Introduction
Many insects can enhance their performance through partitioning activities, such as feeding, resting, and dispersal, relative to spatial and temporal variation in local abiotic conditions. Advantages of such partitioning may reflect the direct influence of abiotic conditions, in particular temperature and humidity, as these parameters often mediate rates of physiological processes and thus impact insect survival and developmental rate (Berridge et al. Reference Berridge, Treherne and Wigglesworth1983; Bardoloi and Hazarika Reference Bardoloi and Hazarika1994). Insects may also accrue indirect benefits from linking activity patterns to abiotic variation because abiotic cues can help balance rates of food intake and digestion (van Frankenhuzen and Espinasse Reference van Frankenhuzen and Espinasse2010), limit exposure to natural enemies (Fitzgerald et al. Reference Fitzgerald, Casey and Joos1988), and help to synchronise social behaviours (Thornhill and Alcock Reference Thornhill and Alcock1983; Quiring Reference Quiring1994). Aside from weather, intrinsic, hormonally mediated influences such as molting, circadian rhythm, and hunger may affect the timing, frequency, and duration of activities (Wellington and Cameron Reference Wellington and Cameron1947; Nigam Reference Nigam1995). A better understanding of the factors influencing insect activities could help to increase efficiency of pest-control programmes, especially those employing control products that must be ingested to kill the target insect (e.g., Bacillus thuringiensis Berliner strain kurstaki (Btk) and tebufenozide). Deploying such products during peak periods of feeding would be expected to increase the uptake of insecticide, and could thus significantly enhance the efficiency and efficacy of control agents.
We investigated how variation in abiotic conditions, in particular weather, influences activity patterns of larvae of spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae), the major defoliator of balsam fir (Abies balsamea [Linnaeus] Miller) and spruces (Picea Dietrich spp.) in eastern Canada. In western New Brunswick, where this study was carried out, second-instar budworm larvae emerge in early to mid-May and mine mature needles or swelling buds. At all instars, larvae use silk to link adjacent needles and shoots, forming protective silk shelters in which to feed and rest. Because both shoots and larvae grow continuously throughout the season, larvae continuously repair and expand their shelters. If resources within a shelter become depleted during late instars, the occupant may roam to neighbouring shoots to acquire fresh needles (foraged needles are generally clipped at the base and dragged on a silken thread back to the feeding shelter for consumption). In a recent laboratory study, van Frankenhuzen and Espinasse (Reference van Frankenhuzen and Espinasse2010) reported that mid- to late-instar larvae feed discontinuously throughout the day. Overall, less than 10% of activity time was allocated to feeding (active ingestion), while resting and silk spinning comprised the majority of their daily activity time budget (together 28–52%) (van Frankenhuzen and Espinasse Reference van Frankenhuzen and Espinasse2010). Although several studies have investigated how temperature, and sometimes humidity, influence adult activity patterns (Sanders Reference Sanders1975) and juvenile developmental rate (Reichenbach and Stairs Reference Reichenbach and Stairs1984; Régnière Reference Régnière1987) of spruce budworm, no field studies have asked how variation in local abiotic conditions influences budworm larval activity. We describe results from a 2-year field study carried out to determine the influence of daily and hourly variations in temperature, humidity, and solar insolation on diurnal activity patterns of late-instar larvae of spruce budworm on mature balsam fir.
Methods
Experimental setup
We used video recording to observe the association between larvae activity and abiotic conditions. We recorded for 24 hours a day for 29 days (May 31–June 28) in 1987 and for 12 days (June 15–26) in 1991. We analysed data only for well-lit daylight hours (05:00–20:00), because review of the videos suggested that timer-controlled flashlights used to illuminate larvae at night might influence their activity; to our knowledge, nocturnal activity patterns of budworm larvae have not been studied. Furthermore, we retained data only for days for which both activity and weather data were available from 05:00 to 20:00 hours (1987, 10; 1991, 11).
Our observations were made in natural stands of mixed balsam fir and red spruce (Picea rubens Sargent) (Pinaceae) ∼10 km south of Upper Blackville, New Brunswick (46°33′N, 65°55′W) (1987) and about 36 km west of Miramichi, New Brunswick, near the Fraser Burchill Road (47°08′N, 65°59′W) (1991). Tree density was ∼1000–1100/ha and most trees had a height of 6.6–11.0 m. Budworm populations in the region were in the declining phase of outbreak and were generally present at low densities in the study sites (Speight Reference Speight1987). In a relatively open section of each stand, scaffolding was erected adjacent to groups of balsam fir and five (1987) or eight (1991) video surveillance cameras (Panasonic WV-1554, Panasonic Canada, Mississauga, Ontario) were set up to record larval activities on one to two mid-crown branches per tree. We positioned each camera slightly above and 10–20 cm away from a branch and monitored a specific microhabitat for as long as at least one larva was present within the field of view. At a daily check, if a camera no longer had a larva in its field of view we shifted it to an adjacent branch occupied by a larva. Cameras were connected to a central control system that regulated recording intervals, with a sequential switcher (Panasonic WJ-527) automatically initiating each camera in sequence for an interval of 3 (1987) or 4 (1991) continuous minutes of video recording. Incoming video was recorded using a time-lapse video cassette recorder (Panasonic NV-8050) in the 12-hour mode, which permitted 12 hours of continuous recording on a 2-hour T120 VHS tape; we replaced tapes every 12 hours.
We reviewed video tapes and one of several observers scored the primary activity of each larva within each 3–4-minute video clip (i.e., one ‘observation’) as follows: (1) feeding – cutting, moving, and chewing needles, (2) resting – immobile or motionless, (3) spinning – spinning silk in or around the microhabitat in the course of building or maintaining a feeding/resting shelter, (4) roaming – wandering or movement not clearly associated with either feeding or spinning. Video tapes and associated observations were spot checked periodically to ensure consistency of scoring among the various observers.
Working in stands adjacent to the study sites, we assessed larval instar every 2 days for the duration of video recording. We randomly collected 50 larvae on each sampling date, and scored developmental stages (second to sixth larval instars = 2–6, prepupa = 6.5, pupa = 7) following McGugan (Reference McGugan1954). From the beginning to the end of each video recording period, larvae ranged from mean instar 4.6–5.9 (1987) and 5.6–6.6 (1991).
To assess local climates, we installed a 13 m tall tower with a 1 m mast extension in the midst of each study stand. We measured insolation (direct and diffuse combined) with a silicon pyranometer (LI-200SZC, Li-Cor, Inc., Lincoln, Nebraska) attached to a mounting arm with a levelling fixture near the middle of the tower, about mid-canopy level. We measured air temperature and relative humidity using a probe (model 207, Campbell Scientific, Inc., Logan, Utah) housed within a radiation shield located below the pyranometer. We also recorded temperature near each microhabitat videotaped using thermocouples (copper constantan) placed beneath the observed shoots. Air and microhabitat temperatures were highly correlated (Pearson's r = 0.95, P < 0.001, y = 1.07x − 3.64), and for simplicity we used only microhabitat temperatures in subsequent analyses. All instruments were connected to a battery-operated Campbell Scientific 21x micrologger (Campbell Scientific Canada Corp., Edmonton, Alberta), which was housed inside a weather-proof enclosure bolted to the weather tower. Weather data were recorded at 1-minute intervals and saved onto the internal memory of the micrologger until it could be downloaded for storage and backup. We used these fine-scale data to estimate mean insolation, temperature, and humidity for each day (pooling across hours) and for each hour (pooling across days). Not surprisingly, temperature and relative humidity were strongly (negatively) correlated, especially across hours within days. We report only activity patterns with temperature but caution that effects of temperature and humidity cannot be separated in our data.
Statistical analyses
For each study year, we fit generalised linear models (GLM) (proc GENMOD, dist = bin, link = logit; SAS Institute 1999) to test the influence of day and hour on each activity. We treated day and hour as continuous, interval-scale variables, and initially fit models including a quadratic term in day or hour. We were particularly interested in testing for quadratic fits with hour, as activities might well peak at midday (quadratic, concave down) or at dawn and dusk (quadratic, concave up). When the quadratic term was not significant (at α = 0.05), we report the linear analysis instead. In several cases, GLM diagnostics such as scaled deviance indicated over-dispersion or under-dispersion of data relative to the modelled error structure, and where over-dispersion or under-dispersion was strong (deviance/df > 3.0 or <0.33) we do not report any fit. More stringent values for these deviance cutoffs, such as described in Atkinson (Reference Atkinson2001), would reduce the number of fitted relationships we show, but not alter any of our major conclusions. We used the same procedure to test the influence of mean daily and hourly temperature, and solar insolation on each activity, again beginning with a model including a quadratic term in temperature or insolation but reporting the linear fit instead when the quadratic term was nonsignificant.
Results
Of the total 1847 and 2969 observations in 1987 and 1991, respectively, only 43% and 68% of larvae were visible in video footage for activity identification. In both years, <10% of visible larvae were observed feeding during the daytime (Fig. 1). Most larvae in 1987 were observed roaming (∼50%) with less than half as many either resting or spinning silk (Fig. 1). Comparatively few larvae in 1991 were observed roaming and were generally observed to be either resting or spinning silk (Fig. 1).
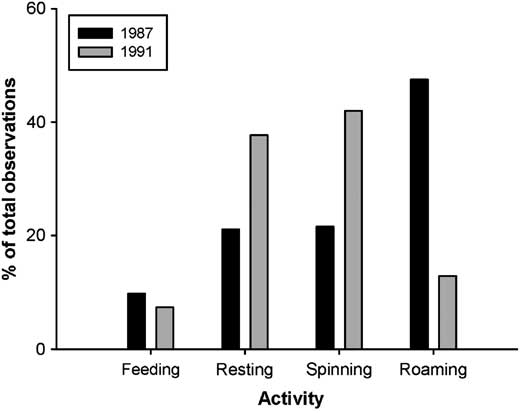
Fig. 1 Overall mean percentages of late-instar budworm larvae feeding, resting, spinning silk, or roaming on mid-crown branches of mature balsam fir in 1987 and 1991.
Activity patterns often differed significantly among days, although effects were rarely consistent between years (Fig. 2, Table 1). Feeding increased linearly throughout 1987 but did not change significantly in 1991 (Fig. 4, Table 1). Resting in 1991 only increased positively with day, whereas roaming in both years decreased with day (Fig. 4, Table 1). Relationships could not be fitted for silk spinning in either year (Table 1). Daily variations in activities were generally not associated with corresponding variations in abiotic conditions, except in 1991 when spinning and roaming were negatively related with temperature (Fig. 4G, 4H, Table 1) and resting was positively related to solar insolation (Fig. 4N, Table 1). As temperature, humidity, and insolation are all interrelated (Pearson's correlations: range of r = 0.51–0.98, P < 0.05), it is not possible to determine the independent influence of each factor on activities.
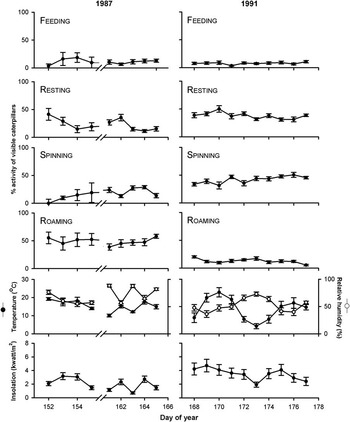
Fig. 2 Daily mean percentages (±1 SE) of late-instar budworm larvae feeding, resting, spinning, or roaming on developing shoots of mature balsam fir in 1987 and 1991; and mean (±1 SE) relative humidity, solar insolation (diffuse and direct combined), and temperature measurements for the same days. Days 156–160 in 1987 are omitted due to unavailability of either abiotic or activity data for those days.
Table 1 Results from GENMOD analyses assessing relationships between daily mean % of late-instar budworm larvae feeding, resting, spinning silk, or roaming and day, temperature, and SI.
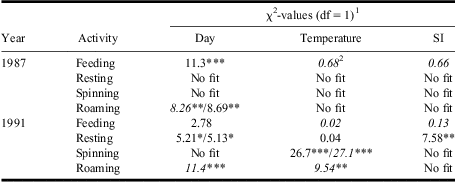
1Where two values are shown, the first is for a linear term and the second for a quadratic. Where a single term is shown, the quadratic term was not significant.
2Italicised χ2-values indicate negative parameter estimates (for quadratic term, negative is convex up).
*, **, *** tests significant at P < 0.05, P < 0.01, P < 0.001.
“No fit”, deviance/df > 3.0 or <0.33 for both linear and quadratic fits.
SI, solar insolation.
In both years, feeding, resting, and silk spinning—but not roaming—varied significantly among the hours of the day (Fig. 3, Table 2). Although feeding was negatively related to temperature only in 1991 (Fig. 5E, Table 2), resting and silk spinning were positively related to temperature in both years (Fig. 5B, 5F, Table 2). Roaming generally decreased with increasing temperature, although the relationship was only significant in 1991 (Fig. 5H, Table 2). Only resting in 1987 was significantly related to solar insolation (Fig. 5J, Table 2).

Fig. 3 Hourly mean percentages (±1 SE) of late-instar budworm larvae feeding, resting, spinning silk, or roaming on mid-crown branches of mature balsam fir in 1987 and 1991; and mean (±1 SE) relative humidity, solar insolation (diffuse and direct combined), and temperature for the same hours.
Table 2 Results from GENMOD analyses assessing relationships between hourly mean % of late-instar budworm larvae feeding, resting, spinning silk, or roaming and time of day (hour), temperature, and SI.
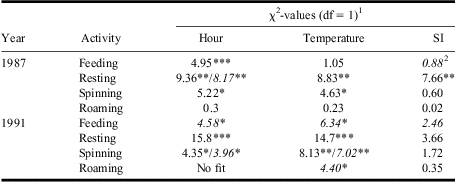
1Where two values are shown, the first is for a linear term and the second for a quadratic. Where a single term is shown, the quadratic term was not significant.
2Italicised χ2-values indicate negative parameter estimates (for quadratic term, negative is convex up).
*, **, *** tests significant at P < 0.05, P < 0.01, P < 0.001.
SI, solar insolation.
Discussion
Less than 10% of budworm larvae were observed feeding during this study; most were observed to be either resting, spinning silk, or roaming (Fig. 1). Resting, of course, is not necessarily unproductive as budworms, similar to other herbivores that feed on low-quality foliage, may require additional time after consuming foliage for digestion and other post-ingestion processes (here included in “resting”). Indeed, van Frankenhuzen and Espinasse (Reference van Frankenhuzen and Espinasse2010) found that in vitro lengths of budworm resting bouts were correlated with lengths of the preceding feeding periods, likely because larger meals required more processing time. Our results for feeding activity are consistent with those of van Frankenhuzen and Espinasse (Reference van Frankenhuzen and Espinasse2010), who reported that budworm larvae allocate less than 10% of their time during the day to feeding.
Activity patterns for silk spinning, roaming, and resting differed between the 2 years of our study. In 1991, roaming was fairly infrequent, while nearly 40% of larvae were observed either resting or spinning silk. In 1987, however, nearly half of all larvae observed were roaming with a comparatively small percentage resting and spinning silk (Fig. 1). Slight differences in larval age between the two study years are unlikely to explain these differences as activity patterns appear to be similar from fourth to sixth instar (van Frankenhuzen and Espinasse Reference van Frankenhuzen and Espinasse2010). It is more likely that the higher proportion of larvae roaming in 1987 was due to lower site quality, possibly caused by reduced foliage availability coupled with higher larval densities (Blais Reference Blais1952). In 1987, budworms were still abundant in the region and trees in the area had sustained heavy defoliation over the preceding 3 years (Speight Reference Speight1987). By 1991, populations in the region had declined such that there were few obvious signs of larvae and almost no recent defoliation. The lower frequency of “visible” larvae in 1987 (43% versus 68% in 1991) may support this hypothesis as highly active larvae would have been more likely to move out of the limited field of view of cameras.
Past laboratory studies have reported that gradients in temperature and humidity can modify budworm larval activity (Wellington Reference Wellington1950a, Reference Wellington1950b), however, we did not detect these effects on budworm in field conditions. The occurrence of feeding by larvae was generally not influenced by daily or hourly variations in abiotic conditions, except in 1991 where there was a slight decreasing trend relative to increasing temperature (Fig. 5e). We emphasise that this does not imply feeding is unaffected by temperature; Retnakaran (Reference Retnakaran1983) demonstrated previously an almost fivefold increase in larval feeding rate between 12°C and 27°C, presumably because warmer temperatures facilitate more rapid consumption and digestion of foliage. Instead, our results indicate that the frequency of feeding by mid- to late-instar budworm is fairly stable across a range of weather conditions and that variations in abiotic conditions have a limited impact on the relative frequency of different activities. Even where relationships were significant, such as those between daily silk spinning or roaming and temperature (Table 1), we could see only slight changes in activity in response to quite dramatic variations in temperature and insolation (Figs. 4G, 4H). Similarly, hourly variations in weather, although significantly related to several activities, yielded relatively subtle changes in larval activity (Fig. 5), especially when compared with the large variations in larval activity reported in a number of other Lepidoptera (e.g., Edwards Reference Edwards1964; Leonard Reference Leonard1970; Fitzgerald et al. Reference Fitzgerald, Casey and Joos1988).

Fig. 4 Relationships between mean daily temperature or solar insolation and the % of larvae feeding (A, E, I, M), resting (B, F, J, N), spinning silk (C, G, K, O), and roaming (D, H, L, P) in 1987 and 1991. Bold symbols represent relationships that were significant (see Table 1).
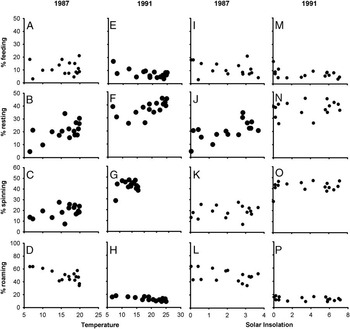
Fig. 5 Relationships between mean hourly temperature or solar insolation and the % of larvae feeding (A, E, I, M), resting (B, F, J, N), spinning silk (C, G, K, O), and roaming (D, H, L, P) in 1987 and 1991. Bold symbols represent relationships that were significant (see Table 2).
Interestingly, larvae maintained similar activity patterns on most days, and during mornings and evenings, even when temperatures averaged below 10°C; below 10°C (and above 30°C) larval growth slows significantly in both field and laboratory colonies (Reichenbach and Stairs Reference Reichenbach and Stairs1984; Régnière Reference Régnière1987). It is possible that the silken tunnels in which larvae spend much of their time buffer ambient climatic conditions, allowing larvae to continue activities despite low temperatures outside the shelter. For other Lepidoptera species that develop primarily in silken shelters, the internal enclosure temperatures can be as high as 2–4°C above ambient (Fitzgerald et al. Reference Fitzgerald, Casey and Joos1988; Ruf and Fieldler Reference Ruf and Fieldler2002). Budworm may also possess adaptations similar to those of other Lepidoptera that allow for continued locomotion and feeding during low temperatures. A large number of Lepidoptera species feed predominantly at night to avoid natural enemies (Raubenheimer and Browne Reference Raubenheimer and Browne2000; Shiojiri et al. Reference Shiojiri, Ozawa and Takabayashi2006) and some, such as the tent caterpillar (Malacosoma americanum (Fabricius)), are able to continue nighttime activities even at temperatures ≤7°C (Joos Reference Joos1992). Although we were not able to analyse nocturnal activity, budworm could also be feeding at night, accruing benefits from maintaining normal activity, even when temperatures are less favourable for growth. Such behaviour could help larvae avoid exposure to predators and parasitoids that favour warm, daytime conditions.
Understanding budworm activity patterns has important implications for the integrated pest management of this intermittent pest as the two main insecticides used to protect trees, Btk and to a lesser extent tebufenozide (Mimic®, Valent BioSciences Corporation, Libertyville, Illinois, U.S.A.), must be ingested to be effective. As such, conditions that influence budworm activity are likely to influence the uptake and associated efficacy of control agents. Although ours and past studies (e.g., van Frankenhuzen and Espinasse Reference van Frankenhuzen and Espinasse2010) show that budworm clearly partition their day to accommodate several essential activities, we found only weak dependence of feeding behaviour on environment and it is not yet clear the extent to which activities besides feeding influence Btk efficacy. Cleaning and repairing the silken shelter occurs more frequently than feeding and may provide an alternative route for Btk to enter the gut as larvae use their mouthparts for both these activities. Budworm on branches sprayed with Btk have been observed collecting spray droplets in their mouths while cleaning their shelter, although whether this behaviour led to greater mortality was not tested (P.C. Nigam, unpublished data). Extensive roaming may further increase exposure to control agents, particularly if particles are accidentally transferred into the feeding shelter to be ingested incidentally later during feeding and shelter maintenance. Further investigation may shed light on the importance of each activity for determining Btk ingestion and whether or not factors influencing the proportion of time allocated to each behaviour have a corresponding influence on product efficacy.
Although climate has a dramatic influence on several aspects of budworm biology and ecology, including adult mating activity (Sanders Reference Sanders1975), host-plant selection (Lysyk Reference Lysyk1989), larval diapause break (Bean Reference Bean1961; Régnière Reference Régnière1987), and developmental rate (Reichenbach and Stairs Reference Reichenbach and Stairs1984; Régnière Reference Régnière1987), the present study detected only minor effects on activity patterns of late-instar larvae, particularly compared with effects documented for other Lepidoptera (e.g., Edwards Reference Edwards1964; Leonard Reference Leonard1970; Fitzgerald et al. Reference Fitzgerald, Casey and Joos1988). We postulate that other factors, such as population density and host-plant quality, are more important mediators of activity in spruce budworm, as has been reported for other outbreaking Lepidoptera (e.g., Lance et al. Reference Lance, Elkinton and Schwalbe1986).
Acknowledgements
We thank G. Burgess, J. Fidgen, J. Fullerton, G. Henderson, M. Mersereau, P. Neilson, G. Speight, B. Sturgeon, B. Tang, and J. Tyre for technical assistance and G. Broderson, D. Carleton, E. Eveleigh, L. Flaherty, S. Fraser, D. Quiring, M. Rhainds, A. Smith, J. Sweeney, G. Thurston, K. van Rooyen, and R. Webster for constructive criticism of the manuscript. Also thanks to D. Quiring for thoughtful discussions on statistical analyses and presentation of results. Financial support was provided by the Canadian Forest Service with substantial logistical support from Forest Protection Limited. We also thank the staff of Instructor Aids Limited (Dartmouth, Nova Scotia) who customised cameras to suit the study objectives and who were instrumental in designing and setting up the video array at field sites.









