INTRODUCTION
In several reference cases of disposal systems envisaged by waste management organizations, radiocarbon (14C) is one of the most important contributors to the dose (Johnson and Schwyn Reference Johnson and Schwyn2008). The FP7 EURATOM project CAST (CArbon-14 Source Term)Footnote 1 aimed to develop understanding of the potential release mechanisms of 14C from radioactive waste materials under conditions relevant to waste packaging and disposal to underground geological disposal facilities. The project focused on the release of 14C as dissolved and gaseous species from irradiated metals (steels, Zircaloys), irradiated graphite and from ion-exchange materials.
The objectives of the CAST project were to gain new scientific understanding of the rate of release of 14C from the nuclear waste and the results will be evaluated in the context of national safety assessments and disseminated to interested stakeholders.
The work-package 4 of the project CAST dealt with one specific type of radioactive wastes containing 14C: Ion Exchange Resins (IERs). IERs are macromolecular copolymers based on styrene monomer cross-linked by divinyl-benzene and functionalized with sulfonic groups (cationic IERs), or quaternary ammonium groups (anionic IERs) (Figure 1). Linear polymer filaments combine forming a three-dimensional finely branched network with many cavities. The surfaces of the polymer filaments are functionalized by chemical groups, which adsorb selectively anions or cations and exchange them with other ions, depending on the nature of their functional groups, resulting in a selective sequestration of components from the solution. These resins have typical spherical shape, with a 0.5 mm ca. diameter and can be used in the single chemical form (anionic or cationic) or in a mixed bed configuration.
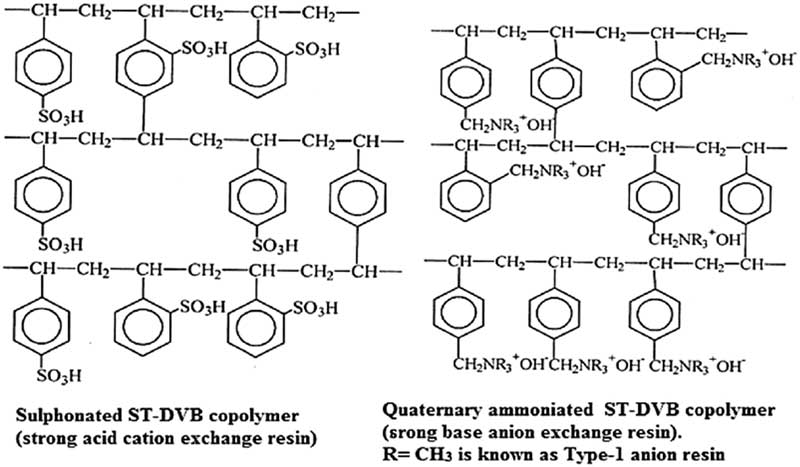
Figure 1 Chemical structure of cross-linked ionic exchange resins (cationic and anionic).
They are widely used in nuclear facilities for the purification of liquid processes or wastes streams. A combination of cationic, anionic and/or mixed bed resins are used depending on the circuits’ physicochemical conditions and on their specific needs.
In general, the main release of 14C from spent ion exchange resins (SIERs) in the gas phase is expected to be in form of inorganic species (14CO2) (Rizzo et al. Reference Rizzo, Bucur, Comte, Källström, Rizzato, Večerník and Reiller2017) and specifically, a disturbance of the acid-base equilibrium of the solution in contact with the resins will result, directly or indirectly, in a release of 14C present in the form of a carbonate. This could also arise through the ingress of foreign anions with selectivity on the resin higher than HCO3 –, e.g. as chloride, nitrate, or hydrogenosulphate anions. Other less known effects leading to 14C release from SIERs are the effects of service/storage temperatures and strong oxidants (thermal and chemical degradation) which could affect resin equilibrium and uptake capacity (Magnusson et al. Reference Magnusson, Stenström and Aronsson2008; Rizzato et al. Reference Rizzato, Rizzo, Heisbourg, Vecernik, Bucur, Comte, Lebeau and Reiller2014).
The density of the functional groups of the resins and the ion-selectivity cross-linking structure are two parameters influencing the decontamination factor (DF), defined as the ratio between the influent activity of the incoming fluid in the IERs and the effluent activity exiting form the IERs. These two parameters are strictly connected with the preservation of morphology in time.
In order to analyze the effect of storage conditions on the potential release of 14C, we investigated the change of the morphology of the IERs upon aging. The present work is a systematic study on two reference IERs, the cationic Lewatitt® MonoPlus S200KR and the anionic Lewatitt® MonoPlus M500 KR, both manufactured by Bayer Chemicals.
METHODS
Two ion exchange resins were chosen for this study:
∙ Lewatitt® MonoPlus S200KR, which is a monodisperse, strong acidic gel type resin, nuclear grade designed. The functional group for H+ exchange is sulphonicon cross type polystyrene beads. The bead size is specified at 0.6 mm ± 0.05 mm. Formerly named as Lewatitt® S100 KR-H (Cl frei);
∙ Lewatitt® MonoPlus M500KR, which is a monodisperse, strongly basic, gelular anion exchange resin, nuclear grade. The functional group of OH– exchange is a quaternary amine, on cross type polystyrene beads. The bead size is specified at 0.64 mm, ± 0.05 mm.
Both resins were provided by SOGIN, the Italian state-owned company responsible for the decommissioning of Italian nuclear plants and the management of radioactive wastes, in the framework of the EU project CAST (Rizzato et al. Reference Rizzato, Rizzo, Heisbourg, Vecernik, Bucur, Comte, Lebeau and Reiller2014).
Instrumentation
Scanning Electron Microscope (SEM)
The morphology of the resin beads was studied by means of a SEM, FEI Inspect S model (FEI Company, ThermoFisher Scientific, Massachusetts, USA). Areas ranging from approximately 1 cm to 5 μm in width can be imaged in a scanning mode using conventional SEM techniques (magnification ranging from 20× to approximately 30.000×, spatial resolution of 50–100 nm). The equipment used is also equipped with EDS probe, for high and low vacuum elemental analyses. A digital imaging analysis tool Macnification®, specifically developed for SEM images has been used for the measurement of the diameters of the beads.
Total Carbon Content
The total carbon content was measured using a benzene synthesis chemical pipeline designed and implemented at the ENEA Research Center of Bologna (Italy). A targeted device (Figure 2), with a double chamber combustion cell, was designed and implemented in order to run stepped combustion of ionic resin samples to obtain different volatile/non-volatile fractions. The inner and the outer tube (shown in the black circle) allows the use of different gas composition (i.e. pure nitrogen, pure oxygen, nitrogen/oxygen mixture, argon) inside and outside the sample crucible, on order to reach different degrees of oxidation potential. The system has been tested with standard samples and has shown recovery of total carbon up to 80% (in terms of yield of benzene synthesis) and up to 98% (in terms of CO2 recovery). Optimized parameters for the combustion of IERs have been fixed as shown in Table 1.
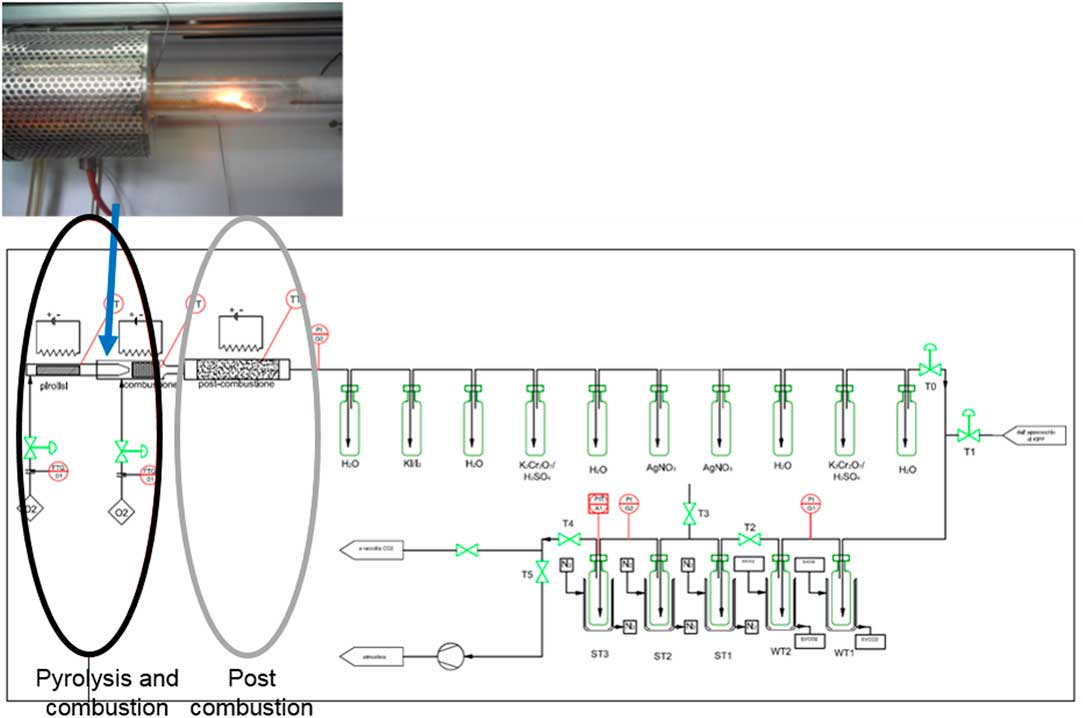
Figure 2 Equipment for stepped combustion/pyrolysis of organic materials for benzene LSC analysis at ENEA 14C laboratory.
Table 1 Parameters for combustion of IERs and 14C total measurement by stepped combustion.

Test Procedure
We prepared two different batches of samples for each type of ionic resins (anionic and cationic: “brand new” and “aged”). The brand new batch was composed by an aliquot of beads as they were supplied by the manufacturer, packed in their original packaging and analyzed shortly after sampling (max 7 days). The “aged” ones were samples of beads fabricated several years ago and stored (without being used in any nuclear process) for 12 yr in the original containers as provided by the manufacturer. The storage conditions are ambient environment T/P without any temperature, humidity, pressure control, so very similar to the temporary storage conditions as could be relevant for radioactive waste (Van Loon and Hummel Reference Van Loon and Hummel1995).
SEM imaging was carried out at various scales, in order to perform both the morphology assessment and the bead diameter statistics. Imaging has been performed at low vacuum (0.4 mbar), with direct imaging detector and 30KV source lamp. The assessment of the sphericity preservation upon aging was applied as a qualitative index. In order to classify the beads, three definitions have been proposed:
1. New: the beads appear as supplied, both for shape and diameter;
2. Degraded: the surface of the beads appears corroded and ruined, but the global spherical shape is preserved;
3. Damaged: a heavy loss of material took place and the beads loose their spherical shape and appear fragmented.
The beads were analyzed with a macro developed upon the image processing software “Fiji” (Van Loon and Hummel Reference Van Loon and Hummel1999a, Reference Van Loon and Hummel1999b) and they were classified as new, degraded and damaged. The identification of the bead shape was done referring to the image at low magnification (50×) and then, if some interpretation difficulties arose, a higher magnification with SEM was provided. An accurate control over the residues above the beads was also performed, in order to avoid false qualitative interpretations (Schindelin et al. Reference Schindelin, Rueden, Hiner and Eliceiri2015). In Figure 3, a sample with 14 as new beads (round symbols), 6 degraded (triangoles) and 4 damaged (square) is shown.
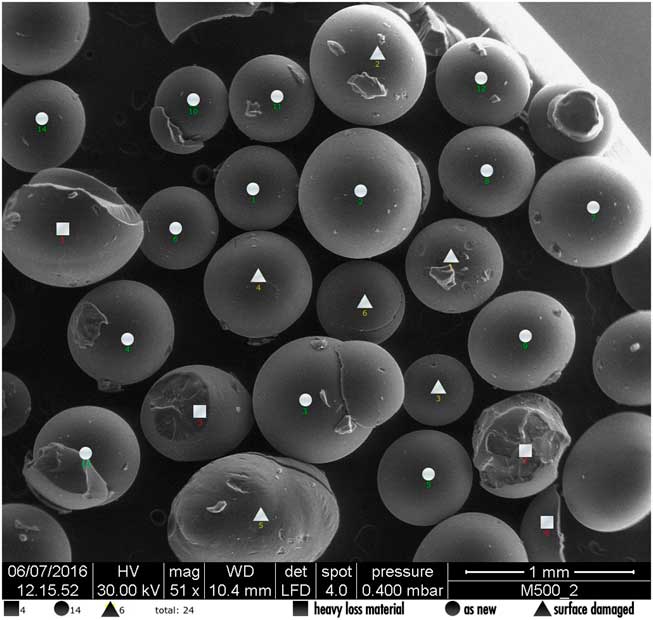
Figure 3 Example of classification of the beads: round symbol means new, triangle symbol means degraded and square symbol means damaged beads.
By means of the image analysis software Macnification®, SEM micrographies were imported, manually analyzed and statistics on diameter variations and bead status were then summarized graphically.
RESULTS
Beads Micrographies
Figure 3 shows the typical appearance of a beads specimen (anionic type). Large-scale images such the one depicted have been used for the assessment of bead diameter. Figure 4a shows a 10-yr aged specimen of the same anionic resin, appearing heavily damaged. The three degrees of sphericity degradation are apparent: some spheres are still intact, the foreground one is still spherical but with a clear surface corrosion and the right one shows a large material loss, ending in a shape loss. Considering the inventory of 14C in such resins, such a loss of materials leads to radioactive material loss too, be it the beads both still used as filters inside primary circuits or already stored as waste. The aged samples appear, macroscopically, as a random dimensions debris and hardly contained by storage filters designed for the original beads diameters.
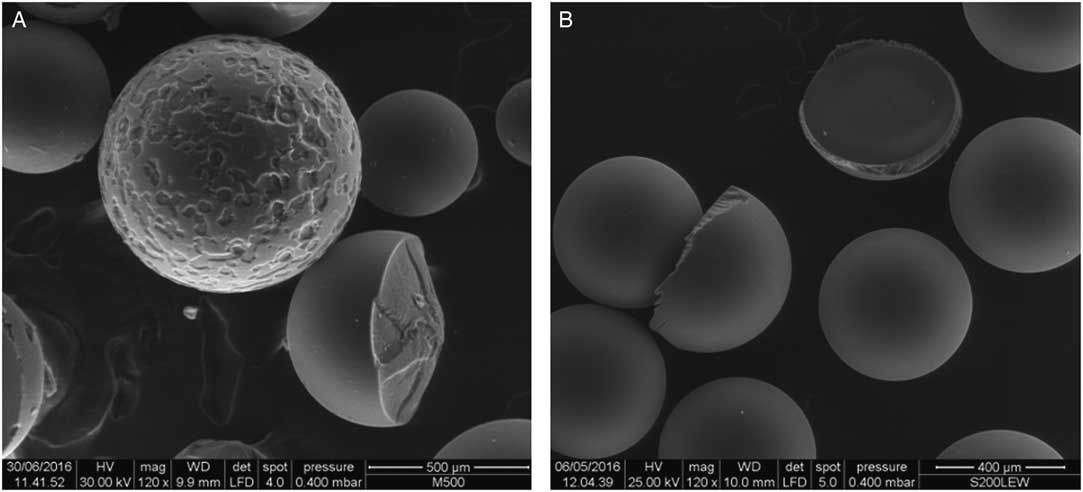
Figure 4 500-μm scale SEM micrography: (A) aged anionic resin; (B) aged cationic resin.
Figure 4b depicts a 10-yr aged sample of the cationic resin, which appears less damaged when compared to the anionic one. Very few spheres are damaged, mainly because of a “half” rupture. No corroded layers were determined, or partial or main material loss. The sample appears with less debris. From a purely qualitative point of view, it can be stated that the main difference between S200 and M500 resin beads is that degradation at first one appears as a half sphere break, while in the latter one as a random corrosion rate.
Graphical Interpretation of SEM Images
Table 2 and Figure 5 show the comparison of bead diameters for the four batches. The manufactured specified diameters are confirmed, with small variations from sample to sample: diameters are globally preserved in time, with small variations. The cationic resins show a better preservation of their sphericity upon aging, compared with the anionic ones. This degradation of the anionic resins is strictly connected to high matter loss.
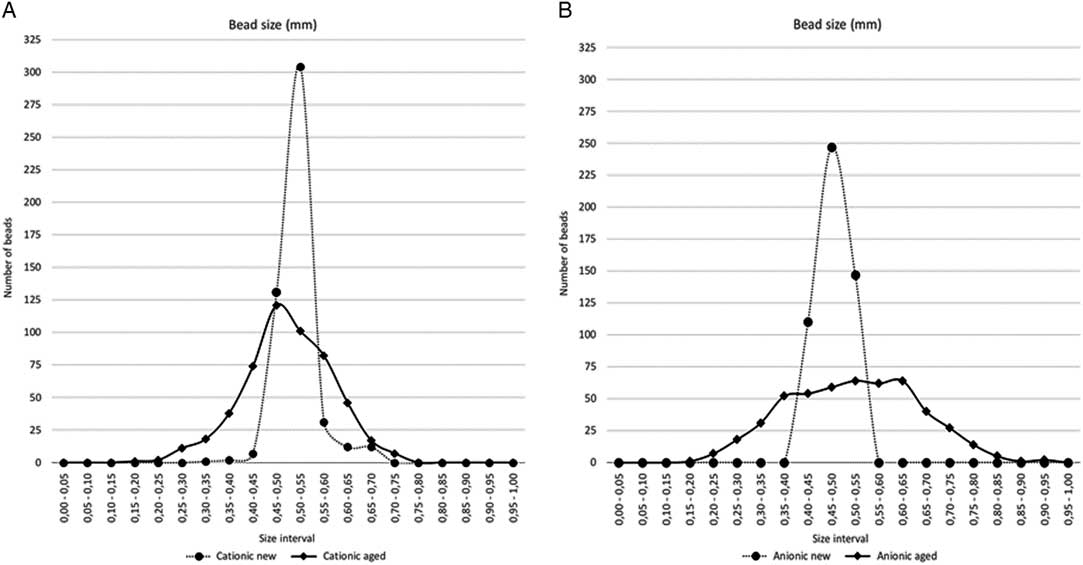
Figure 5 Comparison of bead diameters for the four batches: (A) cationic resins; (B) anionic resins.
Table 2 Diameter percentages and total carbon content of different resin samples.
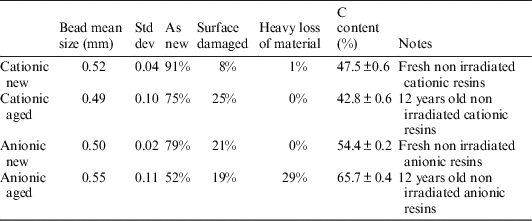
Considering only the two “new” batches (anionic and cationic), the cationic resin exhibits a relatively better quality of the beads: the amount of damaged beads is only 8% in the cationic resin, whilst reaches 21% of the total in the anionic one. The aged resins show a similar trend: after 12 yr of aging, the cationic resin shows that only 25% of the amount of beads suffered damage, while the anionic ones exhibit 19% of the total number of beads damaged and up to 29% of beads with heavy random material loss (Schindelin et al. Reference Schindelin, Arganda-Carreras, Frise, Kaynig, Longair, Pietzsch, Preibisch, Rueden, Saalfeld, Schmid, Tinevez, White, Hartenstein, Eliceiri, Tomancak and Cardona2012; O’Brien et al. Reference O’Brien, Hayder and Peng2016).
Total Carbon Content
Analysis of the total carbon content on non-activated IERs (new and 12 yr old) has been carried out to provide auxiliary data on the composition of the resins and the effect of aging, leading to the following results (Table 2 and Figure 6). The results show that the aging in a closed container caused a decrease in total C content in the cationic resins, which could be quantified in 10% of loss, whereas the anionic resins exhibit a slightly opposite behavior (small increase of C content) that could be due to adsorption of atmospheric carbon dioxide during the temporary storage; this is to be further investigated.
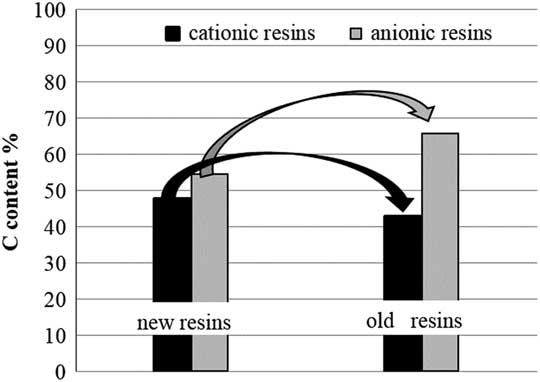
Figure 6 Total carbon content of IERs (new and old).
DISCUSSION
Both resins (cationic and anionic) have shown comparable original quality in terms of statistical distribution of the bead diameters. The SEM images analysis clearly showed that the two resins (anionic and cationic) behaved differently, as far as the sphericity preservation upon aging is concerned.
The anionic beads (M500KR) showed a stronger degradation with aging, with randomly distributed corruptions: several beads showed great material losses, complete cleavage and heavy damages over the spherical surface.
On the other hand, the cationic resin (S200KR) showed only around 20% of damaged beads after aging, probably due to the greater chemical stability of the sulphonyl functional groups. This suggests that the anionic resin beads show a lower overall quality, in terms of average sphericity.
Moreover, considering the application of these products inside primary circuits of Nuclear Power Plants, the consequences of such macroscopic fragmentation lie not only in an efficiency loss in their 14C filtrating power, but also in a possible debris production, with random size. This latter aspect could also affect a potential radioactive material loss during the resins’ storage after their removal from the reactor.
The purpose of this morphological investigation was to compare the results of degradation with the measurement of the inventory of 14C content in different irradiated SIERs, which were carried out by the CAST project consortium members and that have been reported in a final deliverable (D4.5) (Rizzo et al. Reference Rizzo, Bucur, Comte, Källström, Rizzato, Večerník and Reiller2017). These measurements have shown a large distribution of the amount of 14C in the SIERS, because of the difference in the reactor type, in the storage condition, in the sampling and the aging.
A morphological investigation of all the SIERs analyzed by the CAST project and contributing to the 14C inventory has not yet been carried out but, according to the preliminary results of the present study, the different degree of degradation could explain the scattering of the 14C data.
A 14C inventory, upon aging, and an exhaustive morphological analysis of the SIERs discharged from the nuclear reactors, will be of upmost importance in order to have a reference database and to provide data for input to the safety case and the performance assessment of radioactive waste repositories.
CONCLUSION AND OUTLOOK
The tools used to assess the sphericity preservation upon aging were effective in a quality assessment of the ionic exchange resins sent for the temporary storage and final disposal in the radioactive waste repository. The differences between two widely used commercial products Lewatitt® MonoPlus S200KR and Lewatitt® MonoPlus M500KR resin beads were clearly spotted in terms of diameters measurements, surface detection and fragmentation detection.
This qualitative analysis is quite effective for a preliminary assessment of the possible 14C debris loss and can be used as a supporting analytical tool for modeling of potential 14C release. The next step for a better definition of the beads’ property variations in time is the analysis of resin after real operation in a nuclear power plant. The impact of the moderator (water, pressurized or boiling) presence, as well as the chemical equilibria driven by pH, dissolved species in the coolant, fission products and neutrons, must be assessed with a similar experimental campaign, in a hot cell. This would lead to a complete understanding of resin beads behavior, at least as far as their morphology preservation upon aging.
The analysis of C content upon aging and the relative 14C content measurement need to be completed with the morphological study in order to definitively correlate the poor preservation of sphericity with the potential release of 14C. Once the correlation is well assessed, the sphericity could be used as a leading parameter for evaluation of 14C release for the performance assessment of spent ion exchange resin disposal in a radioactive waste repository. The sphericity analysis by means of SEM could become an equivalent, but less time consuming and less expensive, method for the evaluation of the potential release of 14C from SIER, providing valuable input for the performance assessment of the radioactive waste repository.
ACKNOWLEDGMENT
This study has been carried out within the framework of the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 604779, the CAST project.










