Anaemia is a significant public health problem in Vietnam. The 2000 National Nutrition Risk Factor Survey in Vietnam showed an anaemia prevalence of 34 % in children under 5 and 25 % in women (Ninh et al. Reference Ninh, Khan and Khoi2001). No nationally representative data are available on the prevalence of anaemia among primary schoolchildren in Vietnam; however, a few local studies showed an anaemia prevalence of around 30 % (Le, Reference Le1999; Hoa, Reference Hoa2002). Although various nutrients and cofactors are involved in maintaining the normal synthesis of Hb, iron deficiency is the most frequent cause of anaemia on a worldwide basis (Osório, Reference Osório2002). However, infection and inflammation (Yip & Dallman, Reference Yip and Dallman1988; Means, Reference Means2000), malaria (Fleming, Reference Fleming1981), intestinal parasite infection (Stoltzfus et al. Reference Stoltzfus, Chwaya, Tielsch, Schulze, Albonco and Savioli1997, Reference Stoltzfus, Chwaya, Montresor, Albonico, Savioli and Tielsch2000), as well as haemoglobinopathies (Dugdale, Reference Dugdale2001; INACG, 2002) may play a role. Their relative importance in Vietnam is still unclear. A previous cross-sectional study conducted in Tam Nong district, Phu Tho province, a poor rural area of North Vietnam, showed a high prevalence of anaemia among primary schoolchildren with low iron deficiency as measured by serum transferrin receptor (TfR) and serum ferritin (SF) with 2 % of children with TfR>8·5 mg/l and 0·5 % of children with SF < 12 μg/l (Le et al. Reference Le, Brouwer, Verhoef, Khan and Kok2005). Further, Trichuris infection was associated with a doubled risk of anaemia, probably not mediated through iron deficiency. Food fortification is often suggested as one of the most effective and sustainable strategies for increasing iron intake in the general population (Hurrell, Reference Hurrell1997). Also in the nutritional strategies for prevention and control of micronutrient deficiencies in Vietnam, food fortification is considered as a sustainable solution to combat iron deficiency anaemia (Ninh et al. Reference Ninh, Khan and Khoi2001). However, based on the previous study, it is hypothesized that de-worming is more effective than iron fortification in an anaemic, infection-prone population that was not considered iron deficient. Using a trial with iron-fortified noodles and de-worming, we assessed the changes in iron and anaemia status among anaemic schoolchildren, and tested whether the present data are consistent with this hypothesis.
Subjects and methods
Study design and population
The study was implemented from November 2004 to May 2005 in six primary schools in Tam Nong district, Phu Tho province, situated 90 km from Hanoi. Selection was based on high prevalence of anaemia and absence of interventions to control iron-deficiency anaemia in schoolchildren. Children recruited into the study were in Grades 1–3 with Hb concentrations < 110 g/l but not < 70 g/l in an initial Hb screening study. We excluded children with Hb level less than 70 g/l because these children were considered to be severely anaemic and received treatment immediately. The study concerns a randomized, placebo-controlled double-blind parallel trial with a 2 × 2 factorial design plus standard treatment (iron supplementation and de-worming) and an intervention period of 6 months. A total of 425 eligible children were randomly assigned to one of five groups receiving: (1) iron-fortified noodles and mebendazole (Fe+MEB); (2) noodles without iron fortificant and mebendazole (MEB); (3) iron-fortified noodles and placebo (Fe); (4) noodles without iron fortificant and placebo (placebo); and (5) iron supplementation and mebendazole (Fe tablet+MEB) (Fig. 1). Randomization was carried out by a researcher from the Division of Human Nutrition, Wageningen University, The Netherlands, who did not know the children and could not introduce bias in the randomization. Stratified randomization was applied based on the Hb levels and age of the children to ensure equal distribution of Hb and age across groups. Sample size was assessed to achieve a statistical power of 95 %, based on an α error of 0·05, a between-group difference in treatment effect of 5 g Hb/l in Hb concentration being clinically relevant, a standard deviation of 11 g/l (Hoa, Reference Hoa2002) and accounting for 10 % of children being lost in the course of the intervention. In this paper we focus on the placebo-controlled parallel trial with a 2 × 2 factorial design to assess the effect of iron-fortified noodle and de-worming on iron and anaemia status of schoolchildren. The extent of the effect of the iron-fortified noodles compared to the standard treatment and its policy relevance is discussed in another paper (Le, Reference Le2006). Children were invited for the study and a written informed consent was obtained from their parents. The study was approved by the Scientific Committee of the National Institute of Nutrition and the Ethics Committee of Hanoi Medical University – Ministry of Health.

Fig. 1 Study profile: initial screening to enrol anaemic children in the study, followed by a 6-month intervention of: Fe, iron-fortified noodles and placebo; Fe+MEB, iron-fortified noodles and mebendazole; MEB, noodles without iron fortificant and mebendazole; Placebo, noodles without iron fortificant and placebo; Fe tablet + MEB, iron supplementation and mebendazole.
Products and field procedures
Fortified instant noodles were produced at the Hanoi Food Company. Noodles were fortified with a water-soluble, highly bioavailable iron compound (NaFeEDTA: Ferrazone®; Akzo Nobel Chemicals Pte Ltd, Arnhem, The Netherlands) to a fortified level of 10·7 mg iron/52 g noodles calculated based on the JECFA recommendation of an acceptable daily intake of 2·5 mg EDTA/kg body weight (JECFA, 1974) and an average body weight of 29 kg (Food & Agriculture Organization & World Health Organization, 1998). Before intervention retention of iron after production and preparation of fortified instant noodles was checked in laboratories at the National Institute of Nutrition Hanoi, Wageningen University and Akzo Nobel Chemicals Pte Ltd. Capillary zone electrophoresis analysis (Sheppard & Henion, Reference Sheppard and Henion1997) showed that 70 % of the NaFeEDTA dissolves within 5 min into the soup independent of extraction time. No degradation products of NaFeEDTA were found.
Noodles were prepared in school by caretakers trained by field staff. The whole content of a package of instant noodles (52 g) was broken into smaller pieces and put into a plastic heat-resistant bowl together with seasoning. Hot boiled water from a thermos flask (200 ml) was added to the noodles and given to children at break time (09.00 hours) 5 d/week for 6 months under the supervision of teachers and field staff. Children were encouraged to consume all the noodles and water. During the intervention, 100 % of the study population consumed the given noodles or iron tablet. More than 95 % of children were present every school day (5 d/week) and more than 95 % finished the whole portion of the noodles given to them daily under the supervision of caretakers and field staff (based on notes taken by caretakers in a monitoring book).
MEB (500 mg) and an identical placebo were given to children at the beginning of the intervention and after 3 months. Children, teachers and researchers were blinded to the treatment.
Data collection
Capillary blood samples were taken from children's fingers during screening for Hb measurement by a cyanmethaemoglobin method. Children's weight was measured to the nearest 100 g using an electronic scale (Seca 890; Seca, Birmingham, UK) and standing height was measured to the nearest 1 mm with a microtoise (Stanley Mabo no. 191; Stanley Mabo, Poissy, France) during baseline and after intervention. Venous blood (5 ml) was collected in the morning at baseline (T0) and after intervention (T6); 20 μl whole blood was pipetted immediately before coagulating into a tube containing 5·0 ml Drabkin's reagent with a Sali pipette for Hb measurement. An aliquot of whole blood was taken for analysing haemoglobinopathies. The remaining blood was allowed to clot for 30 min at room temperature, centrifuged at 3000 g for 15 min and transferred to five plastic labelled vials (0·5 ml Eppendorf tubes). The vials were stored in a box protected from sunlight and put into an ice box for transfer to the laboratories and kept at − 80°C until SF, TfR, C-reactive protein (CRP) and IgE analysis at the end of the intervention.
For assessment of intestinal parasite infection before and after intervention, containers for collection of stools were distributed to each class and children were asked to collect and deliver a sample of their faeces to school the next day. In cases where children were unable to return a sample, one of the field workers returned the next day to collect the rest of the samples.
Laboratory analysis
Hb concentration was measured in whole blood within 12 h of sampling by a cyanmethaemoglobin method using a Sigma Kit (Sigma Chemical Co., St Louis, MO, USA) in the Tamnong District Health Centre. The CV of intra-assays and inter-assays was 4·0 ± 1·2 and 5·0 ± 2·0 %, respectively. SF, TfR, CRP and IgE analysis was carried out at the same time for both samples of baseline and after intervention at the National Institute of Nutrition and the laboratory of Hanoi Medical University in May and June 2005. Concentrations of SF and TfR were analysed by an ELISA method (catalogue numbers S-22 and TF-94; Ramco Laboratories Inc., Houston, TX, USA), with inter-assay variability of 4–7 % and 4–8 %, respectively. Serum CRP was measured by nephelometry using Epress plus, with an inter-assay variability of 4–8 %. Serum IgE was determined by ELISA using the Kallestad Total IgE Microplate Kit from Kallestad GmbH (Mannheim, Germany), with an inter-assay variability of 4–6 %. A 10 % subsample was re-examined for quality control. Haemoglobinopathies analysis was performed by using the Variant Beta-Thalassemia Short Program (Bio-Rad Laboratories Inc., Hercules, CA, USA) within 24 h of sampling in the Children's Hospital, Hanoi, Vietnam. Stools samples were examined using the Kato-Katz Technique – a cellophane faecal thick smear method (World Health Organization, 1991). Hookworm, Trichuris and Ascaris eggs were counted. A 10 % subsample of smears was re-examined for quality control.
Data analysis
Anthropometric indices were calculated using WHO/NCHS reference data (World Health Organization, 1995). Being wasted, stunted and underweight was defined by z-scores < − 2sd for weight-for-height, height-for-age and weight-for-age, respectively. Anaemia was defined as Hb concentrations < 115 g/l (World Health Organization, 2000). Iron deficiency was defined as SF concentrations < 12 μg/l (World Health Organization, 2000), and tissue iron deficiency was defined as TfR concentration >8·5 mg/l (Skikne et al. Reference Skikne, Flowers and Cook1990). Body iron content was calculated using the following formula: body iron (mg/kg) = − (log(TfR/SF ratio) − 2·8229)/0·1207 (Cook et al. Reference Cook, Flowers and Skikne2003).
CRP and IgE concentrations were considered to be elevated when ≥ 8 mg/l (Hoffbrand & Pettit, Reference Hoffbrand and Pettit1993) and >90 IU/ml (Heil et al. Reference Heil, Koberstein and Zawta2000), respectively. Hb type was determined in each subject on the basis of haematological indexes: HbAA (normal haemoglobin type), HbF (haemoglobin F); HbA2 (haemoglobin A2); HbAE (trait for haemoglobin E disease) or HbEE (haemoglobin E disease). Severity of intestinal worm infections was expressed as the number of eggs/g faeces using the WHO classification system (World Health Organization, 1987).
Data were entered into the computer, cleaned and managed using Epi Info version 6 (Dean et al. Reference Dean, Dean and Coulombier1995) and analysed using SPSS version 11.0 for Windows (SPSS Inc., Chicago, IL, USA; Field, Reference Field2005). Data were checked for normality by visual inspection. One-way ANOVA was used to determine differences in Hb concentration and other biochemical indicators between groups. Paired t test was used to compare Hb and other biomarkers before and after intervention. χ2 test and McNemar test were used to assess the differences in proportions between and within groups. To assess the effect of iron fortification and de-worming on indicators of iron status, we calculated dummy variables for each treatment (iron fortification and de-worming), as well as their interaction. The effect on change in Hb concentration, SF, TfR and body iron, respectively, was then assessed by including them in a general linear model, so as to simulate a two-way ANOVA. In all regression analyses, we adjusted for concentration at baseline, sex and age, and sometimes CRP or thalassaemia. Logistic regression was used similarly to study the effect of iron fortification and de-worming on anaemia prevalence, adjusting for baseline characteristics.
Results
At baseline, the mean age of children was 87·4 (sd 10·2) months. The four groups did not significantly differ in age, Hb concentration, iron status (SF, TfR and body iron) and nutritional status (Table 1) nor in CRP, IgE status and parasite infection (Table 2). The prevalence of iron deficiency was very low as only 1·2 % of children showed SF concentration below 12 μg/l and 5·5 % of children showed TfR above 8·5 mg/l. Mean body iron was around 6·3 mg/kg body weight (Table 1). Two-thirds of the children were infected with Ascaris and/or Trichuris, and about 8·6 % were infected with hookworm. However, the intensity of Ascaris and Trichuris infection was mainly ‘light’ or ‘average’, and only 27 % and 2 % among infected children showed severe infection with Ascaris or Trichuris, respectively. The hookworm infection was ‘light’ for most of the cases (data not show). Very few children showed elevated CRP levels (1·8 %) and 99 % of the children showed an elevated IgE level (Table 2). The prevalence of thalassaemia (HbA2, HbAE, HbF) was around 7 % (Table 1).
Table 1 Baseline characteristics by treatment group in rural Vietnamese schoolchildren (n 409)§

WAZ, weight-for-age z-score; HAZ, height-for-age z-score; WHZ, weight-for-height z-score.
§ Values are means and standard deviations unless otherwise indicated.
‖ Fe, iron-fortified noodles and placebo; Fe+MEB, iron-fortified noodles and mebendazole; MEB, noodles without iron fortificant and mebendazole; Placebo, noodles without iron fortificant and placebo; Fe tablet+MEB, iron supplementation and mebendazole.
¶ Values are medians with 25th and 75th percentiles.
Table 2 Parasite infection, inflammation and nutritional status before and after intervention in rural Vietnamese schoolchildren

CRP, C-reactive protein.
Values were significantly different between groups (one-way ANOVA): ***P < 0·001.
Values were significantly different within group before and after intervention (McNemar): †P < 0·05; ††P < 0·01; †††P < 0·001.
§ Fe, iron-fortified noodles and placebo; Fe+MEB, iron-fortified noodles and mebendazole; MEB, noodles without iron fortificant and mebendazole; Placebo, noodles without iron fortificant and placebo; Fe tablet+MEB, iron supplementation and mebendazole.
Hb concentration increased after 6 months of intervention in all four groups; however, a larger significant increase was seen in the two groups receiving iron-fortified noodles (17·8 and 17·5 g/l, respectively) compared to 14·6 g/l in the group receiving de-worming only and 15·4 g/l in the placebo group (Table 3). Prevalence of anaemia significantly decreased in all four groups with a larger reduction observed in the two iron-fortified groups; however, no significant differences were found between groups (Table 3).
Table 3 Change in Hb, anaemia and iron status during intervention in rural Vietnamese schoolchildren

TfR, serum transferrin receptor.
Values were significantly different between groups (one-way ANOVA): *P = 0·037; **P < 0·01; ***P < 0·001.
Values were significantly different within group before and after intervention (McNemar): †††P < 0·001.
Values were significantly different within group before and after intervention (t test): ‡P = 0·02; ‡‡P = 0·01; ‡‡‡P < 0·001.
§ Fe, iron-fortified noodles and placebo; Fe+MEB, iron-fortified noodles and mebendazole; MEB, noodles without iron fortificant and mebendazole; Placebo, noodles without iron fortificant and placebo; Fe tablet+MEB, iron supplementation and mebendazole.
SF concentration increased significantly in the two groups receiving iron fortification (15·0 and 17·9 μg/l, respectively). This was not the case in the other two groups: the group receiving only de-worming even showed a decrease in SF concentration compared with data at baseline ( − 7·9 μg/l; Table 3).
TfR concentration was slightly improved after 6 months of intervention in all groups (Table 3).
Body iron significantly increased in the two groups receiving iron fortification (1·0 and 1·4 mg/kg) with smaller changes in the de-worming only ( − 0·1 mg/kg) and the placebo group (0·4 mg/kg).
Prevalence of Ascaris and Trichuris infection dropped significantly in two groups receiving MEB (Table 2). Ascaris infection slightly increased in the placebo group, however, the prevalence of Trichuris infection also decreased in the group receiving iron-fortified noodles without de-worming. Prevalence of hookworm infection decreased in all four groups; however, the prevalence of hookworm infection was very low at the baseline. The proportion of children with elevated CRP levels decreased in all three groups except the placebo group, where the number of children with elevated CRP level even increased. Prevalence of elevated IgE significantly decreased in all four groups, with no differences between groups. In the ANOVA, iron-fortified noodles showed a larger change in Hb level (2·6 g/l, P = 0·003) than the non-fortified group after taking into account Hb at baseline, thalassaemia, age and sex (Table 4). Increase of Hb level was greater in younger children compared to older children (β − 1·22; 95 % CI − 2·2, − 0·2) and sex showed no effect on the improvement of Hb level (β − 0·49; 95 % CI − 2·27, 1·28). There was no interaction effect of iron fortification and de-worming on the improvement of Hb level (P = 0·93). Children with haemoglobinopathies showed a reduced improvement of Hb of 4·2 g/l.
Table 4 Differential change in Hb, serum ferritin (SF), serum transferrin receptor (TfR) and body iron during the intervention period by treatment, compared to no treatment, from four different models (n 320)§
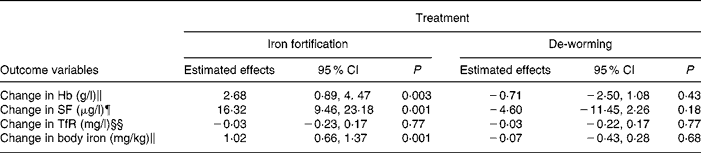
§ Six children with CRP ≥ 8 mg/l were excluded.
‖ Adjusted for Hb baseline, thalassaemia, sex and age.
¶ Adjusted for SF baseline, C-reactive protein (CRP), sex and age.
§§ Adjusted for TfR baseline, sex and age.
‖ Adjusted for body iron baseline, CRP, sex and age.
In the two-way ANOVA, iron fortification showed a significant increase of SF (16·3 μg/l) while de-worming slightly decreased SF levels ( − 4·6 μg/l) after excluding children with CRP ≥ 8 mg/l and adjusting for serum SF at baseline, age and sex (Table 4). Body iron increased 1 mg/kg after 6 months of intervention in children receiving iron-fortified noodles (Table 4). Both iron fortification and de-worming showed no effect on TfR.
After adjustment for baseline Hb concentration, thalassaemia, age and sex in the logistic regression analysis, the prevalence of anaemia at the end was considerably smaller after iron fortification (OR 0·37; 95 % CI 0·17, 0·80; P = 0·01), while de-worming showed no effect (OR 0·98; 95 % CI 0·46, 2·05; P = 0·96).
Discussion
Results of the present study did not support the hypothesis that de-worming is more effective than iron fortification in an anaemic, infection-prone population that was not considered iron deficient. Remarkably, Hb concentrations increased and anaemia reduced in all groups, including the placebo group, during the 6 months intervention period.
Data from the present study reconfirmed the previous finding of existence of anaemia with low iron deficiency in infection-prone schoolchildren in rural Vietnam as indicated by SF and TfR. However, a positive response of Hb levels to iron treatment in anaemic subjects is considered to be a clear indicator of pre-existing iron deficiency (Malope et al. Reference Malope, MacPhail, Alberts and Hiss2001). Also in the present study population, children receiving iron-fortified noodles showed a significant improvement in Hb, which indicates iron deficiency. SF is an acute-phase protein that may be elevated due to infection or inflammation masking iron deficiency during infection. Malope et al. (Reference Malope, MacPhail, Alberts and Hiss2001) supposed that SF values between 12 and 100 μg/l can still indicate iron deficiency especially in the presence of infection and inflammation. However, considering the low prevalence of elevated CRP in the present study population, it seems unlikely that acute infection is affecting the SF levels. TfR has an advantage over SF as it is unaffected by the acute-phase response (Ritchie et al. Reference Ritchie, McNeil and Brewster2004) and is therefore considered to be a sensitive indicator of iron status in children (Asobayire et al. Reference Asobayire, Adou, Davidsson, Cook and Hurrell2001). However, the diagnostic cut-offs for TfR used to identify iron deficiency were derived from studies in adults (Anonymous, 2000). These cut-offs may not apply to children due to their increased erythropoeisis during growth (Kling et al. Reference Kling, Roberts and Widness1998). Based on a study conducted in Morocco and Côte d'Ivoire, Zimmerman et al. (Reference Zimmermann, Molinari, Staubli-Asobayire, Hess, Chaouki, Adou and Hurrell2005) suggest a lower cut-off point for white compared to black Africans although the differences between suggested cut-off points are small. In addition, researchers indicate, regardless of diagnostic cut-offs, modest sensitivity and specificity of TfR in identifying iron deficiency in children (Ritchie et al. Reference Ritchie, McNeil and Brewster2004; Zimmermann et al. Reference Zimmermann, Molinari, Staubli-Asobayire, Hess, Chaouki, Adou and Hurrell2005).
Based on the previous study indicating that Trichuris infection was associated with a doubled risk of anaemia, we expected an improved Hb level after de-worming. The results of the present study, however, did not support this hypothesis as de-worming showed no effect on Hb levels and iron status. The Trichuris infection in the present study population was classified as mild. Therefore, the absence of effect is in line with the previous studies showing that blood loss can occur in Trichuris infection, but probably becomes significant only in severe infection (Layrisse et al. Reference Layrisse, Aparcedo, Martinez and Roche1967; Stephenson, Reference Stephenson1987; Stephenson et al. Reference Stephenson, Holland and Cooper2000). MEB treatment was given directly to children at school by researchers and field staff, and the two groups receiving MEB showed a larger reduction in prevalence of all three types of worms. Surprisingly, the effects on Trichuris in both groups receiving iron were remarkable and indicate interplay between certain helminth infections and iron. More data are becoming available suggesting that iron (and other micronutrients) has important effects on the natural resistance to parasites and that iron supplementation may reduce parasite infection. Studying Trichuris suis, Pedersen et al. (Reference Pedersen, Saeed, Friis and Michaelsen2001) found that iron deficiency increased the severity of T. suis in pigs. It has recently been documented that adults given supplemental iron have significantly lower reinfection rates of Trichuris trichiura, Ascaris lumbricoides and Schistosoma mansoni infection (Olsen et al. Reference Olsen, Nawiri and Friis2000). The effect was suggested to be due to reduced risk behaviour, to improved immune function or to unfavourable host gut conditions caused by an increased oxidative stress.
Remarkably, Hb increased and anaemia reduced in all groups, including the placebo group, during the intervention period. Hb levels are mainly a function of red blood cell production and turnover, affected by factors other than iron deficiency in the present study population. A study in schoolchildren in north-east Thailand found a high prevalence of anaemia without iron deficiency, with haemoglobinopathies, suboptimal vitamin A status and age as the major predictors of Hb concentration (Thurlow et al. Reference Thurlow, Winichagoon, Green, Wasantwisut, Pongcharoen, Bailey and Gibson2005). Another study in school-age children in Alaska, with 15 % anaemia and 8 % being iron deficient, showed an association between a bacterial infection (Helicobacter pylori) and anaemia (Baggett et al. Reference Baggett, Parkinson, Muth, Gold and Gessner2006). Data from the present study indicated that the prevalence of thalassaemia was very low and therefore could not explain the high prevalence of anaemia in the study population. Vitamin A status is considered to be a major determinant of anaemia (Gamble et al. Reference Gamble, Palafox, Dancheck, Ricks, Briand and Semba2004), but sub-samples (n 81) analysed for serum retinol levels showed a very low prevalence of suboptimal vitamin A deficiency (8 and 6 % with serum retinol concentration < 0·70 μmol/l at baseline and after intervention, respectively). Other nutrient deficiencies associated with anaemia include deficiencies of vitamins B6, B12, riboflavin and folic acid (Broek & Letsky, Reference Broek N and Letsky2000), although not all of the causal pathways are yet clearly understood. Vitamin B12 and folic acid deficiency are associated with an increased TfR (Gibson, Reference Gibson2005), but we did not observe elevated TfR levels in the present study population. The role of other nutrients could not be verified in the present study. Malaria remains another important cause of anaemia in tropical countries (Phillips & Pasvol, Reference Phillips and Pasvol1992; Cardoso et al. Reference Cardoso, Ferreira, Camargo and Szarfarc1994), however, malaria was not present in the study area.
The increase of Hb levels in all groups, including the placebo group, might be due to an increase in iron intake due to a seasonal change in food habits or an increase in energy intake through the noodles. We do not have reason to believe there is a seasonal increase in iron intake. A food consumption study among a sub-sample of fifty-nine children (data not shown) showed no change in iron intake between October (baseline) and January, but we did not measure iron intake at the end of intervention (May). Although the noodles intervention did increase energy intake in the sub-sample, we do not think this caused the improvement of Hb levels, as the iron supplementation with de-worming group (the ‘standard’ treatment who did not receive noodles) also showed a Hb improvement (data not shown).
In the absence of the aforementioned causes of anaemia and the observed improvements in the placebo group, the present data may suggest the presence of anaemia of inflammation. Firstly, SF in the study population was much higher compared to the 50th percentile SF value (28·7 μg/l) for the age group 6–9 years in NHANES III (Gibson, Reference Gibson2005), being close to the 90th percentile level of 55·9 μg/l. This may indicate that inflammation is of a more chronic nature. Secondly, Malope et al. (Reference Malope, MacPhail, Alberts and Hiss2001) suggest the use of log TfR:SF ratio to differentiate between iron deficiency anaemia (ratio >2·55) and anaemia of inflammation (ratio < 2·55), although the latter ratio was not able to exclude iron deficiency. In the present study the ratio of log TfR:SF was 2·06, indicating the existence of anaemia of inflammation but being unable to exclude iron deficiency. Children from the placebo group not receiving iron showed increased Hb levels, having a log TfR:SF ratio of 2·07, accompanied by absence of increase (in placebo) or even decrease (in de-worming only) in SF. This indicates a shift of iron from storage to Hb and according to Malope et al. (Reference Malope, MacPhail, Alberts and Hiss2001) this suggests that besides iron deficiency another likely cause of anaemia in the children was infection which improved during the supplementation trial. Thirdly, the seasonal burden of infections in developing countries has been recognized for many years by agricultural and health professionals (Tomkins, Reference Tomkins2005). Mild inflammatory conditions such as upper-respiratory infections and otitis media, which remain common in early childhood, may contribute to anaemia (Yip & Dallman, Reference Yip and Dallman1988). In the study population the proportion of children with elevated CRP is very low. However, CRP is a good measure of acute infection or inflammation but less appropriate when conditions are chronic (Looker et al. Reference Looker, Dallman, Carroll, Gunter and Johnson1997). Earlier a relationship between IgE and respiratory disease was found (Hodge et al. Reference Hodge, Mrinaro, Jones, McGhee, Kiyono and Simecka2001). In our previous study, Trichuris infection was associated with IgE levels which could have confounded the association of Trichuris with anaemia and Hb concentration. De-worming apparently did not affect IgE levels and if the assumption holds true that another unknown infection reflected by IgE plays a role in the anaemia in the study population, this would explain the absence of de-worming effect. In the present study, the high level of IgE and the reduction of IgE levels concurrently with reduction of anaemia independent of treatment may suggest that chronic infection may and intestinal parasite infection may not play a role in inflammation anaemia. Finally, the present data indicated that at the baseline 38·7 % children reported fever or respiratory infection in the previous 2 weeks but at the end survey this prevalence reduced to 13·2 % (data not shown). We did not find an association between fever and respiratory infection with anaemia, however, the reduction in fever and respiratory infection may reflect a general reduction of infection status during the intervention period in the study population. This supports our speculation that anaemia is associated with chronic infection in the present study population and that the anaemia reduction observed in the placebo group may be due to a seasonal reduction of chronic infection. However, the role of chronic infections in anaemia needs to be further investigated.
In conclusion, iron fortification slightly improved anaemia and iron status in anaemic schoolchildren in rural Vietnam that were not considered iron deficient. De-worming reduced prevalence of worm infection but had no effect on anaemia and iron status. A positive seasonal effect was seen in all treatment groups. Chronic infection or other unidentified factors including a limited iron uptake for haem synthesis may play an important role.
Acknowledgements
This study was funded by Neys-van Hoogstraten Foundation, Ellison Medical Foundation and the Ministry of Education and Training, Vietnam. Akzo Nobel Chemicals, Arnhem, The Netherlands, is acknowledged for supplying NaFeEDTA. Hospital De Gelderse Vallei, Ede, The Netherlands is thanked for preparation of MEB and identical placebo. The authors acknowledge the assistance of the Directors, teachers and children from the six schools, the Director and staff of the Education Department, and the Director and staff of the District Health Center in Tam Nong district Phu Tho province. Prof. Dr Renger Witkamp is acknowledged for his valuable remarks on earlier versions of the paper.







