Diabetes mellitus was the fourth leading cause of death in Taiwan in 2005 (Department of Health, Taiwan, 2005). It is a metabolic disorder characterized by hyperglycaemia and dyslipidaemia. Many diabetic patients have an increased risk of CHD, peripheral vascular diseases and cerebrovascular diseases (Parillo & Riccardi, Reference Parillo and Riccardi2004). Endothelium dysfunction accompanied by upregulated inflammatory mediators is a major contributing factor to the pathogenesis of diabetic vascular complications (Nystrom et al. Reference Nystrom, Nygren and Sjoholm2006). Furthermore, the abnormalities in nutrient metabolism resulting from diabetes mellitus lead to impairment of wound healing and vulnerability to infection and sepsis.
Sepsis is a common clinical problem with extremely high mortality rates. Several components of the immune system are implicated in the process of sepsis, including the release of proinflammatory mediators and activation of endothelial cells and polymorphonuclear leucocytes (PMN; Shimizu et al. Reference Shimizu, Newman, Tanaka and Shaw1992; Williams & Hellewell, Reference Williams and Hellewell1992). On activated endothelium, members of the Ig family of adhesion molecules – intercellular adhesion molecules (ICAM) and vascular cell adhesion molecules (CAM) – are expressed. CAM are important in the adhesion of leucocytes to activated endothelium (Carlos & Harlan, Reference Carlos and Harlan1994). CD11a/CD18 and CD11b/CD18 are members of the leucocyte adhesion molecules-β2 integrin. CD11a and CD11b are thought to play central roles in mediating the firm adhesion of leucocytes to endothelial cells (Henderson et al. Reference Henderson, Lim, Tessier, Gavins, Mathies, Perretti and Hogg2001). Overexpressions of adhesion molecules facilitate leucocyte–endothelial interactions which result in endothelial dysfunction and thus aggravate PMN accumulation and tissue damage (Ulbrich et al. Reference Ulbrich, Eriksson and Lindbom2003; Nolte et al. Reference Nolte, Kuebler, Muller, Wolff and Messmer2004). One study showed that plasma ICAM-1 levels increase in septic patients with multiple organ failure (Whalen et al. Reference Whalen, Doughty, Carlos, Wisniewski, Kochanek and Carcillo2000). Also, plasma ICAM-1 in diabetic patients was significantly higher than that in healthy controls (Glowinska et al. Reference Glowinska, Urban, Peczynska and Florys2005).
Fish oils are rich sources of n-3 PUFA, especially EPA and DHA. A number of clinical trials have shown that fish oil has immune modulatory effects (Grimm et al. Reference Grimm, Mayer, Mayser and Eigenbrodt2002). The major advantages of n-3 fatty acids are related to their postulated reductions in proinflammatory effects. Several studies have shown that dietary fish oil has beneficial clinical effects on diseases including rheumatoid arthritis, inflammatory bowel diseases, multiple sclerosis and insulin-dependent diabetes mellitus (Calder, Reference Calder1997, Reference Calder2006). However, a previous study revealed that fish oil supplementation suppresses lymphocyte proliferation and has immunosuppressive properties (Virella et al. Reference Virella, Fourspring, Hyman, Haskill-Stroud, Long, Virella, La Via, Gross and Lopes-Virella1991). An ex vivo study also showed that n-3 fatty acids inhibit proliferative response and IL-2 production in lymphocytes obtained from diabetes mellitus patients (Alnajjar et al. Reference Alnajjar, Sari, Abuharfeil, Hudaib and Aburjai2006). In accordance with such observations, laboratory animals fed fish oil exhibited lower survival and higher viable bacteria numbers than those fed other types of fat when infected with bacteria (Chang et al. Reference Chang, Dulloo, Vladoianu, Piguet, Arsenijevic, Girardier and Pechere1992; Puertollano et al. Reference Puertollano, Puertollano, Ruiz-Bravo, Jimenez-Valera, De Pablo and De Cienfuegos2004). Conflicting results were also observed in septic conditions when n-3 fatty acids were administered (Fritsche et al. Reference Fritsche, Shahbazian, Feng and Berg1997; Lanza-Jacoby et al. Reference Lanza-Jacoby, Flynn and Miller2001). Most animal studies done previously were seldom performed with co-morbidities, the studies concerned with the influence of fish oil on the inflammatory response focused exclusively on the condition of diabetes mellitus or sepsis. Studies investigating the effects of dietary fish oil on diabetes mellitus complicated with sepsis are rare. Therefore, we induced polymicrobial sepsis after treating diabetic mice with fish oil to investigate the effect of n-3 fatty acids on adhesion molecules and inflammatory cytokines in diabetic mice complicated with sepsis. Because oxyradicals released from leucocytes that accumulate in organs may damage organ cells and induce organ dysfunction (Klebanoff & Seymour, Reference Klebanoff and Seymour2005), we analysed the myeloperoxidase (MPO) activities in organs as an indicator for identifying the extent of tissue injury resulting from diabetes mellitus with sepsis.
Materials and methods
Animals
Male ICR mice weighing approximately 25–30 g were purchased from the Animal Center of National Taiwan University, College of Medicine. Mice were maintained in a temperature-controlled (23 ± 2°C) and humidity-controlled (55 ± 15 %) room with a 12 h light–dark cycle. All mice were allowed free access to a standard chow diet and water for 1 week before the study. Care of the laboratory animals was established by Taipei Medical University, and protocols were approved by the Animal Committee. Diabetes was induced in the mice by a single intraperitoneal injection of streptozotocin (Sigma Chemical Co., St Louis, MO, USA) at a dose of 150 mg/kg body weight as previously described by Oguri et al. (Reference Oguri, Motegi and Endo2003). Streptozotocin was dissolved immediately before use in saline to a concentration of 15 mg/ml. Three days later, blood was obtained by piercing a needle into the tail vein of the mice; it was directly applied on to a strip of a blood glucose monitor to determine the glucose levels. Mice were considered diabetic only if their blood glucose levels exceeded 2000 mg/l (Ackerman & Leibman, Reference Ackerman and Leibman1977). The average blood glucose levels for normal mice were 1195 (sd 124) mg/l (n 8). Diabetic mice were not treated with insulin in the present study.
Experimental design and procedures
The diabetic mice were divided into two groups according to the weight and blood glucose of the animals to make average weights and blood glucose levels among groups as similar as possible. All mice were maintained for 3 weeks on a medium-fat (10 %, w/w) semi-purified diet. The diets fed to the two experimental groups were identical except for the sources of the fat (Table 1). The soyabean oil group (SO, n 30) was exclusively fed soyabean oil (Taiwan Sugar Co., Taipei, Taiwan), while the fish oil group (FO, n 30) had 23 % fish oil (Denofa Co., Fredrikstad, Norway) and 77 % soyabean oil (Table 1). The fish oil contained 34 % EPA, 27 % DHA and 72 % total n-3 fatty acids, while the mixed tocopherols was 2·4 mg/g. The soyabean oil contained 6·5 % n-3 fatty acids and 55 % n-6 fatty acids according to the manufacturer. The n-3/n-6 ratio in the FO diet was 1:2 in the present study. After feeding the respective diet for 3 weeks, polymicrobial sepsis was induced in the mice. Sepsis was induced by caecal ligation and puncture (CLP) according to the method of Ayala et al. (Reference Ayala, Deol, Lehman, Herdon and Chaudry1994). Briefly, mice were lightly anaesthetized with diethyl ether and their abdomens were opened through a midline incision below the diaphragm. The caecum was isolated and ligated just below the ileocaecal valve. The caecum was then punctured twice with a 22-gauge needle and was slightly compressed until a small drop of stool appeared. After CLP was performed, the caecum was replaced into the abdominal cavity and the wound was closed in layers. Diabetes mellitus–sepsis mice were killed at 0, 6 and 24 h after the CLP, with ten mice at each time-point. Mice in 0 h groups were killed immediately after CLP. All mice were anaesthetized with intraperitoneal pentobarbital sodium (50 mg/kg body weight) and were killed by heart puncture. The abdomen was opened and the peritoneal cavity was lavaged with 2 ml PBS. The peritoneal lavage fluid was collected for TNF-α and PGE2 analyses. Fresh blood samples were collected in tubes containing EDTA-Na2 for analysing leucocyte CD11a/CD18 and CD11b/CD18 expressions. Plasma samples were stored at − 80°C until glucose and ICAM-1 was measured. Tissues including the liver, kidneys, intestines and lungs were rapidly harvested and stored at − 80°C for the measurement of MPO activities.
Table 1 Composition of the experimental diets (g/kg)
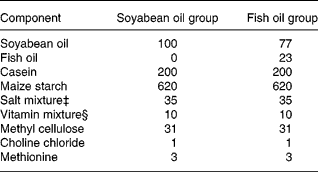
‡ The salt mixture contained the following (mg/g): calcium phosphate diabasic, 500; sodium chloride, 74; potassium sulphate, 52; potassium citrate monohydrate, 20; magnesium oxide, 24; manganese carbonate, 3·5; ferric citrate, 6; zinc carbonate, 1·6; cupric carbonate, 0·3; potassium iodate, 0·01; sodium selenite, 0·01; and chromium potassium sulphate, 0·55.
§ The vitamin mixture contained the following (mg/g): thiamin hydrochloride, 0·6; ricoflavin, 0·6; pyridoxine hydrochloride, 0·7; nicotinic acid, 3; calcium pantothenate, 1·6; d-biotin, 0·05; cyanocobalamin, 0·001; retinyl palmitate, 1·6; dl-α-tocopherol acetate, 20; cholecalciferol, 0·25; menaquinone, 0·005.
Measurements and analytical procedures
Measurements of plasma glucose and intercellular adhesion molecule-1 concentrations
Glucose levels were determined by colorimetric methods after an enzymatic reaction with peroxidase (Randox Co., Antrim, Ireland). Procedures followed the manufacturer's instructions. Concentrations of ICAM were measured by using a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA). Antibodies specific for mouse ICAM-1 were coated on to the wells of the microtitre strips provided. The minimum detectable dose for ICAM-1 was 17 pg/ml. The within-assay CV was 6·3 % in the present study.
Measurement of TNF-α and PGE2 levels in peritoneal lavage fluid
Concentrations of TNF-α were measured using a commercially available ELISA. Antibodies specific for mouse TNF-α were coated on to the wells of the microtitre strips provided (R&D Systems). PGE2 concentrations were also measured by ELISA. The surfaces of the microtitre plates were precoated with mouse monoclonal antibody. Acetylcholinesterase covalently coupled to PGE2 was used as the enzymatic tracer (R&D Systems). The detection limit for TNF-α was 5·1 pg/ml and for PGE2 was 8·5 pg/ml. The within-assay CV for TNF-α and PGE2 were 6·8 % and 7·2, respectively.
Analysis of the CD11a/CD18 distribution in lymphocytes and CD11b/CD18 in polymorphonuclear leucocytes
Lymphocytes and PMN in blood were gated on the basis of the forward scatter and side scatter profiles by flow cytometry (Coulter, Miami, FL, USA), and were analysed for the expressions of CD11a/CD18 and CD11b/CD18, respectively. Fresh blood (100 μl) was incubated with 10 μl fluorescent isothiocynate-conjugated rat monoclonal anti-mouse CD11a (I21/7) and phycoerthrin-conjugated rat monoclonal anti-mouse CD18 (C71/16; Serotec, Oxford, UK) for 15 min at 4°C. Fluorescent isothiocynate-conjugated rat IgG2a and phycoerthrin-conjugated rat IgG2a were used for isotope control (Serotec). Subsequently, erythrocytes were lysed with lysing buffer (Serotec). The percentages of CD11a/CD18 expressed on lymphocytes were analysed by flow cytometry. Fluorescence data were collected and the results are presented as a percentage of CD11a-presenting cells in 1 × 105 lymphocytes. To measure CD11b/CD18 expressions on PMN, fluorescent isothiocynate-conjugated rat monoclonal anti-mouse CD11b (M1/70.15) and phycoerthrin-conjugated rat monoclonal anti-mouse CD18 (C71/16; Serotec) were added into 100 μl fresh blood. Fluorescent isothiocynate-conjugated rat IgG2b and phycoerthrin-conjugated rat IgG2a were used for isotope control (Serotec). Fluorescence data were collected on 1 × 105 PMN which were also analysed by flow cytometry. The results are presented as a percentage of CD11b/CD18 expression in 1 × 105 PMN. Non-specific fluorescence was determined on cells incubated with isotype and fluorochrome-matched control antibodies (Hsu et al. Reference Hsu, Chiu, Yeh, Hou, Chou and Yeh2006).
Measurement of myeloperoxidase activity in organs
The method of measuring MPO activity was modified as previously described (Hillegass et al. Reference Hillegass, Griswold, Brickson and Albrightson-Winslow1990). Tissue samples were homogenized in 50 mm-potassium phosphate buffer (pH 6.0), and centrifuged at 2000 g at 4°C for 15 min. After discarding the supernatant, the pellets were suspended in a solution containing 0·5 % hexadecyl-trimethyl-ammonium bromide dissolved in potassium phosphate buffer (pH 7.0) and centrifuged for 30 min at 15 000 g and 4°C. An aliquot of the supernatant was then allowed to react with a solution of tetra-methyl-benzidine (1·6 mm) and 0·1 mm-H2O2. The absorbance at 650 nm was measured for 3 min and the rate of change in the absorbance was used to calculate the activities of MPO. MPO activity was defined as the quantity of enzyme degrading 1 μmol peroxide per min at 37°C and the data were expressed in units/g wet tissue.
Statistical analysis
Data are expressed as means and standard deviations. All statistical analyses were performed with SigmaStat version 3.1 software (SYSTAT Software Inc., Chicago, IL, USA). Differences among groups were analysed by two-way ANOVA using Fisher's post hoc test. P < 0·05 was considered statistically significant.
Results
Body weights and plasma glucose levels
There were no differences in the initial body weights and body weights after feeding the respective diets for 3 weeks between the two experimental groups (data not shown). There were no differences in fasting plasma glucose concentrations between the FO and SO groups at various time-points after CLP (Table 2).
Table 2 Plasma glucose concentrations (mg/l) of the fish oil group (FO) and the soyabean oil group (SO)‡ (Mean values and standard deviations)
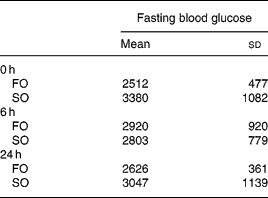
‡ For details of procedures, see Materials and methods section.
Plasma intercellular adhesion molecule-1 levels
Plasma ICAM-1 levels were higher at 24 h than 0 and 6 h after CLP in both groups (P < 0·001 for time effect). There were no differences in ICAM-1 concentrations between the SO and FO groups at various time-points after CLP (Fig. 1).

Fig. 1 Plasma intercellular adhesion molecule-1 (ICAM-1) levels in the fish oil group (□) and the soyabean oil group (■). For details of procedures, see Materials and methods section. Values are means with their standard deviation depicted by vertical bars. Mean values were significantly different from those of the other time-points in the same group: †P < 0·05. CLP, caecal ligation and puncture.
PGE2 and TNF-α concentrations in peritoneal lavage fluid
The PGE2 levels in the FO groups were significantly lower than in the SO groups at each time-point after CLP (P < 0·001 for diet effect). The PGE2 levels were lower at 0 h than at 6 and 24 h after CLP in both groups (P < 0·001 for time effect; Fig. 2(A)). Concentrations of TNF-α increased with the progression of sepsis in the SO group, whereas no differences were found among the FO groups at various time-points (P < 0·001 for time effect). The SO group had higher TNF-α concentrations than the FO group 24 h after CLP (P = 0·039 for diet effect, P = 0·013 for diet and time interaction; Fig. 2(B)).

Fig. 2 Concentrations of PGE2 (A) and TNF-α (B) in peritoneal lavage fluid in the fish oil group (FO, □) and the soyabean oil group (■). For details of procedures, see Materials and methods section. Values are means with their standard deviation depicted by vertical bars. Mean values were significantly different from those of the the FO group at the same time point: *P < 0·05. Mean values were significantly different from those of the other time-points in the same group: †P < 0·05; ‡P < 0·001. CLP, caecal ligation and puncture.
Expressions of CD11a/CD18 on lymphocytes and CD11b/CD18 on polymorphonuclear leucocytes
CD11a/CD18 expressions on lymphocytes were higher at 6 h, whereas the percentage was lower at 24 h in the SO group than in the FO group. SO groups had the highest CD11a expression at 6 h, while the highest CD11a percentage was found at 24 h after CLP in the FO group (P = 0·001 for time effect, P < 0·001 for diet and time interaction; Fig. 3(A)). The expressions of CD11b/CD18 on PMN decreased in the SO group as sepsis progressed. CD11b/CD18 expressions were significantly higher in the SO group than in the FO group at 0 h. There were no differences in the expressions of CD11b/CD18 among the various FO groups (P < 0·001 for diet effect, P = 0·006 for time effect, P < 0·001 for diet and time interaction; Fig. 3(B)).

Fig. 3 Expressions of lymphocyte CD11a/CD18 (A) and neutrophil CD11b/CD18 (B) in the in the fish oil group (FO, □) and the soyabean oil group (■). For details of procedures, see Materials and methods section. Values are means with their standard deviation depicted by vertical bars. *Significantly different from the FO group at the same time point. Mean values were significantly different from those of the the FO group at the same time point: *P < 0·05. Mean values were significantly different from those of the other time-points in the same group: †P < 0·05; ‡P < 0·001. CLP, caecal ligation and puncture.
Myeloperoxidase activities in the liver, lungs, kidneys and intestines
The activities of MPO increased as sepsis progressed and reached a peak at different time-points depending on the type of oil in various organs. The FO groups had lower MPO activities at 0 h in the liver, at 0 and 6 h in the kidneys, and at 24 h after CLP in the lungs compared with those in the SO groups (P < 0·05; Table 3).
Table 3 Myeloperoxidase activities (U/g tissue) in organ homogenates of the fish oil group (FO) and the soyabean oil group (SO)‡ (Mean values and standard deviations)
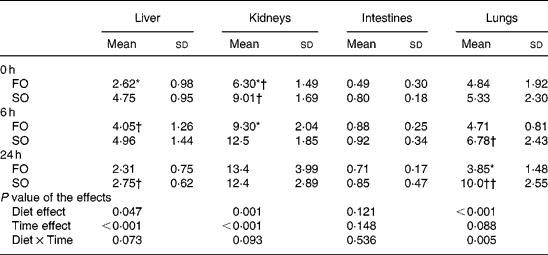
Mean values were significantly different from those of the SO group at the same time-point: *P < 0·05.
Mean values were significantly different from those of the other time-points in the same groups: †P < 0·05; ††P < 0·001.
‡ For details of procedures, see Materials and methods section.
Discussion
In the present study, n-3 fatty acids provided 4·3 % of the total energy in the FO diet. This amount of n-3 fatty acids is comparable to values used in studies with beneficial results, which showed that n-3 fatty acid supplementation reduced inflammatory-related mediators and improved survival in rodents in a CLP model (Johnson et al. Reference Johnson, Griswold and Muakkassa1993; Lanza-Jacoby et al. Reference Lanza-Jacoby, Flynn and Miller2001). The n-3/n-6 ratio was adjusted to 1:2, because this ratio was considered to exert the most favourable modulation of lipid mediator synthesis (Morlion et al. Reference Morlion, Wrenger, Puchsiein and Furst1997). In the present study, we used streptozotocin to induce diabetes, a model frequently used to stimulate non-insulin-dependent diabetes in animal studies (Chyi & Yeh, Reference Chyi and Yeh2000), and CLP is a clinically relevant model of gut-derived sepsis in mice (Maier et al. Reference Maier, Traeger, Entleutner, Westerholt, Kleist, Huser, Holzmann, Stier, Pfeffer and Heidecke2004).
PGE2 is a product of arachidonic acid metabolism. Numerous studies have shown that an increased dietary intake of n-3 fatty acids suppresses PGE2 synthesis (Mayatepek et al. Reference Mayatepek, Paul, Leichsenring, Pfisterer, Wagner, Domann, Sonntag and Bremer1994; Calder, Reference Calder2006). The previous results are in good agreement with the present finding that diabetic mice fed FO had lower PGE2 levels than those fed SO after CLP. TNF-α is an important mediator involved in the onset and regulation of inflammatory and immune response. Circulating TNF-α is associated with significant pathologic change, possibly leading to mortality (DiPiro, Reference DiPiro1997), suggesting that the TNF-α synthesis must be controlled. A study by Blok et al. (Reference Blok, de Bruijn, Leenen, Eling, van Rooijen, Stanley, Buurman and van der Meer1996) showed that the plasma TNF-α concentration was significantly increased in mice fed n-3 fatty acids. A previous study performed by our laboratory also showed that compared with safflower oil, feeding diabetes mellitus–sepsis rats with FO produced lower PGE2 and higher TNF-α in peritoneal lavage fluid (Chyi & Yeh, Reference Chyi and Yeh2000). These earlier results are inconsistent with the findings in the present study that the TNF-α concentrations in peritoneal lavage fluid paralleled the PGE2 concentrations in diabetes mellitus–sepsis mice with FO administration. Those studies which found elevated TNF-α levels provided more than 10 % of total energy as n-3 fatty acids, while in the present study, a relatively lower dose of n-3 fatty acids was administered. It is possible that, although PGE2 was suppressed, the amount of n-3 fatty acids used in the present study was not sufficiently potent to enhance excessive TNF-α production.
CD11a/CD18 are exclusively expressed on leucocytes and CD11b/CD18 are abundant in PMN (Henderson et al. Reference Henderson, Lim, Tessier, Gavins, Mathies, Perretti and Hogg2001). We analysed lymphocyte CD11a/CD18 expression in the present study, because the function of T lymphocyte subsets is important on influencing the type of immunity and the inflammatory response to diabetes mellitus–sepsis (DiPiro, Reference DiPiro1997). CD11a is important for lymphocyte trafficking and activation. Although lymphocytes constitute a relatively small population of the total lymphocyte pool in normal conditions (Westermann & Pabst, Reference Westermann and Pabst1990), the total numbers of lymphocyte subsets in blood were greatly increased under inflammatory conditions. In the present study we found that compared with the diabetic mice fed SO, FO administration resulted in lower CD11a and CD11b expressions in the early stage of sepsis. An in vitro study showed that endothelial cells treated with n-3 fatty acids inhibited cytokine-induced expression of adhesion molecules (De Caterina & Libby, Reference De Caterina and Libby1996). A study by Miles et al. (Reference Miles, Wallace and Calder2000) showed that dietary FO reduced CAM expression by murine peritoneal macrophages. The findings of the present study suggest that leucocyte adhesion and migration may be attenuated when FO is administered in a diabetes mellitus–sepsis condition.
ICAM-1 is a cell surface protein expressed on the vascular endothelium. ICAM-1 and its ligands CD11a and CD11b are important mediators of host defence localized in the earliest lesions of inflammation (Weber, Reference Weber2003). In the present study, we observed that plasma ICAM-1 levels increased as sepsis progressed, however, the changes in plasma ICAM-1 were inconsistent with the alteration in the integrin expressed on leucocytes. Since ICAM-1 plays an important role in transendothelial migration of leucocytes, we speculate that blood leucocytes have transmigrated into the tissue especially at the late stage of sepsis so that only limited amounts of leucocytes can be measured in the blood.
MPO is an enzyme synthesized by neutrophil and monocyte precursor cells. MPO plays an important role in leucocyte-mediated vascular injury responses in inflammatory vascular diseases (Klebanoff & Seymour, Reference Klebanoff and Seymour2005). Previous studies showed that MPO activities increased in vessels of diabetic rats (Zhang et al. Reference Zhang, Yang and Jennings2004) and in type 2 diabetic patients (Moldoveanu et al. Reference Moldoveanu, Tanaseanu, Tanaseanu, Kosaka, Manea, Marta and Popescu2006). Also, a study performed by our laboratory found that MPO activities increased in the lungs, liver, intestines and kidneys at an early stage of sepsis (Hsu et al. Reference Hsu, Chiu, Yeh, Hou, Chou and Yeh2006). In the present study, we found that FO administration resulted in lower MPO activities in the liver, kidneys and lungs at 0, 6 or 24 h after sepsis in diabetic mice. The present finding may indicate that diabetic mice with FO administration have less neutrophil infiltration in these organs after sepsis. A previous study showed that proinflammatory cytokines upregulate CAM expression (Myers et al. Reference Myers, Wertheimer, Schembri-King, Parks and Wallace1992). It is possible that FO decreased proinflammatory cytokine production and thus decreased CAM expression and neutrophil migration.
In summary, the present study demonstrated that diabetic mice with low-dose n-3 fatty acid supplementation resulted in lower PGE2 and TNF-α levels after CLP. Also, leucocyte CD11a and CD11b expressions and MPO activities in various organs were decreased at different time-points after sepsis when FO was administered. The present findings suggest that dietary FO supplementation may attenuate leucocyte adhesion and infiltration into tissues, thus producing a favourable effect in diabetic mice complicated with sepsis.
Acknowledgements
This study was supported by research grant NSC95-2320-B-038-042 from the National Science Council, ROC.








