INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is an important bacterial pathogen which is responsible for a high level of morbidity and mortality. There are over 90 pneumococcal serotypes differing in the composition of the capsular polysaccharide, with varying capabilities to cause colonization of the nasopharynx and invasive disease [Reference Hausdorff, Feikin and Klugman1–Reference Sandgren3]. The 7-valent pneumococcal conjugate vaccine (PCV7, Prevenar®, Pfizer, UK), which protects against invasive pneumococcal disease (IPD) and carriage caused by seven serotypes (4, 6B, 9V, 14, 18C, 19F, 23F), was introduced into the routine childhood immunization programme in the UK in September 2006 following positive reports of marked reductions in IPD incidence from the USA [Reference Pilishvili4–Reference Lexau6]. In the USA, overall IPD has been reported to have reduced by 45% from 24·4 cases/100 000 population to 13·5 cases/100 000 population post-PCV7 [Reference Pilishvili4].
Many countries have subsequently incorporated PCV7 into their childhood immunization programmes [7]. However, differences in pre-existing serotype-specific incidence, baseline IPD incidence, vaccine schedules, vaccine uptake and antibiotic use could all impact on the effectiveness of PCV7 in different populations. In the UK, PCV7 was implemented as a 2 + 1 schedule in September 2006. Children are offered two primary doses at ages 2 and 4 months, followed by a booster dose at 13 months, with a catch-up campaign including children born after 4 September 2004 [8]. A high level of PCV7 uptake was rapidly achieved and maintained; figures compiled by the Health Protection Agency (HPA) for October to December 2007 showed that the percentage of children that had received two doses of PCV7 in the UK by age 12 months was 90·1%, and 79·9% had received a booster dose by 24 months [9]. In spring 2010 the UK was among the first countries in the world to implement the 13-valent pneumococcal conjugate vaccine (PCV13, Prevenar13®, Pfizer). PCV13 directly replaced PCV7 in the routine childhood immunization programme and protects against PCV7 serotypes plus serotypes 1, 3, 5, 6A, 7F and 19A [10].
A population-based enhanced IPD surveillance system was established in North East England in April 2006 to monitor changes in IPD epidemiology post-PCV7. Data from this surveillance system were used to investigate the distribution of serotypes causing IPD and to measure the proportion of IPD caused by serotypes included in PCV7 and PCV13 in North East England.
METHODS
Setting
North East England has a stable population of about 2·6 million, which includes about 146 000 children aged <5 years [11]. North East England is serviced by ten local NHS microbiology laboratories and one Health Protection Unit (HPU). For the purpose of this study North East England comprises the following local authorities: Darlington, Durham, Gateshead, Hartlepool, Middlesbrough, North Tyneside, Northumberland, Newcastle, Redcar & Cleveland, South Tyneside, Stockton and Sunderland.
Case definition
A case of IPD was defined as a North East England resident of any age hospitalized with a clinical diagnosis of invasive disease (including bacteriaemic pneumonia, meningitis, septicaemia, septic arthritis, etc.) and with either S. pneumoniae isolated or detected by polymerase chain reaction (PCR) from a normally sterile site such as blood, cerebral spinal fluid (CSF) or pleural fluid. Only cases with a specimen date between 1 April 2006 and 31 March 2010 inclusive were included in the study.
Enhanced surveillance of IPD in North East England
Active enhanced surveillance of IPD in North East England was established in April 2006 by the North East HPU. All local NHS microbiology laboratories agreed to report laboratory-confirmed cases of clinical IPD of all ages to the HPU by post, telephone or electronically through the HPA laboratory reporting system (CoSurv). Monthly reminders to report IPD cases together with feedback of reported cases and requests to check that all cases had been reported were sent to consultant microbiologists to maintain consistency of ascertainment. Further reports come from hospital clinicians. Each case of IPD was investigated by HPU staff by contacting laboratories, hospital clinicians and primary-care staff to complete an enhanced surveillance proforma including risk factors, immunization history and outcome.
National surveillance of IPD for England and Wales is carried out by the HPA and is based upon laboratory reporting. The case definition in our study differs from England and Wales data as recently reported by Miller et al. [Reference Miller12] in that our study was restricted to cases with confirmed clinical illness, but included cases confirmed by PCR whereas Miller et al. did not include a clinical illness criterion and included culture-positive cases only [Reference Miller12]. The methodology of the two studies differed in that our study used active enhanced surveillance compared to passive laboratory surveillance used by Miller et al. [Reference Miller12]. NHS microbiology laboratories send clinically significant isolates of S. pneumoniae to the HPA Respiratory and Systemic Infections Laboratory (RSIL) for serotyping [Reference Miller12]. RSIL share pneumococcal serotype results with the North East HPU. The percentage of specimens that were serotyped did not change significantly throughout the study for any age group (χ2 for trend = 0·092, P = 0·76123).
Data analysis
The study included IPD cases from 1 April 2006 to 31 March 2010 inclusive, and used the two periods: 1 April 2006 to 31 March 2007, and 1 April 2009 to 31 March 2010 to test for changes. Due to the seasonal pattern of IPD, yearly data is presented in seasons spanning 1 April to 31 March. Age groups investigated were <5, 5–64 and ⩾65 years. The denominators used for calculating incidence of IPD were based upon mid-year resident population estimates from the Office for National Statistics (ONS) included within each season (e.g. mid-2006 for 2006/2007) and are reported as cases/100 000 population. Changes between periods were assessed by comparing 2006/2007 with 2009/2010 expressed as percentage change and incidence rate ratio (IRR). P values were calculated by comparing incidence proportions from 2006/2007 with 2009/2010 using Fisher's exact test (Stata Statistical Software: Release 11, StataCorp LP, USA). Two-tailed P values <0·05 were considered statistically significant.
The 2006/2007 period is used in this study as a proxy for a pre-vaccine period. PCV7 was introduced in September 2006, but the observed reduction in IPD incidence caused by PCV7 serotypes between September 2006 and March 2007 in England and Wales was restricted to children aged <2 years from January 2007 onwards [13]. The effect on the data for the 2006/2007 period in this study was small (see Discussion).
Serotypes contained in PCV7 are herein described as PCV7 serotypes and all other serotypes described as non-PCV7 serotypes.
RESULTS
Between 1 April 2006 and 31 March 2010 there were 1088 cases of IPD reported to the North East HPU. IPD in children aged <5 years accounted for 124 (11·4%) cases, persons aged 5–64 years 500 (46·0%) cases and persons aged ⩾65 years 464 (42·6%) cases. The annual all-age incidence of IPD in 2006/2007 was 12·1 cases/100 000 (308 cases). The incidence decreased each year to 9·7 cases/100 000 (250 cases) in 2009/2010, a statistically significant reduction of 20% [95% confidence interval (CI) 5–32]. There was a reduction in IPD incidence for individual age groups, but these reductions were not statistically significant (Table 1). The greatest reduction was in the <5 years age group, decreasing by 30% (95% CI −20 to 59). In the 5–64 years age group there was an 18% reduction (95% CI −6 to 36) and in the ⩾65 years age group a reduction of 20% (95% CI −3 to 39) (Table 1).
Table 1. Invasive pneumococcal disease cases by age group in North East England April 2006 to March 2010

IRR, Incidence rate ratio; CI, confidence interval.
* All cases includes non-typed cases.
† PCV7 type includes serotypes 4, 6B, 9V, 14, 18C, 19F and 23F.
‡ Non-PCV7 serotype includes all serotypes not present in PCV7.
§ Percent change and IRR are calculated using incidence in 2009/2010 and 2006/2007.
¶ P values were determined using Fisher's exact test comparing the incidence proportion in 2009/2010 to 2006/2007. All P values are two-sided, italics indicates statistically significant differences (P < 0·05).
Pneumococcal serotype was known for 986 (91%) cases. During 2006/2007 the six most frequent serotypes were serotypes 1 (17%), 14 (9%), 8 (8%) followed by serotypes 3, 4 and 9V (7% each) (Table 2). In 2009/2010 the six most common serotypes were serotypes 7F (13%), 3 (11%), 19A and 22F (10% each), followed by serotypes 1 and 8 (6% each) (Table 2).
Table 2. Pneumococcal serotypes causing invasive pneumococcal disease in North East England April 2006 to March 2010

IRR, Incidence rate ratio; CI, confidence interval.
* Only serotypes causing >6 cases of IPD over the study period are shown.
† Percent change and IRR are calculated using incidence in 2009/2010 and 2006/2007.
‡ P values were determined using Fisher's exact test comparing the incidence proportion in 2009/2010 to 2006/2007. All P values are two-sided, italics indicates statistically significant differences (P < 0·05).
§ Serotypes 6A and 6C were not routinely distinguished until May 2009.
¶ Other serotypes that caused ⩽6 cases of IPD (others) included serotypes 5, 7, 9, 9A, 10A, 10F, 12B, 15A, 15B, 15C, 16A, 16F, 17F, 21, 23, 24F, 25F 29, 31, 34, 37 and serotype 38.
Changes in PCV7 serotype IPD incidence
Between 2006/2007 and 2009/2010 the annual all-age incidence of IPD caused by a PCV7 serotype in North East England fell by 66% (95% CI 49–78) from 3·9 cases/100 000 to 1·3 cases/100 000. There were statistically significant reductions in all age groups: <5 years (90%, 95% CI 61–99); 5–64 years (50%, 95% CI 4–75) and ⩾65 years (66%, 95% CI 40–82) (Table 1, Fig. 1).

Fig. 1. Changes in incidence (cases/100 000 population) of invasive pneumococcal disease caused by (a) PCV7 and (b) non-PCV7 serotypes by age group in North East England, 2006–2010.
The all-age incidence of IPD caused by serotypes 9V, 4 and 14 reduced statistically significantly by 84% (95% CI 47–97) for serotype 9V, 79% (95% CI 37–95) for serotype 4 and 75% (95% CI 38–92) for serotype 14. The all-age incidence of IPD caused by serotypes 6B, 18C, 19F and 23F also reduced but these changes were not statistically significant (Table 2, Fig. 2). In the <5 years age group there was a statistically significant reduction in serotype 14 (100%, 95% CI 35–100). In the 5–64 years age group there was a statistically significant reduction in serotype 4 (73%, 95% CI 3–95). In the ⩾65 years age group there were statistically significant reductions in serotype 4 (88%, 95% CI 9–100) and serotype 9V IPD (93%, 95% CI 54–100).

Fig. 2. Changes in incidence (cases/100 000 population) of individual PCV7 serotypes causing invasive pneumococcal disease in North East England, 2006–2010.
Changes in non-PCV7 serotype IPD incidence
Between 2006/2007 and 2009/2010 there was a non-statistically significant 12% increase (95% CI –9 to 38) in the all-age annual incidence of IPD caused by non-PCV7 serotypes from 6·8 cases/100 000 in 2006/2007 to 7·6 cases/100 000 in 2009/2010. There was an 88% increase (95% CI –10 to 312) in non-PCV7 IPD in the <5 years age group and a 12% increase (95% CI –19 to 50) in the ⩾65 years age group with no statistically significant differences observed (Table 1, Fig. 1).
The all-age incidence of IPD caused by serotypes 22F, 19A and 7F increased statistically significantly with a 987% increase (95% CI 167–9434) in serotype 22F, a 262% increase (95% CI 42–992) in serotype 19A and a 91% increase (95% CI 2–275) in serotype 7F (Table 2). In the 5–64 years age group there were statistically significant increases in serotype 7F (161%, 95% CI 11–581) and serotype 22F IPD (1192%, 95% CI 94–54 817) (Fig. 3). In the ⩾65 years age group there were statistically significant increases in serotype 19A (437%, 95% CI 17–4887) and serotype 22F IPD (681%, 95% CI 5–34 565) (Fig. 3). Serotypes 3 and 8 IPD did not change, but were among the most commonly reported serotypes (Table 2).
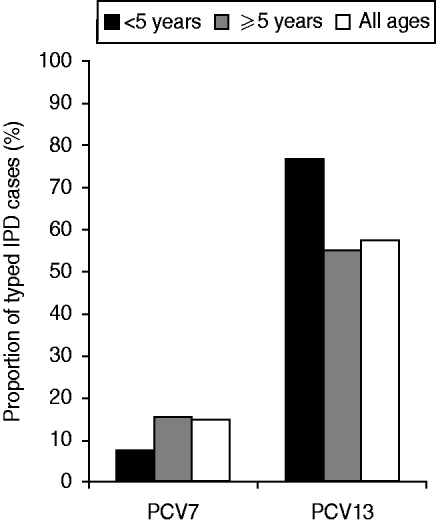
Fig. 3. Proportion of invasive pneumococcal disease cases caused by PCV7 and PCV13 serotypes by age group in North East England, 2009/2010.
IPD caused by serotype 1 decreased statistically significantly by 68% (95% CI 41–83) (Table 2); in the 5–64 years age group the reduction was 66% (95% CI 30–84); and in the ⩾65 years age group 100% (95% CI 56–100) (Fig. 3). If serotype 1 IPD cases are removed from the non-PCV7 serotype analysis, there was a year-on-year increase in all-age IPD caused by non-PCV7 serotypes with a statistically significant rise of 40% (95% CI 11–78) from 5·0 cases/100 000 in 2006/2007 to 7·1 cases/100 000 in 2009/2010 (Table 2).
Proportion of IPD cases caused by PCV7 and PCV13 serotypes
The proportion of all-age IPD cases caused by a PCV7 serotype in 2006/2007 was 36% (99/273 cases), falling to 15% (34/231 cases) in 2009/2010 (P< 0·0001). In the <5 years age group the proportion fell from 63% (20/32 cases) to 8% (2/26 cases) (P < 0·0001). In 2009/2010 the proportion of IPD cases caused by serotypes in PCV13 was 58% (133/231 cases) in all-ages and 77% (20/26 cases) for the <5 years age group (Fig. 4). The additional six serotypes present in PCV13 (serotypes 1, 3, 5, 6A, 7F, 19A) were responsible for 37% (100/273) of IPD cases in all ages in 2006/2007 increasing to 43% (99/231) in 2009/2010. For the <5 years age group the additional serotypes in PCV13 were responsible for 22% (7/32) of IPD cases in 2006/2007 increasing to 69% (18/26) of IPD cases in 2009/2010.

Fig. 4. Changes in the number of invasive pneumococcal disease cases caused by serotypes 1, 7F, 19A and 22F by age group in North East England, 2006–2010 (note scales differ).
DISCUSSION
The introduction of PCV7 into the UK routine childhood immunization programme in September 2006 has been followed by a significant overall reduction in the incidence of IPD in North East England along with important changes in the distribution of pneumococcal serotypes causing IPD. We have observed a 20% reduction in IPD from 12·1 cases/100 000 to 9·7. Reductions occurred across all age groups (<5 years, 30%; 5–64 years, 18%; ⩾65 years 20%), although these were not statistically significant.
In common with most populations where PCV7 has been introduced, we have seen marked reductions in PCV7-type IPD with an overall reduction of 66%. A herd immunity effect occurred with significant reductions in PCV7-type IPD in both vaccinated children and the unvaccinated population. Reductions in the unvaccinated population are most likely a result of reductions in nasopharyngeal colonization of PCV7 serotype pneumococci in vaccinated children and consequently reduced transmission [Reference Huang14]. This reflects reports from the USA [Reference Pilishvili4–Reference Lexau6] and England and Wales [Reference Miller12], the latter reporting a 98% decrease in PCV7-type IPD in children aged <2 years and an 81% decrease in adults aged ⩾65 years. In the UK PCV7 was implemented as a 2 + 1 schedule, unlike the USA and some European countries who adopted a 3 + 1 schedule [Reference De Carvalho Gomes15]. The 2 + 1 schedule appears to be very effective at preventing vaccine serotype IPD in North East England, consistent with England and Wales as a whole [Reference Miller12] and Norway and Denmark which also use a 2 + 1 schedule [Reference Vestrheim16, Reference Harboe17].
An increase in non-PCV7 IPD of 12% in all ages was non-significant given the numbers in the study. Serotypes 7F, 19A and 22F were largely responsible for the increases, consistent with England and Wales [Reference Miller12]. We have observed a significant reduction in serotype 1 IPD of 68%, which is substantially greater than that reported for England and Wales (–11% estimated from adjusted data) using a baseline of 2000/2006 rather than 2006/2007 in this paper [Reference Miller12]. Incidence of serotype 1 has previously been described as volatile in nature [Reference Black18]. If serotype 1 infections are removed from the analysis, non-PCV7 serotype IPD incidence increased significantly by 40% in all ages. This suggests that serotype 1 may have behaved differently in North East England. Whether or not this is the case, the reduction in serotype 1 disease masked the extent of the increase in other non-PCV7 IPD.
Assessing the causes of increases in non-vaccine serotype IPD after the introduction of limited valency pneumococcal conjugate vaccines is difficult because serotype-specific incidence is subject to various factors. Serotype replacement is a widely accepted consequence of vaccine pressure due to reduced vaccine serotype pneumococcal carriage, with non-vaccine serotype pneumococci filling the newly available ecological niche [Reference O'Brien19, Reference Weinberger, Malley and Lipsitch20]. However, serotype behaviour can also change as a result of capsular switching [Reference Brueggemann21] and secular trends can occur without any vaccine pressure. For example, serotype 1 IPD was increasing and serotype 14 IPD was decreasing in England and Wales [Reference Trotter22] and Scotland [Reference Jefferies23] pre-PCV7. In England and Wales serotype 19A IPD was increasing prior to PCV7; however, serotypes 7F and 22F IPD did not appear to increase until after PCV7 implementation [Reference Miller12, Reference Lepoutre24]. It is therefore difficult to assess the true impact of PCV7 on serotype-specific IPD.
In North East England, as in England and Wales as a whole and some European countries [Reference Miller12, 15–Reference Harboe17] we have seen a smaller reduction in the overall burden of IPD post-PCV7 compared to that reported in the USA. The pre-PCV7 incidence reported for children aged <2 years in the USA (188 cases/100 000) [Reference Whitney25], was substantially higher than reported in England and Wales (41·4 cases/100 000) [Reference Trotter22] and in our study (31·7 cases/100 000). Differences in healthcare systems, surveillance systems, antimicrobial susceptibilities, blood culturing practices, vaccine-serotype coverage and vaccine uptake could all contribute to differences in observed IPD rates between countries. For example, incidence is not directly comparable because US data includes outpatients and UK data is restricted to hospitalized cases.
The findings of this study differ in some respects from those reported by Miller et al. using laboratory data from England and Wales [Reference Miller12] which presented both crude data and data adjusted for changes in ascertainment, with adjusted figures preferred. The main trends were similar with overall decreases in IPD, decreases in PCV7 IPD and increases in non-PCV7 IPD. The IRRs for all-age IPD (0·80 for our study, 0·66 for Miller et al.), and non-PCV7 IPD (1·12 for our study, 1·19 for Miller et al.) were similar in both studies. The present study found a slightly lower 2009/2010 incidence [9·7 cases/100 000 for our study, 10·6 cases/1 000 000 (adjusted) for Miller et al.] and the reduction in PCV7 all-age IPD was less in our study (IRR 0·34 for our study, 0·14 for Miller et al.). It should be noted that the pre-PCV7 periods differ (2000/2006 compared to 2006/2007) [Reference Miller12]. Rates are not directly comparable as the studies cover different although overlapping populations and have different case definitions and methodologies. Miller et al. also adjusted data for missing serotypes to avoid bias in trend estimates caused by differing proportions of cases being typed over periods in which incidence of individual serotypes also varies, and also to obtain an adjusted estimate of serotype-specific incidence [Reference Miller12]. However, the assumption behind this adjustment (that untyped cases have the same serotype distribution as serotyped cases) is to the best of our knowledge untested. The proportion of cases serotyped in our study has not been subject to a trend over the study period (K. E. Chapman et al., unpublished data), therefore this adjustment has not been made in our study.
Differences in rates of change are of greater interest than incidence estimates produced by differing methodologies for case capture. Those reported by Miller et al. are adjusted using modelled trends to inflate previous years' data to 2009/2010, based on Flasche et al. [Reference Flasche, Slack and Miller26] who identified a trend of increasing laboratory reporting of certain non-pneumococcal bacteriaemias in England and Wales. Use of a model to estimate ascertainment introduces its own assumptions which introduce uncertainty to the results produced. The number of laboratories reporting in North East England has remained constant (100% throughout the study) and all laboratories report to a consistent protocol. Data (unpublished) from North East England laboratories do not show a trend in blood-culturing practice throughout the study for the organisms studied by Flasche et al., which suggests any such effect in North East England is small [Reference Flasche, Slack and Miller26]. As our study is not based on passive laboratory surveillance but active surveillance it is considered that our data will have enhanced reporting, without affecting reporting of other organisms, such that trends in other organism cannot be used as comparators. We have therefore assumed that ascertainment has not changed during this study.
It is important to acknowledge that this study has other limitations. It would be preferable to base trends on data unaffected by PCV7 implementation. However, PCV7 effects in 2006/2007 were limited to children aged <2 years (estimated from HPA laboratory data) [13]. If replicated in North East England, this effect would have reduced the observed 2006/2007 incidence in children aged <5 years by about 4%, and by 0·5% for all ages (K. E. Chapman et al., unpublished data). Therefore our 2006/2007 data remain a reasonable proxy for the pre-PCV7 period. Moreover, the number of IPD cases in individual age and serotype subgroups is small. Both of these issues limit the power to detect statistically significant differences.
In April 2010 PCV7 was replaced with PCV13 in the UK, which also covers serotypes 1, 3, 5, 6A, 7F and 19A. Prior to PCV13 implementation, PCV13 serotypes caused 58% of all IPD in North East England and 77% of IPD in children aged <5 years. Therefore, PCV13 has the potential to markedly reduce rates of IPD. However, herd immunity is believed to result from reductions in carriage in vaccinated children [Reference O'Brien19, Reference Hammitt27] and serotypes vary in case: carrier ratio. Serotype 1, for example, is responsible for a high proportion of IPD but is rarely found in carriage studies, so PCV13 may not produce effective herd immunity for this serotype [Reference Hausdorff, Feikin and Klugman1]. Further, serotypes 8 and 22F are not included in PCV13 but caused a substantial amount of disease with 22F showing the greatest increase post-PCV7.
In summary, the overall incidence of IPD in North East England fell following the implementation of PCV7 in the UK routine childhood immunization programme in 2006. The serotypes causing IPD have changed; there have been significant reductions in PCV7 serotypes in all age groups, demonstrating herd immunity, along with increases in non-PCV7 serotypes as a whole, although the increases are non-significant given numbers. There has been a substantial reduction in serotype 1 IPD which has partially masked increases in other non-PCV7 serotypes. If PCV13 is as successful as PCV7 at reducing vaccine type IPD it would be expected that the rise in serotypes 7F and 19A will be counteracted by PCV13. However, new non-PCV13 serotypes may become prevalent as a result of this further vaccine pressure. This highlights the importance of continued surveillance of IPD and increased efforts into research and development of new pneumococcal vaccines covering more or all serotypes.
ACKNOWLEDGEMENTS
This work was supported by a grant from the HPA strategic research and development fund. The authors thank the HPA Respiratory and Systemic Infections Laboratory for kindly providing serotype results, all North East local NHS microbiology laboratories for reporting cases of IPD, and members of the North East HPU for their participation in IPD enhanced surveillance.
DECLARATION OF INTEREST
None.








