, marital status and area, frailty, stroke, excessive alcohol consumption, current smoking, physical activity, depressive and anxious symptoms, difficulties in eating an individual's fill, frequency of consumption of dairy products, fruits, vegetables, legumes, starches, oleaginous food, meat/fish/egg and sweet food, death of spouse occurred after the participant reached the age of 65 years and death of a child after the participant reached the age of 65 years.
Discussion
The present study investigated the association between cognitive disorders (MCI and dementia) and undernutrition in a general elderly population from Central Africa, a subject very poorly documented in African countries so far( Reference Guerchet, Mouanga and M'belesso 18 – Reference Ogunniyi, Gao and Unverzagt 20 , Reference Khater and Abouelezz 36 ). The present study confirms our hypothesis that cognitive disorders are associated with undernutrition in this older population even if all the associations did not reach statistical significance.
In ROC, MCI was associated with a higher probability of being undernourished as defined by AMC < 5th percentile. The same tendency was observed for other markers of undernutrition even if they were not statistically significant. In our sensitivity analysis, MCI was also associated with lower BMI, MUAC and AMC. However, in CAR, no association was observed. In the literature, few studies have investigated the relationship between MCI and weight loss, low BMI or undernutrition( Reference Chu, Tam and Lee 17 , Reference Khater and Abouelezz 36 , Reference Orsitto 37 ), and the majority were conducted in clinical settings. All showed a deterioration in nutritional status in MCI subjects compared with those cognitively unimpaired. In addition, several longitudinal studies have shown that low BMI had been associated with preclinical stages of Alzheimer's disease( Reference Barrett-Connor, Edelstein and Corey-Bloom 38 , Reference Johnson, Wilkins and Morris 39 ). The present results observed in a general older population from ROC were thus consistent with the literature. In CAR, the lack of difference in the prevalence of undernutrition between normal and MCI subjects explaining the lack of association observed is unclear. It is unlikely that this could be explained by difference in MCI assessment between countries since (1) the same expert (J.-F. D.) trained neurologists from both countries and (2) this expert reviewed each record. Future longitudinal studies are needed to confirm or refute this specific finding.
Dementia was associated with a higher probability of being undernourished whatever the marker used in both countries, even after adjustment for several confounders. These results are consistent with other studies conducted in African countries( Reference Guerchet, Mouanga and M'belesso 18 – Reference Ogunniyi, Gao and Unverzagt 20 ) and other low- and middle-income countries( Reference Prince, Acosta and Ferri 14 – Reference Albanese, Taylor and Siervo 16 ), but also with several large longitudinal studies conducted in Western countries( Reference Gustafson, Bäckman and Joas 40 – Reference Buchman, Wilson and Bienias 42 ).
A stronger association with AMC was observed in ROC. This suggests that undernutrition is preferentially linked to muscle loss even in the early stages of the disease. MUAC and BMI do not distinguish between lean and fat masses, while AMC specially assesses the muscle mass, main lean mass component. This is consistent with the results of Burns et al. ( Reference Burns, Johnson and Watts 12 ) who found that reduced lean mass was associated with early stage Alzheimer's disease. However, this contradicts the findings of Abellan et al. ( Reference Abellan van Kan, Cesari and Gillette-Guyonnet 13 ) who did not find an association between sarcopenia and cognitive impairment in women aged 75 years or above and Wirth et al. ( Reference Wirth, Bauer and Sieber 43 ) who found no association between mild or moderate cognitive impairment and fat-free mass.
There are no thresholds to define undernutrition with BMI, MUAC or AMC in the older African population. We therefore chose those recommended by the WHO in adults( 22 ). However, body composition changes with age, lean body mass decreases while fat mass increases( Reference St-Onge and Gallagher 44 ). We cannot be sure about the validity of these thresholds in our older population. The use of indicators as quantitative variables in our sensitivity analysis enabled us to overcome the use of cut-offs to define undernutrition. Participants with cognitive impairment had a worse nutritional status than did others even if they were not undernourished as defined by the markers used in the present study. Deterioration of nutritional status seems to affect all body compartments since fat and muscular mass assessed by BMI and AMC, respectively, are both associated with cognitive disorders. This confirms previous results( Reference Burns, Johnson and Watts 12 , Reference Abellan van Kan, Cesari and Gillette-Guyonnet 13 ).
The present study has several other limitations. Due to its cross-sectional design, it is not possible to determine the temporality of the observed associations. Several literature reviews have suggested that weight loss, which could lead to undernutrition, would be more a consequence than a cause of dementia( Reference Sergi, De Rui and Coin 10 , Reference Inelmen, Sergi and Coin 45 , Reference Prince, Albanese and Guerchet 46 ). However, we cannot exclude that undernutrition be linked to others mechanisms. Some potential confounders were not taken into account such as social factors or other comorbidities. In addition, mechanisms of weight loss in dementia are still unknown. We then cannot exclude a reverse causality since some results have shown that higher adiposity at old age would protect against cognitive disorders( Reference Hughes, Borenstein and Schofield 41 ). The low number of prevalent cases of MCI and dementia by country may affect the precision of estimates and lead to non-significant associations. This fact has also precluded the analysis of the effect of the severity of dementia, which was associated with weight loss in several studies( Reference Taylor, Albanese and Stewart 15 , Reference Albanese, Taylor and Siervo 16 ). It has also precluded the analysis of the relationship with dementia subtypes. However, previous studies have failed to show the differences in nutritional status according to dementia subtypes( Reference Orsitto 37 , Reference Faxén-Irving, Basun and Cederholm 47 ). Among the total EPIDEMCA sample, the BMI value was not available for 5·6 % of subjects, the value of MUAC for 4·1 % and the value of AMC for 5·7 %. However, subjects without anthropometric measurements were older than those with measurements, but also more frail as defined by the SOF index( Reference Ensrud, Ewing and Taylor 32 ) and had more cognitive impairment (data not shown). This may have led to underestimation of the observed associations. In addition, we cannot exclude the possibility that the most undernourished people would die before presenting cognitive disorders, leading to a survival bias that underestimated the observed associations. We cannot ensure that we did not miss cases during the first phase since we did not make ascertainment of participants screened negative with CSI-D. However, the threshold used was established from the same population in a previous study( Reference Guerchet, Mouanga and M'belesso 18 ) with a good sensitivity and specificity; therefore, we believed that this number would be very low.
The strengths of the present study are the large sample size and the mix of participants coming from rural and urban areas of two African countries. To our knowledge, this is the first study of this magnitude conducted in French-speaking African countries. Another strength is the richness of the anthropometric measures available and the accuracy with which they have been used.
In terms of public health, the present results highlight the need for weight monitoring in demented people and more generally in elderly people, as their population will substantially grow during the next decades. Unfortunately, the older people are not a priority in the health and nutrition programmes of countries in sub-Saharan Africa, which primarily target children ( < 5 years), women (pregnant and breast-feeding) and people living with HIV/AIDS( Reference Kimokoti and Hamer 48 ). Primary care health workers are not yet trained to manage with older adults who often present several comorbidities. However, monitoring and controlling weight loss in older adults may potentially increase longevity, promote compression of morbidity, hence improving the quality of life of patients and indirectly carers( Reference Taylor, Albanese and Stewart 15 ).
Conclusion
The present results in Central Africa are consistent with an increased probability of undernutrition in cognitively impaired older subjects. Data from the present study add to existing information on the relationship between cognitive disorders and undernutrition and show the same trends as in Western and other low- and middle-income countries. However, longitudinal studies on larger samples seem to be necessary to elucidate this relationship in a context where lifestyle and culture are different from those of wealthier countries.
Acknowledgements
The authors would like to thank the participants in the EPIDEMCA study as well as all the students and nurses involved for their assistance. They also thank the town hall staff, mayors and chiefs of districts for their collaboration, and the Universities of Bangui (CAR) and Marien Ngouabi in Brazzaville (ROC), the Health ministries of the CAR and the ROC for their support.
The present study was funded by The French National Agency (Agence nationale de la recherché; ANR) through the ANR-09-MNPS-009-01 grant. The University of Limoges, Doctoral School 523 of Limoges University, the Limousin Regional Council and the French Ministry of Higher Education and Research funded the doctoral position of S. P.; The funding sources had no role in the design, implementation, analysis or interpretation of the data.
The authors' contributions are as follows: S. P., M. G., B. N.-B. and P. M. supervised the data collection; J.-F. D., B. N.-B. and P. M. were responsible for diagnosing cognitive disorders; S. P. conducted the data analysis and wrote the first draft; M. G. and J.-P. C. were involved in the data analysis and in the interpretation of the data. All authors reviewed the manuscript, provided further contributions and suggestions and approved the final draft. All authors worked collectively to design the EPIDEMCA protocol.
None of the authors has any conflict of interest to declare.








