Lactic acid bacteria are known as typical probiotics which have been defined as viable microorganisms that exhibit a beneficial effect on the health of the host when they are ingestedReference Lee and Salminen1, although in recent years non-viable microorganisms have also been included within the definitionReference Salminen, Ouwehand, Benno and Lee2. Strains of the genera Lactobacillus and Bifidobacterium, mostly isolated from human intestine, are the most common probiotics used for human consumption. Probiotics have been shown to reduce serum cholesterol levelsReference De Rodas, Gilliland and Maxwell3, improve the balance of intestinal floraReference Lidbeck, Geltner-Allinger, Orrhage, Ottova, Brismar, Gustafsson, Rafter and Nord4 and exert immunomodulatory activityReference Kalliomaki, Salminen, Poussa, Arvilommi and Isolauri5, etc. We have previously shown that Lactococcus (Lc.) strains isolated from milk products and plants have probiotic properties, including adhesion to Caco-2 cellsReference Kimoto, Kurisaki, Tsuji, Ohmomo and Okamoto6, tolerance to low pH and bileReference Kimoto, Ohmomo, Nomura, Kobayashi and Okamoto7, cholesterol adsorption in vitro Reference Kimoto, Ohmomo and Okamoto8, and immunomodulatory activity in vitro and in vivo Reference Kimoto, Mizumachi, Okamoto and Kurisaki9. Further, our group showed that strains of Lc. lactis subsp. lactis express glutamate decarboxylase and produce γ-aminobutyric acidReference Nomura, Kimoto, Someya, Furukawa and Suzuki10, Reference Nomura, Kimoto, Someya and Suzuki11, and that these strains can be used to make a γ-aminobutyric acid-enriched fermented milk product that has a hypotensive effectReference Inoue, Shirai, Ochiai, Kasao, Hayakawa, Kumura and Sansawa12, Reference Hayakawa, Kimura, Kasaha, Matsumoto, Sansawa and Yamori13.
As the elderly population increases, the prevalence of ageing-related diseases will increase, and functional foods that provide health benefits to control ageing and prolong health span will become more desirable. Physiological phenomena associated with ageing include impaired memory acquisitionReference Mahncke, Bronstone and Merzenich14, decreased immune responsesReference Solana and Mariani15, increased peroxidation in vivo Reference Voss and Siems16 and loss of bone densityReference Stock, Schneider and Strauss17. Various compounds have been reported to contain functional molecules that have anti-ageing effectsReference Lei, Wang, Li, Yang, Zhou and Chen18–Reference Rubino, Farace, Dessy, Sanna and Mazzarello20; for example, one containing vitexin shows an anti-ageing effect via antioxidant activityReference Kim, Lee, Kim, Sim, Lee, Lee, Yun and Pyo21. It has been reported that the Lactobacillus casei strain Shirota activates immune responses in aged mice, and ameliorates influenza viral infectionsReference Hori, Kiyoshima, Shida and Yasui22, and that Bifidobacterium- and Lactobacillus-fermented milk is beneficial in the treatment of murine inflammatory bowel disease, possibly via stabilization of mucosal immunityReference Matsumoto, Watanabe, Imaoka and Okabe23.
Osteoporosis is one of the diseases associated with ageing. In bone remodelling, the resorption of old bone by osteoclasts and its subsequent replacement by osteoblasts are highly co-ordinated. Disturbances in the process result in an imbalance between resorption and formation and are responsible for most metabolic bone diseases, including osteoporosisReference Sambrook and Cooper24. In postmenopausal osteoporosis, bone resorption proceeds abnormally rapidly compared with bone formation, whereas in senile osteoporosis, bone resorption and formation both decline.
The senescence-accelerated mouse (SAM) provides a good model for the physiological phenomena associated with ageingReference Takeda, Hosokawa and Takeshita25. SAMP6, one of the senescence-prone inbred strains, exhibits an early decrease in bone mass with a reduction in bone modelling, and is used as a model for senile osteoporosis. All SAMP mice develop normally, but then start senescence at approximately 6 months of age and irreversible advancement of senescence, manifested by signs such as loss of hair and increased lordokyphosis. Although there have been some reports on the effects of compounds on physiological phenomena associated with ageing in SAMReference Ohata, Nishikawa, Hirai, Kato and Miyamoto26, Reference Li, Ng, Gao, Li, Fu, Niu, Zhao, Chen and Liu27, the effects of administration of lactic acid bacteria have not yet been investigated in SAMP6 mice.
We recently found that Lc. lactis subsp. cremoris H61 (strain H61) has immunomodulatory activity, as shown by its stimulation of cytokine production in immunocompetent cells in vitro Reference Kimoto, Mizumachi, Okamoto and Kurisaki9. The strain H61 has been widely used over the last 50 years in Japan to produce fermented dairy products. In the present study, we investigated the effects of oral administration of the strain H61 on physiological variables in SAMP6, including bone density loss with ageing.
Materials and methods
Animals
SAMP6 male mice were purchased from SLC Japan Inc. (Shizuoka, Japan) and housed one per cage (27 × 15 × 10 mm) in a 24 h light/dark cycle. Temperature was maintained at 22 ± 2°C, and a basic diet (MM-3; Funabashi Farm, Chiba, Japan; Table 1) and water were provided ad libitum until the start of the feeding experiment. The animal experiments followed the guidelines of the National Institute of Livestock and Grassland Science.
Table 1 Composition of the diets
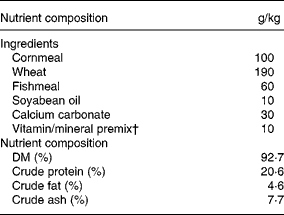
† Takeda Kagaku Shiryou (Tokyo, Japan) supplying (g/kg); FeSO4, 5·44; MnSO4, 2·75; CoSO4, 0·11, CuSO4, 1·26; ZnSO4, 2·47; MgCO3, 19·2 g; Ca(IO3)2, 0·61; menadione sodium bisulphite, 1·92; vitamin A oil, 1 200 000 IU; vitamin D oil, 200 000 IU; dl-α-tocopherol acetate, 6; thiamin nitrate, 0·25; riboflavin, 1·00; pyridoxine hydrochloride, 0·13; calcium pantothenate, 1·36; nicotinic acid amide, 2·48; choline chloride, 57.g; cyanocobalamin, 0·003; pantothenic acid, 0·008; glucose, 90·0.
Preparation of bacterial cells
Strain H61 (No. 400007; Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Japan) was cultured in MRS broth (Becton Dickinson, Cockeysville, MD, USA) by subculturing 1 % inocula for 18 h at 30°C. The bacterial cells were harvested and washed twice with 085 % NaCl and then resuspended in the same solution. Heat-killed cells were prepared by treatment at 100°C for 30 minReference Hori, Kiyoshima, Shida and Yasui22, followed by centrifugation and lyophilization. No colony of the treated strain H61 was observed in MRS agar after 72 h incubation at 30°C. Microscopic observation revealed that the treated strain H61 was intact. Living cells were prepared by resuspending the washed cells in 10 % non-fat milk to 108 colony-forming units/ml. Fermented milk was prepared by inoculating the cells in 10 % non-fat milk and incubating for 18 h at 30°C. This fermented milk contained almost 108 colony-forming units/ml of the strain. Living cells and fermented milk were stored at − 80°C until use and were prepared every 2–3 d.
Experimental schedules
We housed mice for another 2–3 months to ensure that senescence was sufficiently developed. The feeding period was based on that in the report by Hori et al. Reference Hori, Kiyoshima, Shida and Yasui22, in which aged mice were fed for 4 months.
The experimental diet was MM-3 with the addition of 0·05 % (w/w) heat-killed cells of strain H61. The control diet was MM-3 without strain H61. These diets were administered to mice from the age of 9 months (aged SAMP6, six or seven per group) for 5 months or to mice from the age of 1 month (young SAMP6, nine per group) for 2 months.
In separate experiments, living cells of strain H61 or fermented milk were administered to mice from the age of 8 months (aged SAMP6, four or five per group) at 2 × 107 cells/mouse (in a single 200 μl dose via a gastric tube) every 2–3 d for 4 months. Non-fat milk without lactic acid bacteria was administered to mice as a control.
Grading score
The degree of senescence in aged mice was evaluated according to a grading score systemReference Hosokawa, Kasai and Higuchi28. The score included assessment of behaviour (two items; reactivity and passivity) and gross appearance. The appearance criteria consisted of three categories: skin and hair (four items; glossiness, coarseness, loss of hair and skin ulcers), eye (four items; periophthalmic lesions, corneal opacity, ulcers of the cornea and cataract) and spine (one item; lordokyphosis). Blind evaluation was not done.
Detection of enteric bacteria in faeces
Faeces were added to sterile 0·85 % NaCl solution (0·1 g faeces/ml) and mixed by vortexing until suspended. Serial dilutions of the stool samples were spread on acetate agarReference Rogosa, Mitchell and Wiseman29 to detect Lactobacillus sp., bacteroides agar (Nissui Seiyaku, Tokyo, Japan) to detect Bacteroides sp., BL agar (Nissui) supplemented with 0·5 % horse blood to detect Bifidobacterum sp., mannitol agar (BBL; Becton Dickinson) to detect Staphylococcus sp., DHL agar (Nissui) to detect Enterobacteriaceae sp., and azide-citrate broth (Nissui) with 1·6 % agar to detect Enterococcus sp. The acetate agar, bacteroides agar and azide-citrate agar plates were incubated anaerobically using Gas Pak® (Becton Dickinson). After 24–48 h of incubation at 37°C, the numbers of surface colonies were counted.
Determination of oxidation in vivo
Lipid peroxidation status in vivo was determined from the concentration of thiobarbituric acid reactive substances (TBARS)Reference Wozniak, Wozniak, Drewa and Drewa30 in serum. Serum was obtained by incubating blood for 1 h at room temperature, followed by centrifugation at 10 000g for 3 min. The concentration of TBARS in serum was determined according to the method of Naito & YamanakaReference Naito and Yamanaka31.
Preparation of spleen cells
Mice were killed and the spleens were aseptically removed. A single-cell suspension was prepared by pressing the tissue gently. The erythrocytes were depleted with lysing solution (0·15 m-NH4Cl, 10 mm-KHCO3 and 0·1 mm-Na2EDTA, pH 7.2) for 5 min at room temperature, and then fresh RPMI 1640 medium (Sigma Chemical Co., St Louis, MO, USA) was added. After two washes with the medium, cells were seeded in twenty-four-well plates at 1 × 105 cells/ml with 10 μg/ml Concanavalin A (Sigma) as a mitogen, or at 1 × 107 cells/ml without Concanavalin A. We first added 10 and 20 μg Concanavalin A to the spleen cells, and determined the cytokine level in the culture supernatant. We found that cytokine production was similar at the two concentrations, so we used 10 μg Concanavalin A for stimulation. The cells were cultured at 37°C in a 5 % CO2–95 % air atmosphere in RPMI 1640 medium supplemented with 10 % inactivated (56°C for 30 min) fetal calf serum (Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin and 5 × 10− 5m-2-mercaptoethanol. The culture supernatants were collected after 72 h, and the concentration of cytokines (IL-4, IL-6, IL-12p40 and interferon-γ (IFN-γ)) was determined by ELISA using an ELISA Kit (PharMingen, San Diego, CA, USA).
Bone density analysis
The right femur was removed and immersed in 70 % ethanol for 1 week. The solution was changed every 3 d. Bone density was determined by single-energy X-ray absorption (SXA) which measures bone mineral and bone area, using DCS-600R (Aloka, Tokyo, Japan). Osteoclasts in the bone perimeter of cancellous bone tissue were identified and counted by staining with tertrate-resistant acid phosphatase stainReference Faloni, Sasso-Cerri, Katchburian and Cerri32.
Statistical analysis
Differences between the treatment and control groups were analysed using the SAS system version 9.1 (SAS Institute, Cary, NC, USA). Bone mineral densities, osteoclasts numbers and cytokine values were analysed by the General Linear Model procedure with one-way allocation. Changes in body weight were analysed by the General Linear Model procedure as follows: diet was designated as the main plot, and age and diet × age interaction as the subplot; the error term for the main plot was animal or cage within diet, whereas the error term for the subplot was the residual error. Comparisons of least squares means were analysed using the PDIFF option of the General Linear Model procedure. Grading scores for senescence were analysed using non-parametric tests using the NPAR1WAY procedure of the SAS system. The effects of diet were analysed using Mann–Whitney and Kruskal–Wallis tests, using the WILCOXON option of the NPAR1WAY procedure for Tables 2 and 5, respectively. Data are expressed as means with their standard errors. A P value of less than 0·05 was considered statistically significant.
Table 2 Effect of oral administration of heat-killed Lactococcus lactis subsp. cremoris H61 (strain H61) on grading score in aged SAMP6 mice
(Mean values with their standard errors for six or seven mice)
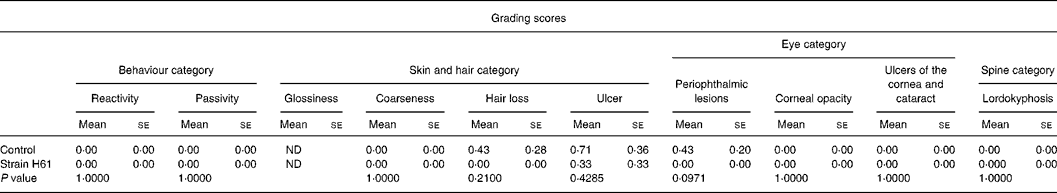
ND, not determined.
Results
Effects of heat-killed cells of strain H61 on physiological changes in aged SAMP6 mice
Body weight
Aged SAMP6 mice (9 months old) were fed either a diet containing heat-killed strain H61 (H61 group) or the control diet (control group). The mean daily dietary intakes ranged from 3·80 to 4·99 g/mouse, and there was no significant difference between the mean intakes of the H61 and control groups. Water consumption did not seem to differ among groups although it was not accurately assessed. The body weight of mice in the control group decreased with ageing, while that of mice in the H61 group increased up to the age of 12 months and then slightly decreased until the end of the experiment (Fig. 1). The effect of diet is dependent on the age of the mice. The body weight of mice in the H61 group was significantly heavier (P < 0·001) than that in the control group at the age of 12 and 14 months old.

Fig. 1 Body weights of aged SAMP6 mice fed heat-killed Lactococcus lactis subsp. cremoris H61 (○) or control diets (●). Values are means with their standard errors depicted by vertical bars (for six or seven mice). Mean values were significantly different between the two groups on the corresponding age: ***P < 0·001.
Grading score
Table 2 shows the grading scores for aged SAMP6 mice at the age of 14 months. In the skin and hair category, there was no difference in the coarseness score between mice in the two groups. Three mice had skin ulcers in the control group, and one mouse had in the H61 group. Loss of hair was observed in mice in the control group, but not at all in the H61 group. In the eye category, the scores for corneal opacity, ulcers of the cornea and cataracts were not different between the groups, but the score for periophthalmic lesions was lower (P = 0·0971) in the H61 group than in the control group. There were no significant differences between the groups in the spine or behaviour categories. The sum of grading scores was 0·33 for the H61 group and 1·57 for the control group. Fig. 2 shows the typical appearance of SAMP6 mice fed control and H61 diets. The mouse that received the control diet (Fig. 2(a)) had skin ulcers and swollen eyelids, whereas the mouse that received the H61 diet (Fig. 2(b)) appeared healthy.

Fig. 2 Typical appearance of aged SAMP6 mice fed control (a) or heat-killed Lactococcus lactis subsp. cremoris H61 (b) diets.
Effect on growth of enteric bacteria
The effect of oral administration of heat-killed cells of strain H61 on the intestinal microflora of the mice was evaluated (Table 3). Administration of strain H61 was not associated with any significant changes in counts for Bacteroides, Bifidobacterium or Enterococcus sp. The number of viable cells of Lactobacillus sp. was significantly lower (P < 0·05) in mice fed strain H61 than the control mice. In addition, viable counts of Staphylococcus sp. were significantly decreased (P < 0·05) in the H61 group than the control mice at 14 months (Fig. 3).
Table 3 Effect of heat-killed Lactococcus lactis subsp. cremoris H61 (strain H61) on viable count of intestinal bacteria of aged SAMP6 mice
(Mean values with their standard errors for three independent trials each with three mice)

ab Mean values with unlike superscript letters within a row were significantly different (P < 0·05).
* P < 0·05, significance of effect.

Fig. 3 Viable count of Staphylococcus sp. in faeces of aged SAMP6 mice fed heat-killed Lactococcus lactis subsp. cremoris H61 (○) or control diets (●). Values are means with their standard errors depicted by vertical bars (three independent trials each with three mice). a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05). cfu, colony-forming units.
TBARS in serum
Lipid peroxidation status in vivo was assessed using the concentration of TBARS. The serum concentration of TBARS was initially similar in the control and H61 groups, and did not change significantly after administration of the diets for 5 months (data not shown).
Cytokine production by spleen cells
We measured the levels of the cytokines IL-4, IL-6, IL-12 and IFN-γ produced by spleen cells from mice in the H61 and control groups. Spleen cells from mice fed strain H61 produced more IL-12 (P = 0·0301) and IFN-γ (P = 0·0839) than the control group (Table 4). There were no significant differences between the groups in IL-4 or IL-6 production. For spleen cells stimulated with Concanavalin A, there were no significant differences between the groups in production of IL-4, IL-6 and IFN-γ.
Table 4 Effect of oral administration of heat-killed Lactococcus lactis subsp. cremoris H61 (strain H61) on cytokine production (pg/ml) in spleen cells or Concanavalin A (ConA)-stimulated spleen cells from aged SAMP6 mice
(Mean values with their standard errors for two independent trials each with three mice)

IFN-γ, interferon-γ.
* Mean values were significantly different from those of the control.
Bone density analysis
Bone density, as determined from the right femurs of the 14-month-old mice, was found to be significantly higher (P < 0·01) in the H61 group than in the control group (Fig. 4). Osteoclasts were counted in the bone perimeter of cancellous bone tissue in two samples randomly selected from each group. The average of bone densities in two samples was 42·6 mg/cm2 for the H61 group and 38·0 mg/cm2 for the control group. The osteoclast count in the samples from the H61 group was 34·4 (se16·4)/100 mm, and 59·0 (se16·4)/100 mm in the samples from the control group. There were no significant differences between the groups.

Fig. 4 Effect of oral administration of heat-killed Lactococcus lactis subsp. cremoris H61 (H61) on bone density of aged SAMP6 mice. Values are means with their standard errors depicted by vertical bars (for five to seven mice). Mean values were significantly different from those of the control group: **P < 0·01.
Effects of heat-killed cells of strain H61 on physiological changes in young SAMP6 mice
Young SAMP6 mice (1 month old) were fed either a diet containing heat-killed strain H61 (H61 group) or the control diet (control group). The mean daily dietary intakes ranged from 4·69 to 6·60 g/mouse, and there was no significant difference between the mean intakes of the H61 and control groups. The body weights of the mice in both groups increased with ageing, but those in the H61 group were heavier (P = 0·0774) than those in the control group (Fig. 5). At the end-point of the experiment, the bone density of the right femur was similar in the two groups (Fig. 6).

Fig. 5 Body weights of young SAMP6 mice fed heat-killed cells of Lactococcus lactis subsp. cremoris H61 (○) or control diets (●). Values are means with their standard errors depicted by vertical bars (for nine mice).

Fig. 6 Effect of oral administration of heat-killed Lactococcus lactis subsp. cremoris H61 (H61) on bone density of young SAMP6 mice. Values are means with their standard errors depicted by vertical bars (for nine mice).
Effects of living cells of strain H61 and fermented milk H61 on physiological changes in aged SAMP6 mice
Aged SAMP6 mice (8 months old) were fed for 4 months with either non-fat milk (control group), living cells of strain H61 suspended in non-fat milk (LH61 group) or fermented milk made with strain H61 (FH61 group). The mean body weight of mice in the control group decreased with ageing, but the mean body weights were maintained in the LH61 and FH61 groups (Fig. 7). Grading scores for glossiness, incidence of skin ulcers, hair loss and periophthalmic lesions tended to be lower in the LH61 and FH61 groups than in the control group (Table 5). Coarseness in the skin and hair category, and corneal opacity in the eye category, were not observed in these three groups. There were no significant differences between the groups in scores for reactivity and passivity in the behaviour category. The sum of grading scores was 0·80 for the LH61 group, 1·20 for the FH61 group and 1·88 for the control group. There were no significant differences in bone density between the three groups (Fig. 8).

Fig. 7 Body weights of aged SAMP6 fed living cells of Lactococcus lactis subsp. cremoris H61 (○) or fermented milk containing strain H61 (●) or control diet (△). Values are means with their standard errors depicted by vertical bars (for four or five mice).
Table 5 Effect of oral administration of living cells of Lactococcus lactis subsp. cremoris H61 (LH61), fermented milk containing strain H61 (FH61) or control diet on grading score in aged SAMP6 mice
(Mean values with their standard errors for four or five mice)


Fig. 8 Effect of oral administration of living cells of Lactococcus lactis subsp. cremoris H61 (LH61), fermented milk containing strain H61 (FH61) or control diet on bone density of aged SAMP6 mice. Values are means with their standard errors depicted by vertical bars (for four or five mice).
Discussion
In the present study we investigated the effects of oral administration of a lactococcal strain with known immunomodulatory activity with regard to physiological changes associated with ageing in SAMP6 mice. Administration of strain H61 as heat-killed cells, living cells or a fermented milk product was found to have anti-ageing effects as shown by reductions in grading scores for appearance items such as hair loss and incidence of skin ulcers. There was no significant difference in the grading score between living cells and fermented milk, indicating that fermented products or components in milk were unlikely to be involved in the anti-ageing effect.
Notably, bone density in aged SAMP6 was significantly higher (P < 0·01) in the H61 group (heat-killed cells fed) than in the control group. In other words, the loss of bone density associated with ageing in the control group was suppressed in the H61 group. This is the first reported study to show that lactic acid bacteria can reduce the loss of bone density associated with ageing.
Suppression of bone density loss was not observed in the aged LH61 and FH61 groups, perhaps because of their lower intakes of strain H61 during the experimental period. In these groups, each mouse received 2 × 107 colony-forming units (about 25 μg) of living cells in non-fat milk (LH61 group) or fermented milk (FH61 group) every 2–3 d for 4 months. In contrast, each mouse in the H61 group ate an average of approximately 2·2 mg heat-killed cells every day for 5 months. This suggests that the dose dependency of the strain H61 should be investigated further. Another consideration is that the non-fat milk used as a control and for delivery of the living bacterial cells may have affected the bone density of the miceReference Huth, DiRienzo and Miller33. The non-fat milk may have compensated for the bone density loss with ageing and interfered with the effect of strain H61.
In the young SAMP6 mice, there was no significant difference in bone density between the H61 and control groups after receiving their respective diets for 2 months. At this age (1–3 months), bone formation exceeds bone resorption. In contrast, as senescence proceeds in the aged mice, bone resorption exceeds bone formation and bone density decreases. We found that in the aged H61 group, the suppression of bone density loss was associated with a lower osteoclast count. Given the important role of the osteoclast in bone resorption, these findings suggest that administration of strain H61 affected bone resorption rather than bone formation.
Because bone density generally increases with increasing body weightReference Wildner, Peters, Raghuvanshi, Hohnloser and Siebert34, the effects of administration of strain H61 on the weight of the mice need to be considered. Mice in the H61 group were heavier than those in the control group, which might account for their higher bone density. However, as shown in Figs. 5 and 7, there was no significant difference in bone density, even though the body weight in the H61 group was heavier than that in the control group. Thus, mice that were fed lactic acid bacteria and had higher body weight did not always have higher bone density than control mice that had lower body weight. However, as mentioned earlier, balance for bone formation and resorption differs between young and aged animals, and the results obtained with the young mice in the present study might not apply to those with the aged mice. Further study is needed to determine whether the increase in body weight is the only factor in the suppression of the bone density loss that occurred with administration of strain H61.
Oral administration of strain H61 may also have anti-ageing effects via an influence on immune responses. Cytokines are produced by immunocompetent cells, such as the antigen-presenting cells, Type 1 helper T (Th1) cells and Type 2 helper T (Th2) cellsReference Lappin and Campbell35. It is widely accepted that the balance between Th1 and Th2 is critical for various diseases in terms of immunological status. In addition, the Th1-associated cytokine, IFN-γ, has been reported to decrease with ageingReference Mascarucci, Taub, Saccani, Paloma, Dawson, Roth, Ingram and Lane36. In the present study, IL-4, IL-6, IL-12 and IFN-γ produced by spleen cells from aged SAMP6 mice were measured by ELISA. In constitutive cytokine production, spleen cells from mice in the H61 group were found to produce more IL-12 and IFN-γ than those in the control group. IL-12 and IFN-γ are Th1-associated cytokinesReference Trinchieri37, so oral administration of heat-killed strain H61 may enhance the Th1-type immune response of aged mice. Administration of strain H61 may therefore have the potential to prevent diseases associated with ageing by improving the balance between Th1 and Th2. As for Th1-type cytokines, they often cause autoimmune diseaseReference Skurkovich and Skurkovich38. In the present investigation, SAMP6 mice fed strain H61 did not show any external manifestations of autoimmune disease. IL-6 may promote osteoporosis by stimulating bone resorption by osteoclastsReference Inanir, Ozoran, Tutkak and Mercerci39, Reference Blair, Robinson and Zaidi40. In the present study, no difference in IL-6 production was observed in spleen cells. The immunological analysis in the present paper was preliminary. Further deep study such as cellular subset analysis should be carried out to examine the immune response, including autoimmune disease, in SAMP6 mice.
It has been reported that intestinal microbiota influence ageingReference Mitsuoka41, and that heat-killed lactic acid bacteria inhibit adhesion of enteroinvasive pathogens to human intestinal cellsReference Coconnier, Bernet, Kerneis, Chauviere, Fourniat and Servin42. In the present study, we investigated the effect of administration of heat-killed strain H61 on the intestinal microflora of aged SAMP6 mice. We found that administration of heat-killed strain H61 did not markedly alter the viable count of intestinal bacteria, besides Staphylococcus sp. Administration of living cells or fermented milk was not associated with any changes in the counts of viable intestinal bacteria (data not shown), even though the reduction of grading scores indicated anti-ageing effects. The present results show that the anti-ageing effects observed in the present study were not mediated by changes in intestinal microbiota.
Lipid peroxide generated by active oxygen in vivo has a role in promotion of disease and cell ageingReference Beckman and Ames43. In the present study we used TBARS in serum as an indicator of lipid peroxidation statusReference Wozniak, Wozniak, Drewa and Drewa30. Serum TBARS concentrations were not significantly different in the aged H61 and control groups, suggesting that differences in oxidation in vivo did not mediate the differences in bone density or grading scores. However, the TBARS index partly represents oxidative damage, so that the limitations of this method should be considered. We have also measured the levels of glutathione (an antioxidant), and superoxide dismutase-like substance in the serum of SAMP6 mice fed a control diet, live cells of strain H61 or fermented milk. We found that the levels of each these substances were similar across the three groups. However, these antioxitant substances were not measured in SAMP6 mice fed heat-killed cells of strain H61. The relationship between the anti-ageing effect of oral administration of strain H61 and the peroxidation status of SAMP6 mice will be the subject of a further study.
In conclusion, the present study has highlighted the beneficial anti-ageing properties of Lc. lactis subsp. cremoris H61. These manifested as prevention of bone density loss and reductions in the incidence of skin ulcers and hair loss. The present findings indicate the potential for the strain H61 to be used by the food industry as a probiotic strain. In the selection of new probiotic organisms, safety is of prime importanceReference Salminen, von Wright and Morelli44. Strain H61 has already been shown to be empirically safe by its use in manufactured dairy products. We expect that intake of lactic acid bacteria as a component of food would meet with less resistance from the consumer than administration as medicine. We showed in the present study that the effect of strain H61 was independent of the viability of the cells, which would enhance its usefulness as a food additive. Given the difficulty that many elderly people have with swallowing solid foods, heat-killed (and lyophilized) cells of strain H61 could be conveniently added to beverages, for example. Strain H61 therefore shows promise for the development of new functional foods with anti-ageing effects.















