Introduction
Peatlands cover vast areas of the central boreal plain of North America, and are widely referred to by their Nêhiyawêwin (Cree) name ᒪᐢᑫᐠ or muskek, anglicized as muskeg. Many are wooded and usually dominated by tamarack (Larix laricina) and/or black spruce (Picea mariana), but the term muskeg has also been applied to sparsely treed or open peatlands. Muskeg has been widely studied for its physiographic properties and vegetation composition, and its different component peatland types have been compared in detail to the nomenclature of peatlands elsewhere in the Northern Hemisphere (Stanek Reference Stanek, Radworth and Brawner1977). Several of these peatland types can be rich in lichen biomass, particularly on hummocks. In muskegs that approach the northern treeline, Cladonia mats become the dominant ground cover over large areas (Harris et al. Reference Harris, Moore, Roulet and Pinsonneault2018). Although these habitats have been included in regional surveys (e.g. Piercey-Normore et al. Reference Piercey-Normore, Brodo and Deduke2016), little attention has been dedicated to the ecological niches that muskegs offer to ecologically specialized lichens and their associated fungi.
Many different kinds of boreal peatland have been described, and some of the more striking physiographic manifestations are patterned fens, which include ‘string fens’ in which water flow and slope interact to produce striking landscape features such as string patterning (Foster et al. Reference Foster, King, Glaser and Wright1983; Zoltai & Pollett Reference Zoltai, Pollett and Gore1983), as well as ‘palsa fens’ or ‘palsa bogs’ in which mounded patterning emerges as a consequence of localized pockets of permafrost (Zoltai Reference Zoltai1972). Depending on the local climate and surrounding fire history, such fens can be largely open with only isolated, old trees, or they can be completely grown in, especially, in North America, with Larix laricina (Fig. 1). It was originally in such habitats that different members of our author team independently discovered what we recognized to be an undescribed species of lichenicolous Calicium in 2015 in Ontario (Canada), in 2017 in Minnesota (USA) and 2018 in Manitoba (Canada), as well as an undescribed sorediate lichen in Canadian string fens that we ultimately recognized to be a new species of Ramboldia. Here, we describe these species and report on their occurrence, ecology and importance for conservation.

Figure 1. Isolated Larix laricina trees in string fens. A, at the type locality of Ramboldia canadensis near Niton Junction, Alberta. B, at the type locality of Calicium poculatum near the hamlet of Hudson Bay, Saskatchewan. In colour online.
Materials and Methods
The two species described here were collected during fieldwork by RTM in Ontario, OG in Minnesota and by GT and TS in Manitoba, Saskatchewan and Alberta. Once we became aware of the species, we began to search for them in numerous fens across the region, as well as in other boreal forest habitats including upland Picea mariana, P. glauca and Pinus banksiana forest.
Specimens were examined using dissecting and light microscopes. Measurements of ascospores and soredia were taken in water mounts with a 1 μm precision. Secondary lichen compounds were investigated with HPTLC (Arup et al. Reference Arup, Ekman, Lindblom and Mattsson1993), using the most stable solvent C (below as TLC). The colour reaction of the thallus was tested using common household bleach (C), 10% aqueous potassium hydroxide (K), Steiner's solution (PD) and short-wave (254 nm) and long-wave (365 nm) ultraviolet light. Calcium oxalate crystals were searched for by applying 10% sulphuric acid to squash preparations of thallus samples. Ascospore and soredia measurements are given as (min.–) x̄ ± SD(–max.), n = number of measurements. Specimens were also examined using scanning electron micrography (SEM) with a Zeiss Sigma 300 VP-FESEM.
DNA extraction and sequencing
We acquired DNA from both species using material from multiple localities (Table 1). For extracting Calicium DNA, ascomata including stalks were broken off and placed in a 1.5 μl Eppendorf tube. For placing the fungus we later realized was a Ramboldia, we removed thallus fragments using sterile razor blades and transferred them to water droplets on a microscope slide, where we removed visible contaminants by transferring the target material to new, clean water drops. This procedure was repeated as often as necessary until a clean piece of agglutinated material was obtained, the excess water wicked away with fine-tipped forceps and placed against the inner wall of an Eppendorf, where it was allowed to air dry. A sterile 3 mm carbide bead was then added and the tube frozen at −80°C for > 1 h in a Qiagen TissueLyser rack. The deep-frozen rack was then subsequently shaken at 27 shakes per second for 25 s, optimizing cell lysis by manual pulsing (5 s on, 5 s off). The pulverized samples were used for a Qiagen Investigator forensic DNA extraction using the Blood & Tissue protocol.
Table 1. Taxa, voucher information and DNA sequences used for the analyses. The new species are shown in bold.

For placement of the species in their respective groups, we amplified four DNA loci from both species using polymerase chain reaction (PCR) and compared these to data from available loci in public databases (Table 1). For Calicium, we decided to target ITS rRNA, 28S rRNA, protein-coding mini-chromosome maintenance complex 7 (Mcm7) and mitochondrial 12S rRNA as loci were already available for numerous taxa in the genus, using primers ITS1F, ITS4, LRlecF, LRlecR, MCM7-709for, MCM7-1348rev, mrssu1 and mrssu3R (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990; Gardes & Bruns Reference Gardes and Bruns1993; Zoller et al. Reference Zoller, Scheidegger and Sperisen1999; Schmitt et al. Reference Schmitt, Crespo, Divakar, Fankhauser, Herman-Sackett, Kalb, Nelsen, Nelson, Rivas-Plata and Shimp2009; Schneider et al. Reference Schneider, Resl, Westberg and Spribille2015). Since we suspected that the sorediate crust we later identified as a Ramboldia could be placed in the order Lecanorales, we targeted the same four loci as used for Calicium as these were broadly informative based on our previous work in that group (Spribille et al. Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020). For Trebouxia, we used ITS1T and ITS4T (Kroken & Taylor Reference Kroken and Taylor2000) with the following modified PCR protocol: an initial denaturation at 95 °C for 5 min, 10 touchdown cycles of 95 °C for 30 s, 66–58 °C for 30 s, and 72 °C for 1 min, followed by 35 cycles of 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 1 min, with a final extension of 72 °C for 7 min. All PCR reactions were run on a Veriti 96-Well Fast Thermal Cycler (Applied Biosystems, Waltham, Massachusetts) where 2 μl dilutions of DNA extract were used in a 22 μl total reaction volume with KAPA3 G Plant PCR Kit (Millipore Sigma, Burlington, Massachusetts) following the manufacturer's instructions.
For placement of the species, we downloaded from NCBI available sequences from the genus Calicium covered by the four loci mentioned above as of March 2023, as well as outgroup taxa from the genera Heterodermia, Physcia and Phaeophyscia. For the placement of Ramboldia, we included sequences of Ramboldia available at the same time plus a selection of representative sequence sets in Lecanoraceae, Miriquidicaceae, Parmeliaceae, Ramalinaceae, Sphaerophorus and Psoraceae, with Rhizocarpon oederi as outgroup. We aligned and concatenated the sequences using MAFFT v. 7.380 (Katoh & Standley Reference Katoh and Standley2013) implemented in phyloscripts (Resl Reference Resl2015). Vertical alignment positions with data in ≤ 10% (in Calicium) and ≤ 20% (in Lecanorales) of sequences were culled for the final analysis. We calculated maximum likelihood trees using IQ-TREE v. 1.6.12 (Nguyen et al. Reference Nguyen, Schmidt, von Haeseler and Minh2015; Minh et al. Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams, von Haeseler and Lanfear2020), inferring substitution models for each locus partition and within Mcm7 by codon position using ModelFinder (Chernomor et al. Reference Chernomor, von Haeseler and Minh2016; Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017). The trees were calculated using 1000 standard, non-parametric bootstrap replicates followed by the generation of a single consensus tree. We only display support values on branches if they have a bootstrap value ≥ 70%.
Results and Discussion
We generated new sequences from six specimens. The final alignment for the Calicium tree minus sites excluded owing to excessive missing data, contained 57 tips and 3469 nucleotide positions divided into six partitions (1–619, 620–1964, 1965–2801 and each codon position of 2802–3469). The result of the substitution model inference for all six partitions in this alignment was TNe + R3, TIM2e + R2, TPM2u + F + I + G4, K2P + I, K2P + I + G4 and TIM2 + F + I. A total of 923 sites were parsimony-informative. The maximum likelihood tree had a log-likelihood value of −20191.621. For the Lecanorales tree for placement of Ramboldia, the final alignment minus sites excluded owing to excessive missing data included 83 tips and 3437 nucleotide positions likewise divided into six partitions (1–582, 582–1860, 1861–2790 and each codon position of 2791–3437), with the following inferred substitution models: TIM2 + F + I + G4, TIM2 + F + R3, TVM + F + R4, TIM2 + F + G4, TPM3 + F + R2 and HKY + F + I + G4. A total of 1366 sites were parsimony-informative. The maximum likelihood tree had a log-likelihood value of −44167.4735. Both trees are unrooted but graphically displayed with distantly related taxa drawn as roots.
The consensus tree for Calicium (Fig. 2) recapitulates the main groups of the genus recovered by Prieto & Wedin (Reference Prieto and Wedin2017), including their Clade A (including Calicium abietinum Pers., C. glaucellum Ach., C. pinicola (Tibell) M. Prieto & Wedin, C. trabinellum (Ach.) Ach.) and their Clade B with its three respective subclades: 1 (including C. adspersum Pers., C. nobile Tibell, C. chlorosporum F. Wilson), 2 (including C. lenticulare Ach., C. notarisii (Tul.) M. Prieto & Wedin, C. tigillare (Ach.) Pers., C. trachylioides (Nyl. ex Branth & Rostr.) M. Prieto & Wedin), and 3 (including C. corynellum (Ach.) Ach., C. lecideinum (Nyl.) M. Prieto & Wedin, C. viride Pers.). In addition, our tree recovers C. sequoiae C. B. Williams & Tibell (Williams & Tibell Reference Williams and Tibell2008) in its own clade within Prieto & Wedin's (Reference Prieto and Wedin2017) Clade B, though with poor bootstrap support, and places several species from the Indian subcontinent described by Tibell (Reference Tibell2006), or yet undescribed but deposited in NCBI, in Clades A and B. The inclusion of sequences from Tibell (Reference Tibell2006) and Tibell & Knutsson (Reference Tibell and Knutsson2016), which include only one locus (ITS), probably contributes to lower bootstrap support for relationships among species, although the main groups and numerous lower-level clades receive bootstrap support > 80%. The new species Calicium poculatum is recovered in Prieto & Wedin's (Reference Prieto and Wedin2017) Clade A in a subclade with 100% bootstrap support, together with C. episcalaris Tibell & Knutsson, C. pinastri Tibell and C. montanum Tibell. Its sibling relationship with C. episcalaris carries 91% bootstrap support. Here, too, the low support is likely related to missing data in the compared taxa: the absence of sequence data for loci other than ITS and mtSSU in C. episcalaris, other than ITS in C. pinastri and other than ITS and LSU in C. montanum.
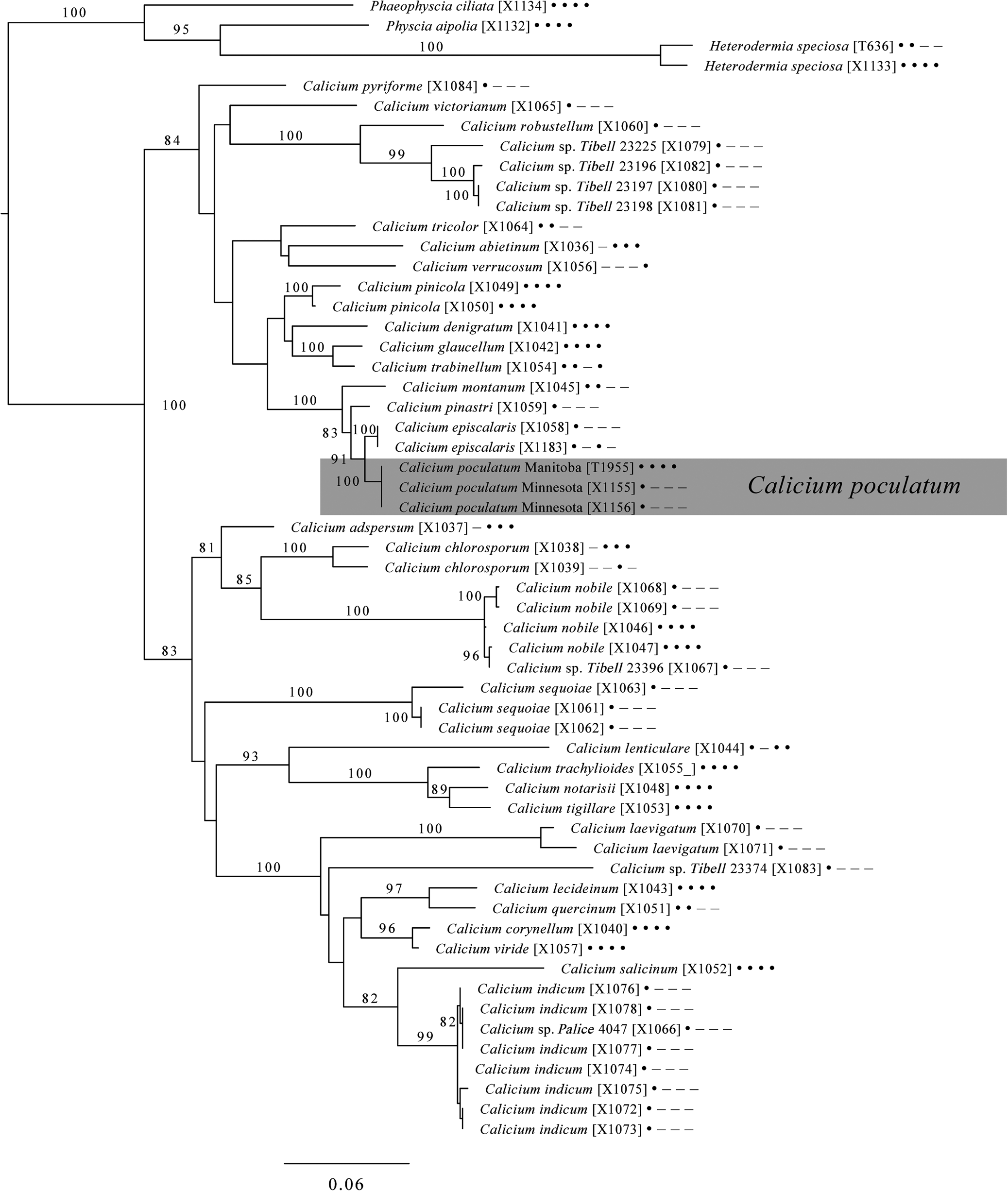
Figure 2. Position of the new species, Calicium poculatum within the genus Calicium based on a maximum likelihood tree of concatenated ITS, 28S and Mcm7 nuclear genes and the mitochondrial SSU gene. Numbers after the taxon names indicate single specimen isolates (Table 1); dots and dashes indicate presence and absence (respectively) of the loci for that sample in the order of loci given above.
The consensus tree for placing Ramboldia in the Lecanorales (Fig. 3) has a weakly supported backbone as is characteristic of recent phylogenies of Lecanorales, with unsupported sibling relationships in much of Lecanora s. lat. The new species Ramboldia canadensis is, however, recovered within a highly supported clade representing the genus Ramboldia, in a subclade together with species with dark apothecia found in Australasia (R. stuartii (Hampe) Kantvilas & Elix, R. brunneocarpa Kantvilas & Elix, R. petraeoides (Nyl. ex C. Bab. & Mitt.) Kantvilas & Elix) and species with the cinnabar-red apothecial pigment russulone, found in the Southern Hemisphere (Australia: R. arandensis (Elix) Kalb et al., R. sanguinolenta (Kremp.) Kalb et al.; Australia and New Zealand: R. laeta (Stirt.) Kalb et al.; Australia and Brazil: R. quaesitica Elix & Kalb; Kalb et al. Reference Kalb, Staiger, Elix, Lange and Lumbsch2008) and pantropical regions of both hemispheres (R. haematites (Fée) Kalb et al., R. russula (Ach.) Kalb et al.). The relationships among these subgroups of Ramboldia are, however, poorly supported, and the nearest sibling of R. canadensis, of the taxa sampled, could not be established with certainty. It should also be noted that more than half of the species in the genus Ramboldia have yet to be sampled using molecular methods. However, such an analysis is beyond the scope of the present study.

Figure 3. Position of the new species Ramboldia canadensis within the order Lecanorales based on a maximum likelihood tree of concatenated ITS and 28S nuclear ribosomal genes, the mitochondrial SSU gene, and the nuclear Mcm7 gene. Numbers after the taxon names indicate single specimen isolates (Table 1); dots and dashes indicate presence and absence (respectively) of the loci for that sample in the order given above.
The new species
Calicium poculatum G. Thor, McMullin & Gockman sp. nov.
MycoBank No.: MB 853516
Similar to Calicium episcalaris in being parasitic, but occurring on Lecanora caesiorubella Ach. subsp. saximontana Imsh. & Brodo and having shorter stalks (up to 0.2 mm tall) and shorter ascospores (8–13 μm).
Type: Canada, Saskatchewan, 41 km by air ENE of village of Hudson Bay, large fen along Silica Sands Road, 52.928°N, 101.788°W, on Lecanora caesiorubella subsp. saximontana on decorticated branches of Larix laricina at edge of large fen dominated by Carex lasiocarpa, in marginal transition zone to Picea mariana muskeg with Andromeda polifolia, 303 m, 26 June 2020, T. Spribille 45911 (CANL—holotype; ALTA, UPS—isotypes).
(Fig. 4)

Figure 4. Calicium poculatum (holotype). A & B, habit in and around the apothecia of Lecanora caesiorubella subsp. saximontana. C, apothecium and associated parasitized thallus (in water). D, ascospores (in water). E, apothecium (SEM). F, ascospore (SEM). Scales: A = 500 μm; B = 200 μm; C = 50 μm; D = 10 μm; E = 20 μm; F = 1 μm. In colour online.
Thallus immersed in the host, associated with a zone of discoloration.
Ascomata parasitic on Lecanora caesiorubella subsp. saximontana, both on the disc and margin of apothecia or sometimes on nearby thallus, often occurring in aggregations of up to five ascomata on a single host apothecium, shining black, ±sessile or on stalks up to 0.2 mm tall and up to 0.2 mm diam.; stalks consisting of dark brown, sclerotized hyphae; capitulum ±doliiform, up to 0.3 mm diam.; excipulum formed by dark brown, sclerotized hyphae; excipulum edge sometimes thinning out and pale, thus mimicking a narrow whitish pruina; hypothecium dark brown, consisting of dark brown, sclerotized hyphae. Asci cylindrical, c. 32 × 7 μm, with uniseriately or some biseriately arranged ascospores. Ascospores ellipsoidal, brown, with a minutely verrucose surface and deep irregular cracks, 1-septate, (8–)9–12(–13) × (5–)5–7(–8) μm (length: x̄ = 10.6 μm, SD = 1.2 μm, n = 120; width: x̄ = 5.9 μm, SD = 0.7 μm, n = 120).
Photobiont not seen; unknown if the fungus interacts with the host photobiont.
Conidiomata not seen.
Chemistry
All parts of the ascomata I−, K−, K/I−.
Etymology
From the Latin poculum (cup, goblet), a reference to the shape of the ascomata.
Notes
Characterized by its parasitic, short-stalked, shining black ascomata and short ascospores. The only other obligately parasitic Calicium described is C. episcalaris, described from Sweden (Tibell & Knutsson Reference Tibell and Knutsson2016) and recently reported for the north-eastern USA (Selva et al. Reference Selva, McMullin, Bell-Doyon, Henderson and Lay2023) and the Czech Republic (published along with the release of Vondrák et al. (Reference Vondrák, Svoboda, Košnar, Malíček, Šoun, Frolov, Svensson, Novotný and Palice2023)). However, this species grows on Hypocenomyce scalaris (Ach.) M. Choisy, and has more distinctly stalked ascomata (up to 0.37 mm tall) and longer ascospores (10–14 × 6–8 μm). Based on the two DNA isolates of C. episcalaris and the three from C. poculatum, the two species differ in seven positions in ITS1, one in 5.8S, and six in ITS2. It is likely an ambiguous base call (N) at one of these positions in C. poculatum isolate X1155 that leads to a bootstrap value of 91% (instead of 100%) between C. episcalaris and C. poculatum. The branch lengths among the species C. montanum, C. pinastri, C. episcalaris and C. poculatum are shorter than those of other species in the tree, suggesting a set of relatively recent speciation events. Chaenothecopsis kalbii Tibell & K. Ryman is another species of calicioid fungus growing on Lecanora caesiorubella (Tibell & Ryman Reference Tibell and Ryman1995), but this species differs by the apothecia not being mazaediate, the stalk being pale at the base, and by having narrower spores (4–5.5 μm) with pointed ends. In addition, Ch. kalbii is described from Brazil and is also known to occur in Australia, north-western Mexico and the south-eastern USA, as well as on the island of Réunion (Tibell & Ryman Reference Tibell and Ryman1995; Lendemer et al. Reference Lendemer, Kocourková and Knudsen2008).
Habitat and distribution
The species appears to be parasitic, causing necrosis and finally the death of the host tissue near the Calicium ascomata. In the Canadian populations, the host, Lecanora caesiorubella subsp. saximontana, was growing on wood in the upper part of Larix laricina snags (up to 2 m high) with some branches still remaining, as well as on the decorticated branches of live trees. The snags are certainly not covered by snow in wintertime. In Manitoba it was intermixed with, for example, Bryoria spp., Buellia arborea Coppins & Tønsberg, Calicium tigillare (Ach.) Pers., Elixia flexella (Ach.) Lumbsch, Evernia mesomorpha Nyl., Flavopunctelia flaventior (Stirton) Hale, Parmeliopsis ambigua (Wulfen) Nyl., Pycnora sorophora (Vain.) Hafellner and Ramboldia canadensis (see below), as well as Lecidella xylophila (Th. Fr.) Knoph & Leuckert which is reported here as new to North America (HPTLC: atranorin; G. Thor 37390, UPS). This might be neglected as a species-rich lichen habitat in Canada and should be studied further. The discovery of a small specimen on Picea mariana twigs suggests the species may have a wider ecology. The Minnesota population is found on the bark of living Larix laricina and Picea mariana in a large peatland complex. At this location, the species and its host were primarily observed in a band of intermixed Larix laricina and Picea mariana situated between a poor, Picea mariana-dominated system in the centre of the peatland and a rich, wet forest dominated by Fraxinus nigra located adjacent to the upland.
Additional specimens examined
Canada: Alberta: Sand River area N of Glendon, 54.541493°N, 111.25033°W, on Lecanora caesiorubella subsp. saximontana on decorticated Larix laricina branch in string fen, 580 m, 2019, T. Spribille 45043 (PMAE). Manitoba: 152 km N of the village Grand Rapids, W of Highway 6, open bog with a few small trees, on 2 m high Larix laricina snag, 54.51352°N, 99.04272°W, 255 m, 2018, G. Thor 37385 (UPS); ibid., T. Spribille 43463 (CANL); Division No. 19, Lake Winnipeg, Long Point, end of Long Point Rd at lake, 52°56.783ʹN, 98°47.664ʹW, on Picea mariana twigs in dense forest, 224 m, 2018, T. Spribille 43506 (PMAE). Ontario: Kenora District, Hudson Bay Lowlands, Ring of Fire, 28 km SE of Kitchie Lake, 19 km WNW of Streatfeild Lake, 14 km SW of Armes Lake, 52.2371°N, 86.1974°W, on Lecanora caesiorubella subsp. saximontana on decorticated Larix laricina, fen, dominant species cover includes Carex chordorrhiza, Chamaedaphne calyculata, Larix laricina, Picea mariana, Trichophorum alpinum and T. cespitosum, 183 m, 2015, McMullin 17450 (CANL). Saskatchewan: 53 km NNW of Hudson Bay, Pasquia Bog, 53.27211°N, 102.00159°W, on branch of Larix laricina in palsa bog, 336 m, T. Spribille 45811 (CANL).—USA: Minnesota: Aitkin County, 7.8 km north-west of McGrath and 15.8 km E of Malmo, on the bark of living Larix laricina and Picea mariana in a large acid peatland system, 46°18.151ʹN, 93°19.150ʹE, 385 m, 2017, Gockman 5477 (MIN); 8.1 km NW of McGrath and 15.35 km E of Malmo, on the bark of living Larix laricina and Picea mariana in a large acidic peatland system, 46°18.181ʹN, 93°19.502ʹE, 385 m, 5 xii 2018, Gockman (MIN).
Ramboldia canadensis G. Thor & T. Sprib. sp. nov.
MycoBank No.: MB 853517
Similar to, for example, Ramboldia elabens (c.ap., Northern Hemisphere), R. farinosa and R. sorediata (both with soralia, Australia), but with a rimose to verrucose-areolate, greyish creamy sorediate thallus with dark brown to blackish, mucilage-encrusted soredia.
Type: Canada, Alberta, Yellowhead County, c. 9.7 km NNE of Niton Junction, c. 10 km NW of Mackay, 53.69486°N, 115.70927°W, on decorticated Larix laricina branch at the edge of a floating string fen, 815 m, 5 August 2021, A. Huereca Delgado 993 & T. Spribille (UPS—holotype [fertile]; CANL—isotype [sterile]).
(Fig. 5)

Figure 5. Ramboldia canadensis. A, habit of thallus and apothecia (holotype). B–D, detail of an areole and its soredia using scanning electron microscopy (Spribille 45910). D is a magnification of the inset of C, which in turn is a magnification of the inset in B. Scales: A = 500 μm; B = 200 μm; C = 100 μm; D = 3 μm. In colour online.
Ascomata apothecia, seen only once, lecideine, shining black, to 0.6 mm diam.; excipulum indistinct and thin, concolorous with the disc, composed of conglutinated, radiating, brown hyphae; hypothecium pale brown; hymenium of sparsely branched paraphyses, pale brown, partly K/I+ blue, K−; paraphyses 1 μm diam.; apices to 4 μm wide, brown. Asci of Lecanora-type, only seen filled with epiplasm, I and K/I+ blue. Ascospores not seen (only one apothecium checked).
Symbiotic thallus crustose, to c. 50 mm wide and to c. 0.5 mm thick, rimose to verrucose-areolate; individual areoles irregular, scattered to confluent, flat to convex, to 1 mm wide or sometimes diffusely delimited, greyish creamy and shiny; soralia excavating from both the upper surface and the sides of areoles, soon ±flat, 0–3 per areole, sometimes confluent; soredia dark brown to blackish contrasting with the white medulla exposed in the soralia, encrusted with secondary metabolite crystals, globose, (20–)26–47(–71) μm diam. (x̄ = 36.3 μm, SD = 10.8 μm, n = 120).
Photobiont Trebouxia simplex Tscherm.-Woess based on ITS rRNA from isolates T1956 (type, Saskatchewan, GenBank PP756436) and T2002 (Alberta, GenBank PP756437); cells globose (8–)9–13(–16) μm diam. (x̄ = 11.3 μm, SD = 2.1 μm, n = 120).
Conidiomata not seen.
Chemistry
Medulla C−, I−, K+ yellow, K/I−, Pd+ yellow, UV−, without calcium oxalate. TLC (solvent system C): two unknown substances, R f 15 and R f 20, both yellow-orange and UV+ green with brown halo. The spots might refer to substances similar to barbatolic acid.
Etymology
Named after its occurrence in Canada.
Notes
Ramboldia is a genus of lecideoid fungal symbionts of lichens that form ascomata that can be black to crimson red, reflecting a rich diversity of pigments (Kalb et al. Reference Kalb, Staiger, Elix, Lange and Lumbsch2008). Most species are described from Australia (Kantvilas Reference Kantvilas2016; Elix & McCarthy Reference Elix and McCarthy2017, Reference Elix and McCarthy2018). The new species is characterized by having a rimose to verrucose-areolate, greyish creamy sorediate thallus with dark brown to blackish corticated soredia. A small number of apothecia were observed once and are apparently rare. It can be confused with Buellia arborea with which it is intermixed (in Manitoba) but B. arborea has a thin or immersed thallus, concave soralia and soredia which are brownish to blackish with a bluish tint. Buellia arborea also has atranorin and placodiolic acid. The only other sorediate Ramboldia species reported in the boreal biome are R. cinnabarina (Sommerf.) Kalb et al. and R. subcinnabarina (Tønsberg) Kalb et al., but these species have a rimose coherent thallus, whitish to greenish soredia, and atranorin and fumarprotocetraric acid or fatty acids as secondary metabolites.
Habitat and distribution
Similar to Calicium poculatum but perhaps extending further west: it was found at several localities in the western part of the boreal forest where C. poculatum could not be found. So far known from central Alberta to central Manitoba. The type locality in Yellowhead County, Alberta, lies just outside the perimeter of a major wildfire that burned several thousand hectares in May 2023.
Additional specimens examined
Canada: Alberta: Sand River area 33 km north of Glendon, 54.541493°N, 111.25033°W, on decorticated Larix laricina branches in string fen, 2019, T. Spribille 45044 (ALTA); between Lac La Biche and Conklin, 55.25558°N, 111.31361°W, on decorticated Larix laricina twigs in large string fen, 670 m, 2019, T. Spribille 45087 & D. Díaz Escandón (ALTA); 11 km S of Conklin, 55.530044°N, 111.076118°W, on decorticated Larix laricina branches in string fen, 655 m, 2019, T. Spribille 45249 (to be issued in Dupla Graecensia Lichenum); Westlock County, fen near Vega, 54.44116°N, 114.27664°W, 17 vii 2021, A. Huereca Delgado s. n. & T. Spribille (ALTA). Manitoba: 152 km N of the village Grand Rapids, W of Highway 6, open mire with a few small trees, on 2 m high Larix laricina snag, 54.51352°N, 99.04272°W, 255 m, 2018, G. Thor 37396 (UPS, CANL); ibid., T. Spribille 43585 (CANL), 43597 (PMAE). Saskatchewan: 53 km NNW of Hudson Bay, Pasquia Bog, 53.27211°N, 102.00159°W, on decorticated branch of Larix laricina in palsa bog, 336 m, T. Spribille 45810 (CANL); 41 km by air ENE of village of Hudson Bay, large fen along Silica Sands Road, 52.928°N, 101.788°W, on decorticated branches of Larix laricina at edge of large fen dominated by Carex lasiocarpa, in marginal transition zone to Picea mariana muskeg with Andromeda polifolia, 303 m, 2020, T. Spribille 45910 (ALTA, CANL, GZU, H).
Significance of the habitat
The two newly described species are the first lichen and lichenicolous fungus to be described with a high habitat specificity for the tamarack tree (Larix laricina), one of the marquee species of muskeg vegetation. However, some differences in the microhabitat amplitude are apparent based on the localities discovered so far. Ramboldia canadensis has so far been found only on hard wood of isolated L. laricina snags. Calicium poculatum, by contrast, has been found in the same habitats in Canada and in more densely grown wooded L. laricina fens in Minnesota, as well as once on its Lecanora caesiorubella host on Picea mariana in western Manitoba.
The fact that the majority of findings of Calicium poculatum and all records of Ramboldia canadensis to date are on isolated Larix laricina snags in string or palsa fens suggests that these habitats play a special role in supporting populations of the new lichen and lichenicolous fungus. What distinguishes these specific trees from the millions of other trees in the boreal forest is likely their special landscape position and associated propensity to escape destruction during wildfires. The central Canadian boreal forest has a relatively dry, continental climate with mean annual precipitation ranging from 418 mm (e.g. Fort McMurray, Alberta) to 491 mm (Grand Rapids, Manitoba) and is an ecosystem shaped by recurrent fire (Larsen Reference Larsen1980). In this region, one of the overarching attributes of the habitats where the new lichens described here occur is that the trees or clumps of trees are relatively isolated (Fig. 1). The ages of L. laricina snags in string fens in Canada has not been systematically investigated, but local studies have found living trees up to 337 years old (see Table S1 in Caners et al. (Reference Caners, Crisfield and Lieffers2024)). One branch in a Manitoba specimen of R. canadensis (Thor 37385) measuring 15 mm in diameter had c. 30 annual rings. We suspect that the isolation of the trees increases the likelihood that they escape the cyclical fires that burn surrounding contiguous forests, and may contribute to tree ages otherwise anomalous in this region and climate type. In more humid regions, it is possible such ages can be achieved without the need for trees to be isolated, as natural fire return intervals may be longer. This may account for Calicium poculatum occurring in Minnesota in a fen complex in which the host trees grow densely together, in a region with higher mean annual precipitation (c. 780 mm).
The occurrence of ancient trees on fen strings or hummocks recalls key lichen habitats known as kelo trees in Fennoscandia. Like the Larix laricina snags we sampled, kelo trees can occur in string fens known regionally as flark-string aapa mires (Laitinen et al. Reference Laitinen, Rehell, Huttunen, Tahvanainen, Heikkilä and Lindholm2007) or string mires in aapa fen complexes (Malmer Reference Malmer1985), where they grow if hummocks are sufficiently elevated (up to 0.5 m) above the water table. Coniferous trees in this habitat are slow growing and die before reaching a specific height. The cause of the death of the trees is not well explained in the literature but one possibility is that trees are killed by rapidly increasing temperatures in spring, causing the trees to start photosynthesizing, while the ground where the roots are located is still frozen. In Manitoba, Saskatchewan and Alberta the main coniferous tree occurring on strings in string fens is Larix laricina. The snags of this species resemble kelo trees in having old, hard, silvery grey and decorticated trunks (Niemelä et al. Reference Niemelä, Wallenius and Kotiranta2002). However, they differ from kelo trees, which in Europe are formed from Pinus sylvestris, in usually being less impregnated with resin. Formation and decay of kelo trees are slow processes but under certain conditions, which probably include repeated wildfires, wood of P. sylvestris becomes hard and resin-impregnated and is then very long lasting (Santaniello et al. Reference Santaniello, Djupström, Ranius, Weslien, Rudolphi and Thor2017). Pinus sylvestris can reach up to 800 years of age in Fennoscandia, and its transformation into kelo trees takes c. 40 years after tree death (Sirén Reference Sirén1961; Leikola Reference Leikola1969). Kelo trees can remain standing for over 700 years (Niemelä et al. Reference Niemelä, Wallenius and Kotiranta2002). Larix laricina snags in string fens can be considered to constitute a North American counterpart to kelo trees, and the discovery of a lichen and lichenicolous fungus largely specialized to this type of structure again highlights the importance of dead wood for lichen diversity (Spribille et al. Reference Spribille, Bunnell, Thor, Goward and Björk2008; Santaniello et al. Reference Santaniello, Djupström, Ranius, Weslien, Rudolphi and Thor2017).
Larix laricina snags in patterned fens clearly represent an overlooked and important habitat for lichen specialists in North American muskegs. Notwithstanding their resilience to natural fire regimes, such habitats are on the decline owing to the increasing intensity of wildfires under anthropogenic climate change, and locally are threatened by peat mining and the disruption of water flow from construction of roads and seismic lines for oil and gas exploration. It is too early to say whether Calicium poculatum and Ramboldia canadensis are rare, as only a tiny fraction of string fens in Canada and the boreal part of the United States have been surveyed, and both species appear to have a rather wide range. However, they were found only in a fraction of the fens surveyed, and the specialized habitats are a tiny area of the overall boreal forest. Furthermore, other specialists can be expected to be discovered with increased survey attention, and L. laricina snags should be treated as priority habitats for survey and stewardship.
Acknowledgements
Special thanks to Nathan Gerein (University of Alberta Earth and Atmospheric Sciences SEM unit) for help with the scanning electron micrographs. Fieldwork by RTM in the Hudson Bay Lowlands was funded by the Ontario Ministry of the Environment, Conservation and Parks. TS was supported by an NSERC Discovery Grant and a Canada Research Chair in Symbiosis.
Author ORCID
Toby Spribille, 0000-0002-9855-4591.
Competing Interests
The authors declare none.









