Introduction
The conventional three-dimensional conformal radiation therapy (3DCRT) techniques are still used in many centres, although important advances have been done on radiation delivery.Reference Purdy1, 2 Some treatments of 3DCRT are still appropriate and the new technologies provide a limited real-added value, such as in the adjuvant treatment of breast cancer, in the neo-adjuvant treatment of rectal cancer and in the postoperative treatment of prostate cancer.
In 3DCRT treatments prolonged from 5 to 7 weeks, it is important to know carefully the levels of uncertainty in the whole process of clinical dose delivery. Over the past decade, a number of papers have analysed the different types of uncertainties that characterise these treatments of conventional 3DCRT.Reference van Herk, Remeijer, Rasch and Lebesque3–Reference Langen and Jones7 A conventional 3DCRT treatment usually consists of one planning phase and multiple irradiation sessions. During planning, the patient geometry is visualised using computed tomography (CT) images. The visualised structures are the basis for the contouring of the target volume and organs at risk, thus being the references for the treatment plan construction; the intent is then to deliver this plan in all irradiation fractions with the aim to get the correct dosage for the actual target. Of course, target delineation errors are the first source of error and they should always be considered.
Other major sources of geometrical uncertainty that may compromise the exact delivery of a plan can be outlined: patient set-up variation, organ motion and deformation and machine-related errors; in addition, random or systematic nature of these errors was examined.8
Starting from the International Commission on Radiation Units and Measurements9 definition for clinical target volume (CTV) and planning target volume (PTV), some authors such as van Herk et al.Reference van Herk, Remeijer, Rasch and Lebesque3, Reference van Herk, Remeijer and Lebesque4 and Stroom and HeijmenReference Stroom and Heijmen5 have reported fairly general recipes to determine CTV–PTV margins as a function of these geometrical uncertainties.
Hurkmans et al.Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 described set-up error verification by means of portal imaging defining the various types of set-up errors and discussing different causes of set-up errors by reviewing the results of a large number of studies regarding patient set-up verification for treatments of head and neck, prostate, pelvis, lung and breast cancer. Moreover, other authors have proposed protocols to be used in clinical practice to reduce systematic set-up errors, as for example, the shrinking action-level (SAL) protocol of Bel et al.Reference Bel, van Herk, Bartelink and Lebesque10, Reference Bel, Vos and Rodrigus11 and the no action-level (NAL) and the extended no action-level (eNAL) protocols of de Boer and HeijmenReference de Boer, van Sornsen de Koste, Senan, Visser and Heijmen12–Reference de Boer and Heijmen14. In particular, NAL protocol is quite used in clinical practice as a good compromise between effectiveness on accuracy gain and limited added workload9. Lozano et al.Reference Lozano, Pérez and Torres15 described advantages of NAL protocol applications in conventional 3DCRT for patients with head and neck cancer and patients with breast cancer. They also evaluated CTV–PTV margins and found significant reductions, pointing out that the margins estimated in their work cannot be readily adopted in other environments, as different techniques, immobilisation devices and procedures can have an impact on margins’ magnitude.
This paper focus on inter-fractions set-up errors in conventional 3DCRT, delivered in a single centre of radiation oncology, reporting random and systematic set-up errors evaluated for different anatomical sites of treatment using an offline verification protocol and verifying the impact of a NAL correction protocol application on the reduction of systematic-setup errors and CTV–PTV margins.
Materials and methods
At the Radiation Oncology Centre of Sassari, about 600 patients are treated yearly using 3DCRT techniques with palliative or curative intent by two Varian (Varian Medical, Palo Alto, CA, USA) linear accelerators, model ‘DBX600’ and ‘2100C’, both equipped with a multileaf collimator with 120 leaves (Varian Millennium 120) and an electronic portal imaging device; the planning patient geometry is obtained using CT scan images, treatment plans are elaborated by means of a Varian treatment planning system Eclipse and overall treatment process is managed by Varian ARIA v8.9 record and verify system. Patients’ set-up is verified through the ARIA utility offline review, comparing orthogonal electronic portal images with digitally reconstructed radiographies (DRRs) from CT planning scans.
Groups of selected patients
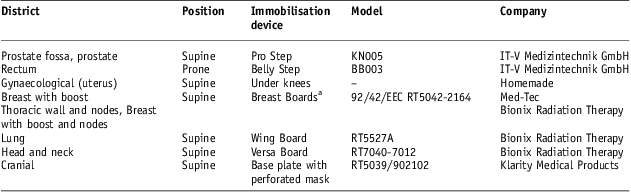
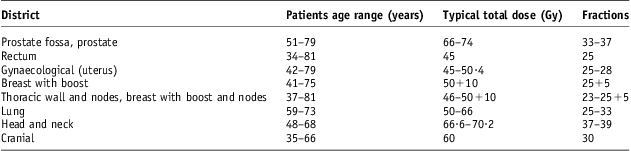
For this study, we considered the last 100 patients treated with curative intent in 2011. Patients were divided into groups according to the anatomical site as summarised in Table 1. In the same table, dose prescriptions and fractionation schemes are reported.
Table 1 Groups of treated patients
Treatment position was supine for all patients groups, except for rectum cancer ones for which the position was prone. Immobilisation devices used for the different patient groups are reported in Table 2. Cancer prostrate patients were positioned with the immobiliser Pro Step model KN005 of IT-V Medizintechnik GmbH (Innsbruck, Austria) that allows the correct positioning of the lower abdomen and lower extremities. Cancer rectum patients were in prone position using Belly Step model BB003 of IT-V Medizintechnik GmbH to keep a comfortable positioning and reduce the amount of irradiated small bowel volume. Gynaecological cancer patients were in supine position with a simple in-house device to be kept under the knees. Breast Board model 92/42/EEC by MEDTEC (now CIVCO Medical Solutions trade mark, IA, USA) was used on Varian accelerator ‘2100C’ and Breast Board model RT5042-2164 by Bionix Radiation Therapy was used on Varian accelerator ‘DBX600’ for breast cancer patients. Lung cancer patients were in supine position with Wing Board model RT5527A device by Bionix Radiation Therapy (OH, USA) and without any breathing gating system. Head and neck cancer patients, only with oropharyngeal locations, were in supine position with Versa Board model RT7040-7012 by Bionix Radiation Therapy and the base plate model RT5039 with thermoplastic perforated mask model 902102 by Klarity Medical Products (Guangzhou, China); cranial cancer patients were in supine position using only the base plate model RT5039 with the same thermoplastic perforated mask model.
Table 2 Position and immobilisation systems

Note :aBreast Boards Med-Tec and Bionix one are used for breast with boost, for thoracic wall and nodes and breast with boost and nodes. Med-Tec one is used on Varian accelerator 2100 C and Bionix one is used on Varian accelerator DBX600.
Verification protocol of set-up
For all patients, orthogonal electronic portal images were acquired during the first three sessions of the first week of treatment in double exposure modality using 10 × 10 cm setup fields. In the subsequent weeks of treatment, portal images were obtained in one session per week. Portal images of each patient were obtained by means of an amorphous silicon detector (Varian AS500). In total, for the 100 patients considered in this study, 668 pairs of orthogonal portal images were acquired and processed comparing with DRRs and evaluating isocenter displacements by ARIA utility offline review.
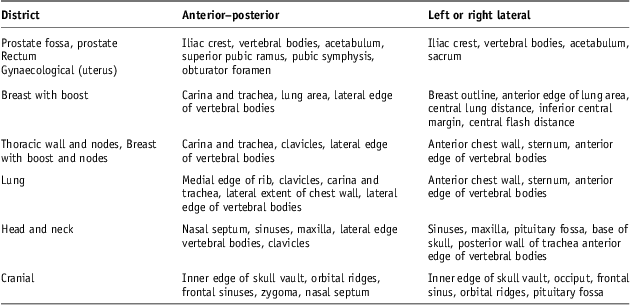
The registration process between portal images and DRRs has been then based on anatomy matching. The anatomy key structures used on anterior–posterior (AP) and lateral (Lat) images are reported in Table 3.
Table 3 Electronic portal images and digitally reconstructed radiographs anatomy matching key structures

Note : It recommended at least three structures, visible within the field outlined.8
Following Royal College of Radiologists Guidelines8 for gynaecological, rectum and prostate cancer patients on AP images, key structures were iliac crest, vertebral bodies, acetabulum, superior pubic ramus, pubic symphysis and obturator foramen; on Lat images: iliac crest, vertebral bodies, acetabulum and sacrum.
On AP images of breast cancer patients, key structure were carina and trachea, lung area and lateral edge of vertebral bodies and in case of sovraclavear nodes they also included clavicles; on Lat images were used for breast outline, anterior edge of lung area, central lung distance, inferior central margin and central flash distance8; in case of sovraclavear nodes, they included anterior chest wall, sternum and anterior edge of vertebral bodies.
On Lat images of lung cancer patients, the same structures as for sovraclavear nodes were used; on AP images, we used medial edge of rib, clavicles, carina and trachea, lateral extent of chest wall and lateral edge of vertebral bodies.
For head and neck cancer patients on AP images, the key structures were nasal septum, sinuses, maxilla, lateral edge vertebral bodies and clavicles; on Lat images: sinuses, maxilla, pituitary fossa, base of skull, posterior wall of trachea and anterior edge of vertebral bodies.
For cranial cancer patients on AP images, inner edge of skull vault, orbital ridges, frontal sinuses, zygoma and nasal septum were used; on Lat images, key structures were inner edge of skull vault, occiput, frontal sinus, orbital ridges and pituitary fossa.
The process yields two measures of displacement of the isocentre for each set-up field incidence: superior–inferior (SI) and left–right (LR) directions for the AP images; and SI and AP directions for the Lat images. Tolerance of displacements was set at 5 mm for all patient groups, except head and neck and cranial ones for whom tolerance has been placed at 3 mm. The basic offline protocol adopted in Radiation Oncology Center implies that any single value of displacement greater than tolerance limit leads to a patient repositioning attempt and a new electronic portal imaging acquisition during each portal imaging sections of treatment, the first three sections of the first week and the once-weekly sections of the subsequent weeks. Protocol did not provide any other correction of set-up.
Elaboration of set-up displacements data
Displacement data from matching of 668 pairs of orthogonal portal images and reference DRRs were elaborated to evaluate set-up errors and random and systematic components using Hurkmans et al.Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 definitions. Random inter-fraction errors are deviations between different fractions and systematic errors are deviations between the planned patient position and the average patient position over the entire course of the treatment. For each patient, the random and systematic errors were calculated for each projection on LR, SI and AP directions. In the SI direction, assessments were made from both the AP and Lat portal images. The ith patient systematic and random errors on any x direction (x: LR, SI and AP), called mi,x and SDi,x were defined, respectively, as the mean and the standard deviation of the projection on x direction of inter-fraction isocentre displacements.
Following Stroom and Heijmen,Reference Stroom and Heijmen5 for each group of treated patients we calculated the population systematic and random set-up errors on x direction called ∑x and σx, respectively, and defined by
In this approach, the mean population errors on x direction, Mx, defined by
is not considered as a contribution to population systematic error on x direction being usually small or limited by controlling devices of the CT planning system.
Random and systematic components of set-up errors were calculated by an in-house software using output data from Varian ARIA utility offline review, through which both patient-specific and population errors could be studied. The in-house software, developed in Visual Basic as an Excel Macro (Microsoft Corporation, Redmond, WA, USA), reads automatically an arbitrary number of text files from offline review, one for each patient, containing set-up displacements data of an entire course of treatment and collected in different directories on the basis of anatomical district. Data timing and coherence are verified and values of set-up displacements are archived in Excel sheets; statistical indexes necessary to CTV–PTV margin determination are computed and summary tables and graphs are produced.
The CTV–PTV margin on LR, SI and AP directions was evaluated following van Herk et al.Reference van Herk, Remeijer and Lebesque4 and Royal College of Radiologists Guidelines8 by
in which omitting the subscript x for simplicity of writing, ∑ and σ are the combined sum of the standard deviations of all contributing systematic and random errors, respectively, defined by
considering patient set-up, differences in transferring image data from CT planning system to linear accelerator measured through a phantom test, change in target position and shape between delineation and treatment, difference between the defined and ‘ideal’ CTV. Following Van Herk et al.Reference van Herk, Remeijer and Lebesque4a value was set at 2·5; σ p is the standard deviation of Gaussian penumbra; b coefficient depends on different beam configuration used in treatment planning; and c term takes into account parameters that affect margin in linear manner, such as breathing.8 The CTV–PTV margins were evaluated for head and neck, prostate and lung cancer patients groups using locally measured systematic and random population set-up errors and literature reported values for other uncertainties affecting accuracy in conventional 3DCRTReference Ausili Cefaro, Gabriele, Genovesi, Rosi, Tabocchini and Viti16 as explained in Table 4. In this study, c term of expression (4) took into account the breathing parameter and a value of 3 mm for Lat–AP and AP–LR directions and one of 4 mm for AP–SI direction were used for lung district; for head and neck and cranial and prostate districts a value of 0 was used on each direction.
Table 4 Literature used values to describe the other uncertainties affecting CTV–PTV margins in conventional 3DCRT different from set-up errors
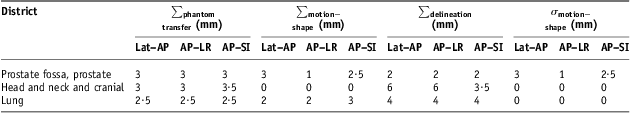
Note: ∑phantom transfer, ∑ motion−shape and ∑delineation are systematic uncertainties components of expression 5; σ motion−shape is random uncertainties component of expression 6.
Abbreviations: CTV, clinical target volume; PTV, planning target volume; 3DCRT, three-dimensional conformal radiotherapy; Lat–AP, anterior–posterior (AP) displacement of the isocentre for lateral (Lat) field incidence of set-up image; AP–LR, left–right (LR) displacement of the isocentre for AP field incidence of set-up image; AP–SI, superior–inferior (SI) displacement of the isocentre for AP field incidence of set-up image.
Statistical t-test and F-test of Microsoft Excel have been applied to explore differences of systematic and random set-up errors on SI direction as estimated with AP images and with Lat images.
The relative importance of the patients’ population systematic set-up errors on CTV–PTV margin was assessed in the absence of other corrective measures.
Then an application of a corrective NAL protocol was simulatedReference de Boer, van Sornsen de Koste, Senan, Visser and Heijmen12–Reference de Boer and Heijmen14 and the effect on systematic set-up errors was calculated. The NAL protocol implies that the systematic error is calculated after a few fractions and a correction is performed, thus corresponding to the total magnitude of the systematic error, regardless of the tolerance for that treatment district; as this NAL approach does not define an action level for corrections, there is a subset of patients in whom the systematic error is so small that applying a correction would be not so much practical, for example, by moving the couch of only 1 or 2 mm. In this study, following The Royal College of Radiologists, Society and College of Radiographers report8 that suggested only systematic error >2 mm should be corrected, a threshold of 3 mm was used; in the NAL approach used here, if the systematic errors were within tolerance, there was no action on set-up displacements evaluated in subsequent weekly set-up imaging; the correction of subsequent weekly set-up imaging was done when the systematic error, estimated from the pairs of orthogonal portal images made during the first three fractions of the first treatment week, was greater than the threshold.
The relative impact on the CTV–PTV margins for the case of patients with tumours of the head and neck, prostate and lung was estimated too.
Results
Systematic and random set-up errors were evaluated for a total of 100 patients treated in 3DCRT with curative intent in 2011. Breast without nodes inclusion patients were 29 with 170 pairs of orthogonal portal images used, and thoracic wall/breast with nodal inclusion patients were 17 with 107 pairs of images; in this second group, a patient with eight pairs of images was excluded because of the detection of a bias in the centring procedure. The other 54 patients were distributed among 16 for prostate cancer, 13 for head and neck and cranial cancer, nine for gynaecological cancer, 8 for rectum cancer and 8 for lung cancer. Head and neck and cranial cancer patients were positioned through a similar immobilisation technique and were considered in the same group.
In Table 5, we report population systematic and random set-up errors as defined in expressions (1) and (2), respectively; in the same table, the corresponding number of patients and pairs of orthogonal portal images are indicated. Systematic and random set-up errors are reported on SI and on LR directions for the AP images (AP–SI and AP–LR, respectively) and AP direction for the left or right (Lat) images (Lat–AP).
Table 5 Systematic and random set-up errors
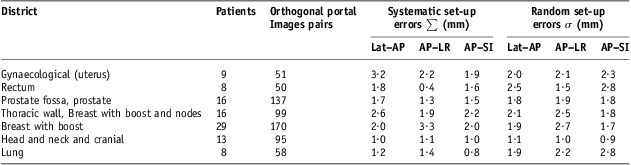
Note : Head and neck and cranial cancers patients showed comparable set-up errors.
Abbreviations: Lat–AP, anterior–posterior (AP) displacement of the isocentre for lateral (Lat) field incidence of set-up image; AP–LR, left–right (LR) displacement of the isocentre for AP field incidence of set-up image; AP–SI, superior–inferior (SI) displacement of the isocentre for AP field incidence of set-up image.
Systematic population set-up errors and random ones for head and neck and cranial cancer population were limited and comparable, about 1 mm on each direction of isocentre shift and the mean population errors on each direction Mx, defined in expression (3) was consistently not significant, resulting in <0·2 mm in absolute value. Correspondingly, also patient systematic and random set-up errors were <3 mm, almost always <2 mm, on each direction for each patient (Figure 1a).

Figure 1 Systematic and random set-up errors using offline correction protocol. (a) Head and neck and cranial cancers patients case and (b) prostate cancers patients’ case.
Abbreviations: AP–SI: superior–inferior (SI) displacement of the isocenter for anterior–posterior (AP) field incidence of set-up image; AP–LR: left–right (LR) displacement of the isocentre for AP field incidence of set-up image; Lat–SI: superior–inferior (SI) displacement of the isocenter for lateral (Lat) field incidence of set-up image; Lat–AP: AP displacement of the isocentre for lateral (Lat) field incidence of set-up image.
For prostate cancer population, set-up errors ranged between 1·3 and 1·9 mm, and, on each direction systematic set-up errors were a little smaller than random ones; the mean population errors on each direction Mx was smaller, in absolute value, than 0·8 mm. For this anatomical district, patient systematic and random set-up errors were <5 mm and systematic ones often <3 mm (Figure 1b).
In addition, for rectum and lung districts, systematic population set-up errors were smaller than random ones; however, in the lung case, the mean population errors Mx were not negligible on AP–SI direction, resulting in about 1·4 mm in absolute value. For those anatomical districts, patient random set-up errors were, on average, larger than those for the prostate group, also with values >5 mm in some patients (Figures 2a and 2b).

Figure 2 Systematic and random set-up errors using offline correction protocol. (a) Rectum cancer patients’ case and (b) lung cancer patients’ case.
Abbreviations: AP–SI: superior–inferior (SI) displacement of the isocenter for anterior–posterior (AP) field incidence of set-up image; AP–LR: left–right (LR) displacement of the isocentre for AP field incidence of set-up image; Lat–SI: superior–inferior (SI) displacement of the isocenter for lateral (Lat) field incidence of set-up image; Lat–AP: AP displacement of the isocentre for lateral (Lat) field incidence of set-up image.
The largest population systematic set-up errors were recorded for breast without nodes inclusion, thoracic wall and breast with nodes inclusion and gynaecological groups, with values ranging between 1·9 and 3·3 mm; however, for the last two groups, significant values for the mean population errors Mx were recorded on AP–LR direction, about 2·1 mm in absolute value, and only for gynaecological cancer also on Lat–AP direction with about 1·5 mm. Frequency distributions for breast cancer patients’ systematic set-up errors were evaluated on each direction (AP–SI, AP–LR, Lat–SI and Lat–AP) with 1 mm isocentre shift steps (Figure 3a); in this case, some systematic set-up errors were >6 mm on AP–SI and AP–LR directions were observed too. Patients’ systematic and random set-up errors for thoracic wall and breast with boost and nodes group and for gynaecological one were calculated on each directions for each patients and respectively reported in Figure 4a and in Figure 5a. Patients’ systematic set-up errors showed, on average, a significant shift from zero, more pronounced in the direction AP–LR and more modest in the Lat–SI and AP–SI directions; on the average, no significant shift resulted in Lat–AP direction for thoracic wall and breast with boost and nodes group, whereas a significant shift was in Lat–AP for the gynaecological group, as evidenced by mean population errors Mx; patient systematic set-up errors >5 mm in absolute value were recorded in both groups in some patients in Lat–AP for gynaecological group and in each direction for thoracic wall and breast with boost and nodes group. For those groups, population random set-up errors were comparable, on average, with those of rectum and lung cases (Table 5).

Figure 3 Breast cancer patients’ systematic set-up errors distribution. (a) Offline correction protocol case and (b) no action level (NAL) correction protocol case.
Abbreviations: AP–SI: superior–inferior (SI) displacement of the isocenter for anterior–posterior (AP) field incidence of set-up image; AP–LR: left–right (LR) displacement of the isocentre for AP field incidence of set-up image; Lat–SI: superior–inferior (SI) displacement of the isocenter for lateral (Lat) field incidence of set-up image; Lat–AP: AP displacement of the isocentre for lateral (Lat) field incidence of set-up image.

Figure 4 Systematic and random set-up errors for thoracic wall and breast with boost and nodes cancer patients. (a) Offline correction protocol case and (b) no action level (NAL) correction protocol case.
Abbreviations: AP–SI: superior–inferior (SI) displacement of the isocenter for anterior–posterior (AP) field incidence of set-up image; AP–LR: left–right (LR) displacement of the isocentre for AP field incidence of set-up image; Lat–SI: superior–inferior (SI) displacement of the isocenter for lateral (Lat) field incidence of set-up image; Lat–AP: AP displacement of the isocentre for lateral (Lat) field incidence of set-up image.

Figure 5 Systematic and random set-up errors for gynaecological cancer patients. (a) Offline correction protocol case and (b) no action level (NAL) correction protocol case.
Abbreviations: AP–SI: superior–inferior (SI) displacement of the isocenter for anterior–posterior (AP) field incidence of set-up image; AP–LR: left–right (LR) displacement of the isocentre for AP field incidence of set-up image; Lat–SI: superior–inferior (SI) displacement of the isocenter for lateral (Lat) field incidence of set-up image; Lat–AP: AP displacement of the isocentre for lateral (Lat) field incidence of set-up image.
Some unexpected differences were observed between the behaviour of AP–SI and Lat–SI; t-test on patient systematic set-up errors, mi,x , e F-test on patient random set-up errors, SDi,x, were done with α = 0·05 threshold for each i-th patient and x direction AP–SI and Lat–SI. Differences of mi,x resulted statistically significant for six patients, four in thoracic districts (two in breast without nodes and two in thoracic wall and breast with nodes) and two in head and neck group; differences of SDi,x, resulted statistically significant for 13 patients in thoracic districts (five in breast without nodes, five in thoracic wall and breast with nodes and three in lung), four in head and neck group and three in pelvic district (two in rectum and one in prostate). Population systematic set-up errors, ∑, AP–SI and Lat–SI resulted statistically significant using F-test for breast without nodes group and thoracic wall and breast with nodes group, with AP–SI systematic errors a little bit greater than Lat–SI ones; also AP–SI population random errors, σ, resulted about equal or a little bit greater than Lat–SI ones and for this reason these latter ones have been omitted in Table 5.
The systematic set-up errors presented so far were obtained only by applying offline review verification protocol. In Table 6, for each group of patients considered in Table 5, population systematic set-up errors, calculated by applying the NAL correction protocol with a threshold of 3 mm, are shown; in the same table, the corresponding percentage reductions are reported. Significant reductions were recorded on each direction for breast and thoracic wall and breast with boost and nodes groups, reaching up to 50% on AP–SI direction for breast group and obtaining a correspondent population systematic set-up error of about 1 mm. Appreciable reductions in population systematic errors were also obtained for gynaecological group on Lat–AP and AP–LR directions, for Lung group on AP–LR direction and for prostate on AP–SI direction. No reduction was recorded for head and neck and cranial cancer groups of patients. Applying NAL correction protocol, the frequency distributions for breast cancer patients’ systematic set-up errors were evaluated on each direction (AP–SI, AP–LR, Lat–SI and Lat–AP) with 1 mm isocentre shift steps and shown in Figure 3b. In this case, benefit from the NAL protocol is shown in comparison with the corresponding frequency distributions in Figure 3a: all breast cancer patients recorded systematic set-up errors of <5 mm on each direction. In Figure 4b and in Figure 5b, patient systematic set-up errors are shown for thoracic wall and breast with boost and nodes group and for gynaecological group, respectively, evaluated by applying NAL correction protocol with a threshold of 3 mm. This was also evident for those groups benefiting from NAL protocol application and patient systematic set-up errors were always obtained with <4 mm in absolute value on each direction.
Table 6 NAL protocol application with 3 mm threshold to reduce systematic set-up errors
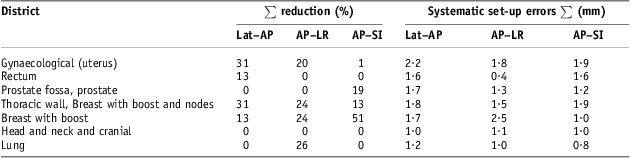
Note : ∑ per cent reduction of set-up errors was calculated starting from systematic set-up errors on Table 5 obtained without NAL protocol application.
Abbreviations: NAL, no action level; Lat–AP, anterior–posterior (AP) displacement of the isocentre for Lat field incidence of set-up image; AP–LR, left–right (LR) displacement of the isocentre for AP field incidence of set-up image; AP–SI, superior–inferior (SI) displacement of the isocentre for AP field incidence of set-up image.
These encouraging results on the reduction of systematic set-up errors arising from the NAL correction protocol application led to analyse the impact on CTV–PTV margins. CTV–PTV margin, as described above, were calculated by the expressions (4), (5) and (6) for prostate cancer, head and neck and cranial cancer and lung cancer patients groups. In Table 7, CTV–PTV margins are shown and evaluated on each direction for those three groups of patients with systematic set-up errors obtained by applying only offline review protocol. Calculated CTV–PTV margins ranged from about 10 mm on AP–LR for prostate cancer group to <20 mm on AP–SI direction for lung cancer group. Applying NAL correction protocol with a threshold of 3 mm, because of the other important uncertainties present in 3DCRT,Reference Ausili Cefaro, Gabriele, Genovesi, Rosi, Tabocchini and Viti16 did not lead to any appreciable reduction in CTV–PTV margins, even in cases of prostate cancer and lung cancer patients.
Table 7 Impact on CTV–PTV margin of NAL protocol application with 3 mm threshold
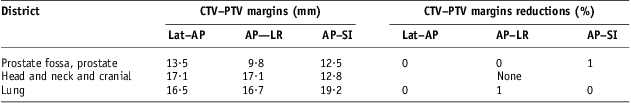
Abbreviations: CTV, clinical target volume; PTV, planning target volume; NAL, no action level; Lat–AP, anterior–posterior (AP) displacement of the isocentre for lateral (Lat) field incidence of set-up image; AP–LR, left–right (LR) displacement of the isocentre for AP field incidence of set-up image; AP–SI, superior–inferior (SI) displacement of the isocentre for AP field incidence of set-up image.
Discussion
The set-up errors presented in this paper were obtained on a limited number of cases, with the last 100 patients treated with curative intent in 2011 in Sassari, in a small Radiation Oncology Centre, using conventional 3DCRT techniques and through the comparison of 668 pairs of orthogonal portal images obtained during treatment sessions with DRRs from CT planning scans. These conventional 3DCRT techniques did not involve any use of breathing control systems, optical tracking of patient surface during treatment sessions or image-guided radiation therapy technologies in verifying organ motions and deformations. Population systematic and random set-up errors, as defined in expressions (1) and (2), respectively, and evaluated using only the offline verification protocol as described above, were compared with those reported in the review presented by Hurkmans et al.Reference Hurkmans, Remeijer, Lebesque and Mijnheer6
In that review, random and systematic set-up errors for head and neck region evaluated through an electronic portal imaging device, ranged between 1·3 and 2·7 mm, with random ones a few tenths of a millimetre smaller than the systematic ones and without a clear and distinct difference between the different directions of isocentre shift; the correspondent set-up errors resulted slightly smaller, about 1 mm. These little more limited set-up errors seemed reasonable in relation to the performance of accuracy and reproducibility provided by the immobilisation system used (Table 2), and no bias was found for Mx, as defined in expression (3). Application of a NAL correction protocol for this group of patients could give a hypothetical significant contribution to the reduction of systematic set-up errors using thresholds as low as about 1 or 2 mm (Figure 1a) and Table 6. However, these thresholds do not appear to be consistent with the accuracy and typical equipments used in conventional 3DCRT.
For prostate cancer patients, population random set-up errors found in this study, about 1·8 mm on each direction, were fully comparable with those reported in the review by Hurkmans et al.Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 (Table 5 and Figure 1b); population systematic set-up errors resulted in the lower part of the range covered by the errors reported in that review,Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 about 1·3, 1·5 and 1·7 mm, respectively, on LR, SI and AP directions, against intervals of 1·2–3.6, 1·0–3·8 and 1·4–3·7 mm, respectively, on LR, SI and AP directions. Under the operating conditions of this study, using NAL correction protocol with a 3 mm threshold gave a modestly appreciable reduction of systematic set-up errors only on AP–SI direction (Table 6). Starting from patients’ systematic set-up errors in Figure 1b, using 2 mm threshold, some more benefit could be effective, but it does not seem a priority looking at the impact on CTV–PTV margins (Table 7). In such conditions, some more reduction on the CTV–PTV margin for these prostate cancer patients could come from adopting corrective strategies to limit bias in transferring image data from CT planning system to linear accelerator and to control organ motion and deformation uncertainties as derived from expressions (5) and (6) in actual conventional 3DCRT.8, Reference Ausili Cefaro, Gabriele, Genovesi, Rosi, Tabocchini and Viti16∑ phantom transfer values of 3 mm on each directions and ∑ motion−shape and σ motion−shape values of 3 mm and 2·5 mm, respectively, on Lat–AP and AP–SI directions (Table 4) used in CTV–PTV margin calculation are more relevant than population systematic errors found in this study (Table 5).
For the group of lung cancer patients, population random set-up errors ranged between <1 mm on AP–SI and about 1·5 mm on AP–LR direction (Table 5 and Figure 2b); they were smaller than those reported in the review by Hurkmans et al.,Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 in which the smallest were 1·8, 2·0 and 2·1 mm, respectively, on Lat–AP, AP–SI and AP–LR directions. The number of patients included in this study is small, and on AP–SI direction the mean population error, Mx, was not negligible, about 1·4 mm in absolute value. However, plausibly, population systematic set-up errors could be between 1·5 and 2·5 mm. Population random set-up errors found in this study were 1·9, 2·2 and 2·8 mm, respectively, on Lat–AP, AP–LR and AP–SI directions (Table 5) and resulted comparable with those reported by Hurkmans et al.Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 (2·2, 2·0–2·9 and 2·7–3·5 mm, respectively, on Lat–AP, AP–LR and AP–SI directions). Application of a NAL correction protocol with a 3 mm threshold gave an appreciable reduction of systematic set-up errors on AP–LR direction (Table 6). CTV–PTV margins’ evaluation, with about 17 mm on Lat–AP and AP–LR directions and <20 mm on AP–SI direction (Table 7), underlined the importance of the ∑delineation value of 4 mm on each direction, ∑motion−shape value of 3 mm on AP–SI directions and the breathing linear contribution of c term (expression 4) with a value of 3 mm for Lat–AP and AP–LR directions and a value of 4 mm for AP–SI direction. Also in such conditions, a NAL correction protocol application with 2 mm threshold could give a significant reduction of systematic set-up error, but an overall negligible impact on CTV–PTV margins without corrective actions on other uncertainties.
For the group of rectum cancer patients, population systematic set-up errors resulted 1·8 and 1·6 mm, respectively, on Lat–AP and AP–SI directions, somewhat smaller than those reported by Hurkmans et al.,Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 respectively, 2·6 and 2· mm (Table 5 and Figure 2a); concerning AP–LR direction, this study reported values of about 0.5 mm, which seems too optimistic against 2·4 mm found by Hurkmans et al.Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 On the contrary, population random set-up errors resulted fully comparable with those reported by Hurkmans et al.,Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 resulting in about 2·5, 1·5 and 2·8 mm in this study against 2·8, 1·8 and 1·7 mm, respectively, on Lat–AP, AP–LR and AP–SI directions. When patient systematic set-up errors were small, as in this study (Figure 2a), NAL correction protocol application with 3 mm threshold did not give significant contribution.
Within the group of breast cancer patients without nodes inclusions, population systematic set-up errors were significant (Table 5 and Figure 3a): 2·0 mm on Lat–AP and AP–SI directions and 3·3 mm on AP–LR direction, and population random set-up errors were somewhat smaller, respectively, 1·9, 1·7 and 2·7 mm on Lat–AP, AP–SI and AP–LR directions. All these population set-up errors were inside the ranges reported by Hurkmans et al.Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 Population systematic and random set-up errors relating to the group of patients with thoracic wall with nodes and breast with nodes inclusion cancer resulted comparable (Table 5 and Figure 4a). However, significant values for the mean population errors Mx, were recorded on AP–LR direction, a little >2 mm in absolute value and about 1·2 mm on AP–SI direction. In these two groups of patients, there were some more significant differences to be observed, although not clearly understandable, on the behaviour of AP–SI set-up errors against the Lat–SI ones, and this fact may suggest further caution. For both groups of patients, application of NAL correction protocol gave significant benefit reducing systematic set-up errors also by using a 3 mm threshold (Table 6, Figures 3b and 4b); plausibly by means of this procedure, a population set-up error between 1·5 and 2·5 mm could be obtained.
Population systematic set-up errors were also significant in the group of gynaecological cancer patients, about 3·2, 2·2 and 1·9 mm, respectively, on Lat–AP, AP–LR and AP–SI directions, and population random set-up errors were about 2·0, 2·1 and 2·3 mm, respectively (Table 5 and Figure 5a); these set-up errors resulted somewhat smaller than those reported by Hurkmans et al.,Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 on AP–LR direction about 2·7 and 3·1 mm, respectively, for systematic and random ones; concerning the other directions, Hurkmans et al.Reference Hurkmans, Remeijer, Lebesque and Mijnheer6 reported higher set-up errors, about 4 mm for systematic and random ones. Also in this group of patients, significant values for the mean population errors Mx were recorded, and in terms of absolute value they resulted on AP–LR direction of about 2 mm, on Lat–AP direction about 1·5 and 1 mm on AP–SI. However, it is reasonable to believe that the application of NAL correction protocol for this group could give a useful contribution on systematic set-up errors by limiting such kind of errors between 1·5 and 2·5 mm too (Table 6 and Figure 5b).
Conclusion
Population set-up errors were found reasonably controlled, from almost 1 mm in the head and neck district to 2–3 mm in the prostate, rectum, lung, breast and gynaecological districts. Application of NAL correction protocol gave significant reductions of systematic set-up errors, even higher than 30% for breast and gynaecological cancer patients; instead, in the head and neck district, using thresholds consistent with typical tolerances of systems and equipment used in basic conventional 3DCRT, NAL did not seem to give any substantial benefit. Moreover, the sole application of NAL correction protocol did not seem to add any significant benefit on CTV–PTV total margins, without the adoption of corrective strategies to reduce other important uncertainties limiting the accuracy of conventional 3DCRT, such as the organ motion and deformation and the target contouring.
Acknowledgements
The authors thank all the staff of the Radiation Oncology Center of Sassari for their support.
Disclosure of Funding
No funding was received for this work.
Conflicts of Interest
The authors declare no conflict of interest.