Antimicrobial resistance (AMR) is a global health issue. It has been identified as one of the top 10 global health threats by the World Health Organization (WHO). 1 AMR occurs when a microorganism evolves mechanisms for resistance to antibiotics over time. This results in infections that are more difficult and expensive to treat and often have higher morbidity and mortality. Although AMR is a natural process, the misuse and overuse of antimicrobials are important drivers of AMR. 1
In Nepal, a number of factors have contributed to AMR: a high burden of infectious diseases, irrational use of antibiotic therapy, use of antibiotics as growth promoters in animals, and a lack of regulation of antibiotic prescribing and use. Reference Acharya and Wilson2 Several studies show unnecessary prescribing of antibiotics in Nepal. Reference Basnyat3 A global action plan implemented by the WHO in 2015 identified antimicrobial stewardship programs (ASPs) as one of the key strategies to address the problem of AMR. Reference Kakkar, Shafiq and Singh4,Reference Hijazi, Joshi and Gould5 A Cochrane review published in 2017 analyzed 221 studies worldwide and found that antimicrobial stewardship programs are successful in increasing compliance to standard antibiotic policy and reducing the duration of antibiotic treatment without adverse effects in mortality. Reference Davey, Marwick and Scott6 Another systematic review published in 2016 revealed that the main components of ASP (eg, adherence to guidelines for empiric therapy, de-escalation of therapy, appropriate switch to oral therapy from parenteral therapy and restriction of use of certain antibiotics) resulted in better clinical outcomes including lower adverse events, costs, resistance rates, or both. Reference Schuts, Hulscher and Mouton7 ASP has been implemented most widely in high-income countries. In many low-income countries where inappropriate use of antibiotics is common and serious drug resistance is widespread, evidence on the effectiveness of ASPs is limited. Reference Cox, Vlieghe and Mendelson8–Reference Ohara, Pokhrel and Dahal11 An executive summary by The Global Antibiotics Resistance Partnership GARP–Nepal National Working Group also acknowledges the role of ASPs to contain AMR in Nepal and recognizes the absence of ASP guidelines in Nepal. Reference Basnyat3
Methods
Setting
Grande International Hospital (GIH) is a 200-bed, tertiary-care hospital established in 2013 in Kathmandu, Nepal. It implemented a comprehensive, multidisciplinary infection control and ASP program in 2014. Our ASP utilizes a primarily front-end approach (restriction on certain category of antibiotics). Reference Dik, Hendrix and Poelman12 ASP started with the formation of a multidisciplinary antibiotic stewardship committee, which included representatives across departments. The ASP at GIH comprises the following components: an ASP committee, an antibiotic prescribing reference guide, educational outreach to prescribers, restriction of antibiotic formulary and prior authorization, and antibiotic dose optimization.
Formation of antimicrobial stewardship program (ASP) committee
An ASP committee was formed involving members from the following disciplines and departments: critical care medicine, internal medicine, nursing, microbiology, infection control, pharmacy, nephrology, hospital administration and an infectious disease specialist. In addition to representation from these departments, the ASP committee includes the heads of several clinical departments including the hospital medical director. This committee was designed to improve uptake and acceptance of any ASP-related interventions in the respective departments. The primary role of the ASP committee was to create, implement, and enforce the hospital’s antibiotic policy which primarily entailed formulary restriction. Regular meetings involving staff across disciplines were conducted to design, pilot test, and implement our ASP program components across patient care units. A multidisciplinary team of clinicians was identified and trained in concepts and antimicrobial stewardship. The team rotated to provide and document the approval of restricted antibiotics. It was critical that staff from all departments involved in ASP had a clear understanding of the necessity and benefits of antibiotic stewardship. This understanding allowed them to be champions of the ASP and educators regarding AMR within their department and helped garner support for the ASP.
Development of an antibiotic prescribing reference guide
The development and use of a standard and simple prescribing guide has been helpful in achieving the objectives of ASP. 13,Reference Pradelli, Risso, de Salvador, Cua, Ruimy and Roger14 Empiric antibiotic therapy guidelines were created for commonly encountered infections with antibiotic dosing guidance. This guide was designed to provide an easily accessible guide for the use of antibiotics for clinicians and pharmacists. Reference Baur, Gladstone, Burkert, Carrara, Foschi, Döbele and Tacconelli15 It was created and reviewed by clinicians from relevant departments and pharmacy to build collaboration and ownership of the guidance. Clinical experience and local microbiological susceptibility patterns were also considered when developing the guide. The reference guide is being revised and updated in regular intervals (every 1–2 years) to incorporate clinician input on the current knowledge of infection treatment, latest updates on antibiotic dosing, and antibiotic susceptibility patterns.
Education of prescribers
Meetings, discussions, and lectures were conducted by an infectious disease expert to justify the importance of ASP to stakeholders including administration, prescribers, microbiology, and pharmacy. It was critical for the infectious disease specialist to be involved early in the development and implementation of the ASP to garner support, answer questions, and train ASP champions in various departments and disciplines because this concept was novel concept to most staff involved in the ASP. The roles of various departments in implementation and continuity of the ASP have been regularly reinforced.
Restriction of formulary and prior authorization
The ASP developed an inpatient antibiotic formulary with 45 restricted antibiotics requiring approval as well as a mechanism for approval (Table 1 and Fig. 1). The list of restricted antibiotics complies with WHO AWaRe classification database. 16 Most of the antibiotics in the “reserve” category are not routinely available in Nepal. For the most part, antibiotics were considered for restriction based on their broad-spectrum activity. The rationale for restriction was to avoid unnecessary use when evidence showed that alternatives could be used with no adverse effect on mortality rate. Reference Kakkar, Shafiq and Singh4,Reference Hijazi, Joshi and Gould5 Other antibiotics (eg, antiretroviral or antifungal agents) were restricted because they may have significant risk of drug toxicity or are specialty medications that would benefit from the input of an infectious disease physician. Urgent initial doses of restricted antibiotics were allowed, but subsequent doses required ASP approval. To date, 16,548 patients (over 5 years) have been prescribed restricted antimicrobials after approval from ASP team. Most interventions taken by the ASP team have been related to dose modification (65%) followed by changes in antibiotic therapy duration (12%) and changes in antibiotic choice (8%).
Table 1. List of Restricted Antimicrobials
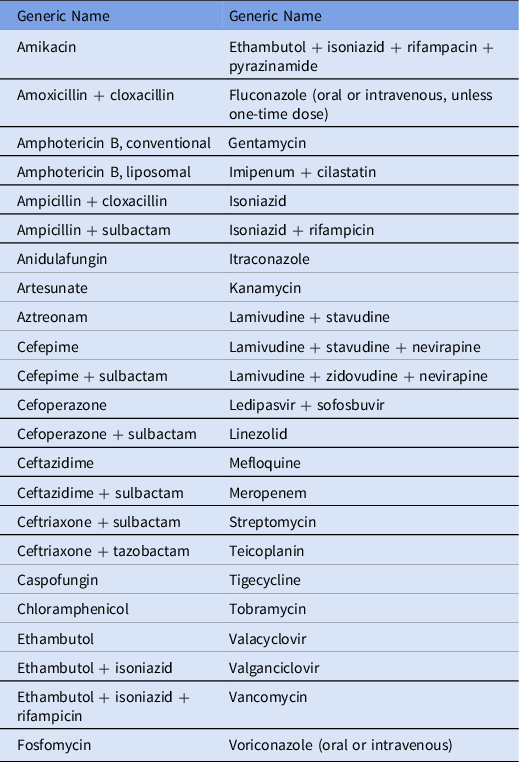

Fig. 1. Steps for ordering restricted antimicrobials from the pharmacy.
Dose optimization based on pharmacodynamics of antibiotics
Time-dependent killing is a characteristic of many antibiotics such as β-lactams, macrolides, and clindamycin. Beta-lactam antibiotics are the most-used agents in clinical practice. Reference Jacobs17 Pharmacodynamics studies suggest that the main parameter that correlates with clinical and microbiological efficacy of time-dependent antibiotics is the time for which serum concentration (fT) is above the minimum inhibitory concentration (MIC) of the pathogen. Based on Monte Carlo simulations, there is a higher probability for attaining fT > MIC when β-lactam antibiotics are administered using extended infusion or continuous infusion. Reference Jacobs17 Many clinical studies have shown advantages of extended infusion. Reference Jacobs17–Reference Bauer, West, O’Brien and Goff22 As part of the ASP program, we developed and implemented an extended infusion protocol for several β-lactam antibiotics (ie, meropenem, piperacillin-tazobactam, cefepime, and ceftazidime) to maximize clinical effectiveness.
Challenges for the ASP
Lack of acceptance from physicians
We encountered reluctance and resistance from many physicians during the introduction and initial implementation of the ASP. One prominent concern was that the ASP undermined professional autonomy. In the setting of specific patient scenarios, concerns were also voiced by the ASP team and/or prescribing physicians regarding the rationale and/or evidence of various proposed antibiotic plans. These concerns provided an opportunity for discussion and review of clinical guidelines, which served a continuing medical education function for all those involved and improved the quality of patient care. Discussions related to how clinical guidelines are applied outside Nepal were especially important in our setting.
We addressed these issues by ensuring that senior members of the medical staff, including the infectious disease specialist, were included in the approval team. Occasionally, discussions with individual physicians were necessary to address concerns; these discussions were often effective in reaching a mutually acceptable plan regarding antibiotic use. High-level administrative support was also key in addressing such situations. One particularly effective strategy was utilizing ASP champions within a physician’s specialty to help engage the physician in discussions regarding antibiotic use for conditions specific to their specialty. Despite these efforts, some instances remained in which the ASP recommendations were not accepted. In such cases, ASP members, the prescribing physician, and administration discussed the specific case to help facilitate a mutually agreeable plan. This experience is not unique to our program or Nepal. Several qualitative studies have also revealed physician behavior toward hospital antibiotic policy as a barrier for ASP. Reference Mol, Rutten, Gans, Degener and Haaijer-Ruskamp23,Reference Pakyz, Moczygemba, VanderWielen, Edmond, Stevens and Kuzel24
Lack of knowledge regarding optimal antibiotics prescribing
Many studies in low- and middle-income countries (LMICs) have demonstrated lack of knowledge among prescribers regarding appropriate use of antibiotics. Several studies have been performed on this subject in Nepal, with similar results. Reference Shrestha25,Reference Sarraf, Rai and Rauniar26 Our program benefitted from having both an infectious disease specialist and a doctor of pharmacy on the team. Educational programming, group discussions, and individual discussions were helpful in addressing this challenge but required significant time and effort.
Lack of support
Interventions like an ASP require substantial time and dedication, especially in the initial implementation phase. The development of a restricted formulary (with rationale) and a reference antibiotics guide tailored to our institution was time-consuming. Because it was a new concept in our practice setting and physicians had limited time given competing professional responsibilities, it was challenging to coordinate and communicate with stakeholders. However, our ASP program benefitted from early and consistent support from high-level hospital administration as well as committed ASP team members, which were key supports to our program.
Diagnostic barriers
The capacity and expertise of the microbiology laboratory is a key support for the ASP because the ASP relies on accurate and timely microbiology data to guide appropriate antibiotic therapy. Our hospital has a clinical microbiology laboratory capable of performing Gram staining, organism identification from culture results, and susceptibility testing via disc diffusion. But the laboratory does not have access to molecular or rapid organism identification testing. The lack of availability of rapid, automated, or molecular testing represents a limitation of identification and antibiotic sensitivity testing of a microorganism. 27 However, the GIH microbiology laboratory provides services that many other hospitals in Nepal do not provide. Lack of microbiological laboratory support is a very significant barrier in LMICs that has been associated with increased mortality. Reference Petti, Polage, Quinn, Ronald and Sande28
Documentation process
The recommendations made by the ASP were documented by the approval team to provide data regarding recommendations and if the recommendations are followed. This step is critical for process analysis and improvement. A dedicated form was developed to record the recommendation, but it was often challenging to use because calls for approval occurred around the clock and sometimes ASP members did not have the forms available. As a result, some gaps in documentation occurred, and the exact recommendation made by the ASP was not always documented.
Availability of antibiotics
Our ASP not only advocated for minimizing unnecessary use of antibiotics but also supported appropriate and prompt use of antibiotics when clinically necessary. Antibiotic unavailability can contribute to the use of broad-spectrum antibiotics when a narrower antibiotic is not available. Reference Kakkar, Shafiq and Malhotra29 In some instances, lack of some antibiotics within the context of Nepal (eg, aztreonam or echinocandins) made the development of empiric guidelines challenging. On the other hand, availability of broad-spectrum β-lactam–β-lactamase inhibitor combination antibiotics (eg, ceftriaxone+tazobactam and cefepime+tazobactam) required sustained educational efforts and discussion to discourage use when alternatives existed. The promotion of the wider use of such broad-spectrum antibiotics, often in unnecessary settings, was often driven by the influence of pharmaceutical companies either directly or indirectly on prescribers. The barriers we faced while implementing our ASP are similar to barriers encountered by other centers. Reference Schuts, Hulscher and Mouton7
Effectiveness of ASPs
Change in carbapenem resistance
The most common microorganisms isolated in our hospital are Acinetobacter, Klebsiella, and Pseudomonas. We detected a decrease in percentage of carbapenem-resistant bacteria immediately after implementing the ASP program (Table 2). Possibly, this decrease in carbapenem resistance may have been due to decreased carbapenem utilization and thus drug selective pressure, among other possible causes.
Table 2. Profile of Carbapenem Resistance Isolates Among the Most Common Microorganisms, 2015 and 2016

Opportunities provided by ASPs
The initiation and implementation of an ASP has opened opportunities to our healthcare workers to gain experience and education in ASP roles within their own professions and opportunities for interprofessional practice. These opportunities help build professional experience and expertise in ASP for future programs.
Decrease in unnecessary use of antibiotics
Basic interventions employed by the ASP have helped to decrease the unnecessary use of antibiotics. Requiring approval for broad-spectrum antibiotics has sensitized clinicians to use such agents carefully and to reconsider whether they are truly necessary. The purchase of combination antibiotics (eg, cefipime+tazobactam, ceftriaxone+sulbactam, and ampicillin+cloxacillin) drastically decreased after the ASP began. For example, 326 vials of ceftriaxone+sulbactam and 132 vials of ampicillin+cloxacillin were procured in 2015, which decreased to 34 and 59 vials, respectively, in 2016.
Improved knowledge and awareness
Formation of guidelines, education to the providers and publication of data have helped to improve knowledge and awareness among healthcare workers. Utilization of culture reports to guide antibiotic prescribing was incorporated into clinician prescribing practices when previously broad-spectrum antibiotics were commonly used for empiric therapy and further antibiotic management were often not guided by culture sensitivity data once available.
Access to safer alternate antibiotics
The availability of antibiotics is limited in LMICs. ASPs, through development and promotion of antibiotic dosing guides, have not only limited the unnecessary use of unwanted antimicrobials but have also helped to obtain and utilize antimicrobials with less adverse effects, such as echinocandins and teicoplanin, as pharmacy has become involved in the ASP.
Improved healthcare facilities
Many factors mentioned here have ultimately helped decrease morbidity and mortality and have likely decreased the overall cost of treatment. We believe that these steps have contributed to the improvement of overall outcomes of patients.
Plans to strengthen ASPs
Improved diagnostic capacity
Implementation of an ASP and its feedback to the hospital and organization can provide an opportunity to establish and improve microbiological laboratory facilities, which can ultimately have a positive impact on patient outcomes. Short-term solutions to improve diagnostic capacity may include partnering of an institution with larger institutions with better microbiology laboratory capabilities. Long-term solutions include expansion of microbiology laboratory facilities via a national action plan with dedicated funding. Global programs such as Strengthening Laboratory Medicine Toward Accreditation (SLMTA) and Stepwise Laboratory Improvement Process Towards Accreditation are working to increase access to microbiology laboratories in LMICs. Reference Nkengasong, Yao and Onyebujoh30 GIH is currently working to purchase an automated system (VITEK-2, bioMèrieux, Marcy-l’Étoile, France) for rapid and accurate identification of pathogen and assessment of antimicrobial susceptibility.
Postprescription review with feedback
Postprescription audit with feedback from the ASP team of other unrestricted antibiotics can be a useful approach for better understanding overall antibiotic utilization in this institution. Studies have shown a higher acceptance rates and improvement in appropriate use of antibiotics when postprescription review with feedback was used compared to preprescription authorization. Reference Tamma, Avdic and Keenan31,Reference Doukas, Cheong, McKew, Gray, McLachlan and Gottlieb32
Regular training to pharmacists and approval team
We plan to continue training for ASP members and to include new ASP members as staff turnover occurs and to diversify our team. We anticipate that this will strengthen the ASP team and enhance long-term team function.
Establishment of ASP as a priority plan in the national action plan
Starting ASP in one healthcare institution can encourage other institutions to develop an ASP in their own center. However, a national policy is essential to standardize and monitor these programs. The WHO provides a framework for the development of a national action plan, which the WHO also notes ideally starts with developing local ASPs. 33 Few LMICs have developed national plans to establish ASPs and limit AMRs, but most notably, India has. Reference Cox, Vlieghe and Mendelson8 According to the US Center for Disease Dynamics, Economics and Policy (CDDEP), Nepal has also partnered with the Global Antimicrobial Resistance Partnership (GARP), which provides tools for LMICs to collect data on nationwide trends in antibiotic use and AMR along with supporting stewardship activities. 34 We hope our experience will help support national policy development to explore how to establish and adapt ASPs in other hospitals.
Importance of ongoing research, publication, and dissemination of results
Monitoring the impact of the ASP is extremely important in demonstrating the effectiveness and value of stewardship. Future research at our institution may include further investigation of reductions in antibiotic use due to ASP; appropriateness of antibiotic selection, dose, and duration; decrease in cost; decrease or change in resistance; and overall improvement in clinical outcome. Research and publication are extremely important to advocate adaptation in other institutions or settings and to describe facilitators and barriers to ASP implementation in a variety of settings. It also opens the door for improvement by identifying potential areas of improvement by others who review ASPs in different settings.
In conclusion, our antimicrobial stewardship program was established with all necessary core elements as outlined by the WHO: leadership commitment, accountability and responsibilities, AMS actions, education and training, monitoring, reporting and feedback. The challenges faced by this program could be utilized by other institutions in LMICs to implement their own ASPs in similar settings. We have many plans for our ASP in the areas of monitoring, surveillance, and reporting to better describe our operations and outcomes and to inform ongoing quality improvement.
Acknowledgments
We thank the ASP physicians for their input and feedback regarding this manuscript. We are grateful for the continued support of leadership of Grande International Hospital in establishing and continuing our ASP program.
Financial support
We have not received any financial support for this study.
Conflicts of interest
All authors report no conflicts of interest related to this article.





