INTRODUCTION
Leptospirosis is a globally distributed zoonosis caused by bacteria of the genus Leptospira [Reference Ko, Goarant and Picardeau1]. This disease has a large impact on public health, accounting for at least one million annual human cases worldwide [Reference Costa2]. Leptospirosis can be transmitted through direct contact with the urine of infected animals and occasionally through rodent bites [Reference Roczek3], and indirectly by exposure to water or soil contaminated with leptospires excreted in the urine of infected hosts [1, 4, 5]. The burden of disease in developing countries is associated with residents of urban slums, as exemplified by studies conducted in Salvador, Brazil, where outbreaks during the rainy season affect the poorest proportion of the population [Reference Felzemburgh6–Reference Sarkar8].
The Norway rat, Rattus norvegicus, is the primary reservoir for pathogenic leptospires in the slums of Brazil [Reference Barocchi9, Reference De Faria10] and in many urban centres in developing and developed countries [Reference Allan11–Reference Thiermann14]. Despite studies describing the prevalence of Leptospira infection in Norway rats in Salvador, Brazil, [Reference De Faria10, Reference Costa15], little is known about the maintenance and intra-specific transmission of this pathogen within rat populations [Reference Himsworth13].
Although environmental transmission is possibly the major route for Leptospira infection in rodents [Reference Cox and Twigg16], a recent study indicated that young non-reproductive rats, which had just left the nest, showed a prevalence of up to 30% of kidney colonization [Reference Costa15]. Infection at a very young age (>2 months) suggests that transmission may occur also in utero or to neonates, possibly through infectious milk, or by other routes within the nest including close contact with an infected mother via fomites or uro-gentital cleansing of neonates by the dam. While intrauterine transmission of leptospires has been documented for humans [Reference Faine17, Reference Shaked18], and occurs in horses [Reference Poonacha19], there has been only a single case report of breastfeeding-associated transmission in humans [Reference Bolin and Koellner20]. There has also been just a single case report of sexual transmission in humans [Reference Harrison and Fitzgerald21], but is likely in horses, based on findings of leptospiral DNA in semen [Reference Hamond22]. Transplacental transmission of leptospires is well recognized in livestock animals (and is related to abortions) [Reference Poonacha19, Reference Higgins23, Reference Leon-Vizcaino, Hermoso and Garrido24]; however, there is no clear evidence of this route of transmission in rodent populations [Reference Lahiri25–Reference Ward and Turner27].
Herein we report the presence of Leptospira spp. in breast tissue and milk indicating the potential for neonatal transmission in R. norvegicus. Our results will inform predictive models of intra-specific maintenance and transmission of leptospires in rodents [Reference Holt, Davis and Leirs28] to better understand and generate new hypotheses regarding the contribution of varied routes of transmission in reservoir populations.
METHODS
Study site
Norway rats (R. norvegicus) were trapped at the study area of Pau da Lima (0·16 km2) in the coastal city of Salvador, Brazil [Reference Felzemburgh6, Reference Reis29]. High human density (>3700 inhabitants/0·16 km2); low socioeconomic level and lack of basic sanitation and garbage collection are features of this area [Reference Costa30]. Prospective cohort studies performed in this setting since 2003 have identified an incidence of leptospiral infection of 36/1000 person-years [Reference Felzemburgh6, Reference Reis29].
Trapping of rats and sample collection
Norway rats were trapped from peridomestic areas in Pau da Lima. Double-bagged traps containing rats were transported to the Zoonotic Control Centre (ZCC) and animals were euthanized following protocols previously described [Reference Costa15]. Only lactating females were selected for this study. After euthanasia and hair removal in the ventral region, the whole body of lactating rats was cleansed with 70% alcohol. Milk was extracted by finger pressure applied to each breast, generally collected from the most enlarged breasts. Small drops (~10–20 µl) of milk were expressed onto poly-l-lysine-coated glass slides for indirect immunofluorescence assay (IFA) and 1–2 small drops collected by sterile swab swipes and stored at −80 °C for quantitative real-time polymerase chain reaction (qPCR). Breast tissue samples were cut from the muscle base and the exposed surface was pressed on glass slides for IFA testing. Five left breast tissue samples were collected and fixed in 10% neutral buffered formalin for immunohistochemistry (IHC). Breast tissue from four animals was separated for scanning electronic microscopy (SEM) and seven breast tissue samples were collected to attempt isolation of Leptospira. Breast tissue from two healthy and non-infected lactating laboratory rats (R. norvegicus Wistar) was used as negative controls in all experiments.
To document chronic leptospiral carriage kidneys were obtained from all animals and tested as described previously [Reference Costa31]. It is important to note that chronic kidney carriage occurs after leptospiral systemic infection is cleared from multiple tissues and blood indicating that any further identification of leptospires in other tissues is indicative of prolonged infection in additional sites.
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the Oswaldo Cruz Foundation (Salvador, Brazil; 03/2012) and Yale University (New Haven, CT; 2012-11 498).
IFA
Slides with imprints of kidney, breast tissue and milk were analysed by IFA as described previously [Reference Chagas-Junior32]. Briefly, slides were fixed in acetone for 3 min and then washed with phosphate-buffered saline (PBS). Blocking with 1% bovine serum albumin (BSA) for 40 min was followed by 1-h incubation with hyperimmune rabbit antisera to whole L. interrogans serovar Icterohaemorrhagiae strain RGA diluted 1:1000 [Reference Chagas-Junior32, Reference Athanazio33]. After drying, the samples were fixed in acetone for 3 min and re-washed with PBS. Samples from non-infected laboratory rats were similarly treated as negative controls. Additional negative controls were generated from kidney-positive wild rats by incubating slides with normal rabbit serum at the same dilution. After washing with PBS, the imprints were incubated with goat anti-rabbit IgG Alexa 488 (Invitrogen, USA) at a dilution of 1:500 for 1 h. The imprints were washed 3× with PBS, mounted with anti-fading medium (ProLong Molecular Probes, Thermo Fisher Scientific, USA) and examined at ×400 and ×1000 (Olympus BX51 microscope, Olympus America, USA). We estimated the presence of leptospires in milk imprint as the mean number of leptospires per four fields of view at a magnification of ×1000.
IHC
Fragments of the breast tissue used for imprints were fixed in 10% formalin, embedded in paraffin according to routine histological procedures and cut into 4–5 µm sections. Sections were stained with haematoxylin and eosin (H&E) for histopathological analyses. Paraffin was removed with xylene and ethanol. Following the methodology described previously [Reference Chagas-Junior32], slides were treated with 3% hydrogen peroxide for 15 min at room temperature, blocked with 1% BSA and then incubated at 37 °C for 1 h with a 1:2000 dilution of pathogen-specific rabbit antiserum against LipL32 [Reference Murray34]. After three washes with PBS, slides were incubated with biotinylated-conjugated anti-rabbit immunoglobulin and then with horseradish peroxidase-conjugated streptavidin (Invitrogen). The chromogen used (3,3-diaminobenzidine; DAB; Invitrogen kit 00–2114) was visualized as a brown deposit. Finally, the slides were stained with haematoxylin, mounted with balsam and cover-slipped. Positive kidney samples from wild rats were processed identically and used as positive controls. Slides were examined in brightfield microscopy (Olympus BX51, at ×1000).
Culture isolation
Breast tissue samples were macerated and inoculated into 5 ml liquid EMJH medium [Reference Faine, Adler and Perolat35]. The cultures were incubated at 29 °C for 24 h. After this period, 0·5 ml supernatant was subcultured in another tube with 5 ml EMJH medium. The cultures were examined weekly over 3 months by darkfield microscopy. In case of contamination by other microorganisms, filtering methodologies were attempted using 0·22 µl pore syringe filters (Sterile Millipore, Millipore Corp., USA).
SEM
Breast tissue samples were cut into 1–2 mm pieces and fixed in 2·5% glutaraldehyde and 0·1 m sodium cacodylate buffer (pH 7·4). Samples were post-fixed with 1% osmium tetroxide for 1 h, washed with 0·1 m sodium cacodylate buffer, dehydrated in a series of ethanol baths and dried using a critical point drying apparatus (Leica EM CPD030, Leica Microsystems, UK). Fragments were mounted on aluminum stubs, sputter-coated with gold (Desk IV, Denton Vacuum, USA) and examined in a scanning electron microscope (JSM6394LV, JEOL, Japan) operated at 12 kV.
qPCR
Frozen breast tissue from each rat was thawed and DNA was extracted from 25 mg tissue previously homogenized with PBS. Frozen rat milk collected with cotton swabs was thawed and homogenized with 500 µl PBS. Both tissue and liquid were extracted using Maxwell 16 Tissue DNA Purification kit (Promega, USA).
The qPCR was performed using 5’-nuclease (TaqMan, Thermo Fisher) assay and primers that amplified a sequence of the pathogen-specific Leptospira lipL32 gene [Reference Stoddard36], using the Applied Biosystems 7500 Fast Real-Time PCR instrument (Applied Biosystems, USA). For the standard curve, genomic DNA obtained from Leptospira serovar Copenhageni strain Fiocruz L1–130, the strain infecting Norway rats and humans in Salvador, was quantified using an ND-1000 spectrophotometer (Nanodrop Technologies, USA). Eight calibrating dilutions [0–107 genome equivalents per millilitre (GEq/ml)] were prepared and served as reference values. The reaction mix consisted of 12·5 µl PCR SuperMix-UDG (Invitrogen), 500 nm of forward and reverse primers, 100 nm of probe, 5 µl DNA extract and ultrapure water (Invitrogen) to a final volume of 25 µl. The amplification protocol consisted of 2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. Cycle threshold <40 was treated as positive. For quality control, each reaction was run in duplicate and non-template controls were included in every column of 96 reaction plates. The rodent housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (gapdh) was used as an internal control to monitor inhibition of PCR amplification and the efficiency of DNA extraction.
Histopathological analyses of breast tissue samples
For histopathological examination, breast tissue slides were stained with H&E, Picrosirius (PIFG) and analysed by light microscopy. The main excretory duct, intralobular excretory duct, acinar tissue/ductal and intralobular stroma were evaluated. In these structures, morphological lesions were scored based on observations of acute and chronic inflammation, fibrosis, calcification, eosinophilic staining and fat content. The intensity score of the lesion was rated on a scale of 0–3 (0 = normal, 1 = mild, 2 = moderate, 3 = severe).
Statistical analyses
We performed descriptive analysis evaluating the proportion of positive animals for each sample and laboratory technique. Concordance between different techniques was assessed by the kappa index statistic.
RESULTS
Samples
Fifty-five lactating rats were caught during the study period. We obtained all tissue samples (kidney, breast and milk) from 24 lactating rats included in this study. These 24 animals did not differ in mass and age from other lactating rats captured but from which milk samples could not be obtained.
Leptospira in kidney
We defined carrier status by evaluating the presence of leptospires in kidney by IFA (Fig 1), IHC (Fig. 2) or qPCR. All 24 rats were positive by one or more tests (Table 1). All control samples from laboratory Wistar rats and wild rat samples incubated with normal rabbit serum were negative.

Fig. 1. Identification of Leptospira by indirect immunofluorescence in milk and breast slide impression samples obtained from chronically infected (kidney positive) wild-caught Rattus norvegicus. (a) Negative kidney control; (b) positive kidney control; (c) positive breast and (d) positive milk. Magnification, ×1000. Bars, 30 µm.
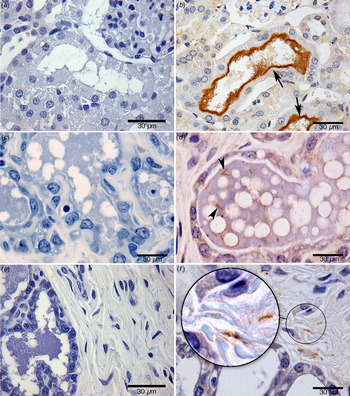
Fig. 2. Identification of Leptospira by immunohistochemistry: (a) negative kidney control; (b) renal tubule positive kidney control (arrow points to a cluster of leptospires); (c) negative mammary gland control; (d) the mammary gland of a wild-caught chronically infected (kidney positive) Norway rat (arrow points to an individual leptospire; several are visible); (e) negative control in connective tissue of a breast sample; (f) leptospires in connective tissue of a breast sample from a chronically infected (kidney positive) wild-caught Norway rat. Zoom highlights the morphological structure of leptospires, sharp helical structure; bar length, 30 µm.
Table 1. Results of laboratory tests for the presence of Leptospira in kidney, breast and milk samples in lactating urban slum rats (Rattus norvegicus)

n.a., Not applicable.
Leptospira in milk
Leptospires, as evaluated by IFA, were observed in 18 (75%) expressed milk samples (10–20 µl milk) from the 24 kidney-positive females (Fig. 1). Leptospires were sparsely distributed and not numerous in milk (Fig. 1d ). The number of leptospires observed in milk ranged from 1 to 31 leptospires (mean 7·5) per four fields of view at a ×1000 magnification. Four samples (17%, 4/24) were positive by qPCR, with a range of 36-1484 GEq/ml. All negative controls were negative. The internal control qPCR reaction using the gapdh gene were all positive, indicating that there was no inhibition or issues with the extraction that could explain the large number of negative samples obtained by qPCR compared to IFA.
Leptospira in breast tissue
Leptospires were sparse but clearly identifiable (in 67% of breast tissue samples by one or more tests (Fig. 1c ). By specific test, leptospires were observed in 12 (50%), 12 (50%) and two (8%) breast tissue samples by IFA, IHC and qPCR, respectively (Table 1, Figs 1c , 2d – f , 3a – d ). The concordance between IFA and IHC findings was fair (kappa = 0·61). The two samples positive by qPCR indicated a bacterial burden ranging from 20 to 1519 GEq/mg, consistent with values obtained from milk. All breast tissue samples tested by qPCR were positive for the gapdh internal control gene. All the negative control breast samples from the two Wistar rats were negative by IFA, IHC and qPCR testing. The positive SEM from one of the four samples examined confirmed the presence of leptospires in breast tissue (Fig. 3). Attempts to isolate leptospires from breast samples resulted in contaminated cultures and contamination was not controlled by filtering and serial passage.

Fig. 3. Scanning electron microscopy reveals clusters of leptospires in breast tissue from a naturally infected wild-caught Rattus norvegicus. (a) Cluster of leptospires, (b) zoom of panel (a) showing visible erythrocytes in breast tissue; (c, d) leptospires densely packed side by side.
Of the 12 positive samples analysed by IHC, sparsely distributed leptospires with few organisms per microscopic field were detected within the mammary gland of 67% (8/12) rats and in 33% (4/12) of samples from the acinar/ductal region and connective tissue. The morphology of leptospires showed well-preserved size and morphological features. Leptospira with a clear helical morphology was more readily distinguished in breast tissue than in kidney tissue. However, visualization of bacteria by SEM showed aggregated leptospires, potentially in the form of biofilm within breast tissue and kidneys.
Histopathological examination of breast tissue samples
Staining by H&E and PIFG identified mild to moderate lesions in breast tissue in 10 (42%) rats (Table 2). Of these 10 animals, nine (90%) had Leptospira in breast tissue or in milk detected by one or more assays. Lesions included: (a) enlarged main excretory duct, (b) enlarged, calcification, and acute or chronic inflammation in the intralobular excretory duct, (c) enlarged and acute inflammation of the acinar/ductal tissue comprising eosinophilic and fat secretion and (d) fibrosis in the intralobular stroma (Table 2). However, there were no statistical differences in the proportion of animals showing breast lesion when compared to Leptospira-positive and -negative females.
Table 2. Histopathological alterations between positive and negative breast samples

Values given are n (%).
DISCUSSION
Lack of knowledge regarding the routes of intraspecific transmission of Leptospira in natural reservoir-host species, such as the Norway rat, are an obstacle to a complete understanding of the epidemiological factors underlying patterns of acquisition, maintenance and shedding of this spirochaete. Herein we describe for the first time the presence of pathogenic leptospires in the milk and breast tissue of lactating wild Norway rats demonstrated to be chronically infected by carriage of leptospires within their kidneys.
Although the outcomes of various assays varied, leptospires were demonstrated to be present by IFA testing of milk and breast tissue impressions, by IHC staining of breast tissue using pathogen-specific LipL32 antisera and by qPCR. Additionally, confirmation of breast colonization was demonstrated by visualization of clusters of leptospires by SEM.
Vertical or neonatal transmission of Leptospira has been suggested by findings of prevalence studies stratified by mass/age that indicate a proportion (up to 30%) of the smallest juvenile rats are already infected by the time they leave their natal nest [Reference Costa15]. As the estimated age of these animals is ~28 days infection must have occurred in the nest at a minimum age of 18 days (A. Minter et al. unpublished data). During the postnatal period of 25–38 days [Reference Calhoun37] transmission from mother to offspring could occur via several routes, including in utero infection, breastfeeding, infectious saliva during grooming and female uro-genital cleansing of pups, urine of the dam or through fomites (e.g. contaminated nest material). The multitude of possible transmission routes limits the possibility to identify any specific route of vertical or neonatal transmission, although in utero transmission has been described [Reference Schnurrenberger, Hanson and Martin26, Reference Ward and Turner27].
The presence of Leptospira in 67% (8/12) of lumen of positive mammary glands sampled by IHC, indicates that pathogenic leptospires could readily be discharged with milk during suckling. The preference of leptospires for lumen compartments is commonly observed in the renal tubules of rat reservoirs [Reference Athanazio33]. Studies focusing on leptospiral colonization in renal tubules propose that preference for the lumen could be explained by the low antibody concentration in this tissue [Reference Athanazio33, Reference Faine, Adler and Perolat35]. We also observed leptospires in the connective tissue of breast samples, and, although not determined, the titre of anti-Leptospira antibodies can be assumed to be low in this immunologically protected tissue. Furthermore, studies have shown that the mother can influence the immune state of pups, as well as the carrier state [Reference Birnbaum, Shenberg and Torten38].
In mammary glands, leptospires were observed predominantly in free form with the length and helical morphology described for this bacterium. Relatively few organisms were present in any given microscopic field in both milk and breast tissue. However, as discussed below the small volumes of milk tested could translate into high levels of leptospire shedding during the course of breastfeeding. In addition, any immunological staining is limited to the restricted viewing of a limited number of sections and the visualization of bacteria by SEM clearly identified clusters of organisms, or potentially biofilm aggregates in breast tissue similar to observations from chronically infected kidneys [Reference Agudelo-Flórez39, Reference Ristow40].
As mentioned above, the frequently reported findings that leptospires are cleared from all rat tissue, other than the kidney by ~10 days, our observations clearly suggest prolonged infection in breast tissue and shedding of organisms in milk. Each of the pregnant rats included in this study were kidney-positive females indicating that a carrier state had been achieved.
The presence of leptospires in wild Norway rat milk, although not previously described, is not biologically unique when considering other mammalian species. Cows infected with serovar Hardjo shed these bacteria in milk and mount an immune response that can result in mastitis [Reference Ellis41]. Potential breastfeeding transmission of leptospirosis has been reported in a human newborn [Reference Bolin and Koellner20], and leptospires have also been isolated from human milk [Reference Chung42]. However, there is no evidence that infection from milk is a common route for transmission.
The number of leptospires in milk samples (range 1–31 per ×100 field) could only be roughly approximated as milk samples were estimated to be less than 20 µl, and concentrations could not be estimated. However, qPCR results indicated similar leptospiral loads of 36–1484 GEq/ml in milk. When milk consumption is taken into account sucking pups could be exposed to high loads of leptospires prior to weaning. An increase in milk uptake occurs during the first 15 days of breastfeeding then declines when young are supplementing their diet with solid foods – preceding weaning at about day 27 [Reference Galef43, Reference Thiels, Cramer and Alberts44]. At postpartum day 15 dams can produce and accumulate up to 14 g (ml) of milk during each 3- to 4-h period prior to feeding pups [Reference Thiels, Cramer and Alberts44]. A single pup in a litter of 10 pups (based on median embryo counts from >100 pregnant rats in Salvador, Brazil (J. A. Panti-May, unpublished data) could thus ingest >8 ml milk per day cumulatively containing hundreds (~300) or many thousands (~12 000) of leptospires based on qPCR results. Furthermore, the cumulative volume of milk ingested over the entire course of breastfeeding would increase the exposure above tenfold.
The oral dose for infecting R. norvegicus is unknown. However, the intraperitoneal ID50 for 4-day-old pups was 102 (the study did not test lower doses) was far lower than the 104 organisms required to infect adults, suggesting pups are more susceptible as mass alone cannot account for such a large difference [Reference Athanazio33, Reference Muslich45]. However, a previous study [Reference Muslich45] indicated feasibility that an inoculum dose of Leptospira in the hundreds or thousands ingested through milk could cause infection.
The pathological consequences of leptospiral infection in breast tissue of Norway rats were minimal, and the pathogenic scores of infected tissue were no different from non-infected tissue. Of note, the presence of leptospires was not accompanied by inflammation and there were no significant pathological changes when breast tissue samples with presence and absence of leptospires were compared. These negative results suggest that colonization does not affect organ function and dilated acinar ducts and accumulation of hyaline material in the acini, compatible with milk, were present in infected animals. It is unlikely that colonized breasts would in any way inhibit the normal release of milk. In contrast, while the acute phase of clinical leptospirosis caused by serovar Hardjo in cows is usually subclinical, lactating cows show a number of pathological changes including agalactia (‘milk drop syndrome’) where small quantities of blood are shed in the milk. Agalactia is associated with a rapid drop in milk production, a soft flabby udder, febrile response and milk with appearance of yellow colostrum, thus affecting the quantity and quality of milk available to calves [Reference Higgins23].
It is no surprise that the results from different diagnostic techniques varied, as none of these have been standardized for breast tissue. The presence of inhibitors or other material in milk could have contributed to low frequency of qPCR positive samples. Additionally, it was only possible to collect low volumes of milk from lactating rats. The IHC and IFA results indicated that leptospires were sparsely distributed in milk and breast tissue samples potentially limiting detection through qPCR sampling of small volumes of milk and breast tissue. However, SEM identified large aggregates of leptospires in breast tissue similar to observations of kidney proximal tubules [Reference Athanazio33]. As a consequence, this aggregation could impact the qPCR or other assays, since it may cause inhibition in the biofilm thus preventing detection.
Laboratory experiments and refinements to assays could shed light on the variation observed and confirm our findings of Leptospira in milk and breast tissue. Irrespective of the limitations mentioned above, our results, when considered in total, confirm the presence of leptospires in the milk and breast tissue of naturally infected Norway rats for the first time. Although leptospiral presence does not translate into demonstrating Leptospira transmission from dams to pups, the possibility of this transmission route deserves attention and additional studies. Characterization of potential transmission pathways is critical to understanding how leptospires are acquired and maintained in the Norway rat and other reservoir species and is essential for informing parameters used in mechanistic models of leptospirosis in rodent hosts [Reference Holt, Davis and Leirs28].
ACKNOWLEDGEMENTS
The authors thank the staff of Zoonosis Control Centre, Salvador for their assistance in conducting the study. We also to thank Mayara Carvalho for her assistance with database processing and Kathryn Hacker for review of the grammar. This work could not have been accomplished without the joint collaborative effort of the resident associations, community leaders and residents, which constitute the Urban Health Council of Pau da Lima. We thank the Global Leptospirosis Environmental Action Network (GLEAN). We also thank the Histotecnologia platforms and electron microscopy of the Gonçalo Moniz Research Center (FIOCRUZ-CPqGM-Bahia). This work was supported by the Oswaldo Cruz Foundation and Secretariat of Health Surveillance, Brazilian Ministry of Health, the National Institutes of Health (grants R01 AI052473, U01 AI088752, R01 TW009504, R25 TW009338 and R01 AI121207) and by the Wellcome Trust (102330/Z/13/Z).
DECLARATION OF INTEREST
None.








