1. Benjamin Franklin and the use of ‘storm oil’
In 1757, Benjamin Franklin was sent by the Pennsylvania Assembly to England, to negotiate on taxation. On the passage with a fleet of sailing vessels, when watching the wind-ruffled and troubled sea around him, he observed that the wakes of some ships were remarkably smooth. He asked the captain about this phenomenon, who apparently considered this to be a very stupid question, as everybody would know that the smoothening is due to oil poured into the water; in this case presumably as the cooks had just emptied their greasy water into the ocean.
Franklin was fascinated by the observation and, after having arrived in England, he was the first to do controlled experiments on it on various English ponds and lakes and to report on it in a scientific publication (Franklin Reference Franklin1774). He wrote: ‘… The oil, though not more than a teaspoonful, produced an instant calm of a space over several yards square, which spread amazingly and extended itself gradually … till is reached half an acre, as smooth as a looking glass’. That paper also included an attempt to explain the phenomenon as being connected to the ‘friction’ between the wind blowing over the water and the water itself, viewing the oil as kind of a thin lubrication layer in between (Tanford Reference Tanford1989; Mertens Reference Mertens2006; Behroozi et al. Reference Behroozi, Cordray, Griffin and Behroozi2007). Though this explanation has major shortcomings, Franklin did realize that it is key for the effect that the oil layer is extremely thin. In the publication he also reported in detail his communication with the sea captain on this matter and that the effect had long been known by seamen in various cultures, who called the oil used in this way ‘storm oil’. The earliest mention of the wave-stilling properties of oil can be found in Pliny's Natural History (Mertens Reference Mertens2006).
Through his exchange with sea captains Franklin also became aware of a letter of the Dutch colonial official Mr. Tengnagel, in which he relates his and the ship's survival in a storm in the Indian Ocean to the captain's action of pouring oil into the sea, ‘to prevent the waves breaking over her, which had an excellent effect’ (Mertens Reference Mertens2006). Based on this, Franklin suggested pouring oil into the rough sea to ease landing on an island, and did corresponding experiments.
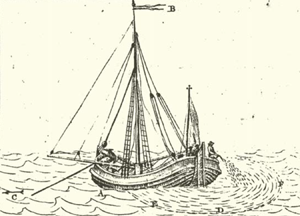
In late 1774, first orally and then by reading Franklin's publication, the Dutch merchant and cloth manufacturer Frans van Lelyveld became aware of Franklin's experiments and his exchange with seamen. He repeated the oil pouring experiments in the Oude Vest canal in Leiden in 1775 and spoke to various Dutch seamen and fishermen, being intrigued by the fact that an effect so well known to them had not been known to the learned. To share his knowledge, and to learn more about the effect, Lelyveld wrote the essay ‘Reports and Prize Questions as to the pouring of oil, whale oil, tar, or other floating substances to reduce shipping dangers’ (Lelyveld Reference Lelyveld1776), see figure 1(a) for an illustration out of that publication. The 17 prize questions were on the history of the practice, on what oil would be best, on how to pour the oil into the sea, on how long the smoothening effect would last, etc. The prize money for the best essay was 30 ducats (Mertens Reference Mertens2006). Lelyveld's publication led to the interesting discussion on whether a scientific approach to the problem would be necessary, or whether the practitioner's knowledge would be sufficient. Joannes le Francq van Berkhey, a lecturer of natural history at Leiden University, considered it to be an offence to the honour of Dutch fishermen and seamen that Lelyveld had asked for more scientific work on the problem and he wanted ‘to uphold the honour of the fishermen and seamen of the Low Countries … who deserve this honour for their ancestral knowledge’ (Mertens Reference Mertens2006).
Figure 1. (a) The effect of storm oil in the North Sea. The drawn experiment was conducted by the Dutch captain Isak Kalisvaar in 1776. He reported about it in a letter to Frans van Lelyveld in 1776. In the illustration, A is KalisvaarÕs ship, B is a flag indicating the wind direction, C shows the current direction, and the arc EDF identifies a circle of whale oil spreading behind the anchored ship. (Adapted from Lelyveld (Reference Lelyveld1776), p. 197, according to Mertens (Reference Mertens2006), from where the figure is taken with permission.) (b,c) Breaker profile images from high-speed movies of the wave crest for the case of clean water (a) and for the case of water with 7.5  $\mathrm {\mu }$mol L
$\mathrm {\mu }$mol L $^{-1}$ Triton-X-100 contamination. In (d) the mechanism of compression of the surfactants at the crest of the waves and dilation elsewhere is illustrated. Panels (b–d) are taken from Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023).
$^{-1}$ Triton-X-100 contamination. In (d) the mechanism of compression of the surfactants at the crest of the waves and dilation elsewhere is illustrated. Panels (b–d) are taken from Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023).
2. Lord Rayleigh, Agnes Pockels and her quantitative measurements of surface films
The next major step in understanding the effect of ‘storm oil’ was done by Strutt (Reference Strutt1890), the later Lord Rayleigh. He dropped 0.81 mg of olive oil on water and found that it spread towards 555 cm $^2$, from which he deduced that the layer thickness was 1.6 nm. This corresponds to a monolayer of oil molecules, though he would not have realized this in those days.
$^2$, from which he deduced that the layer thickness was 1.6 nm. This corresponds to a monolayer of oil molecules, though he would not have realized this in those days.
In parallel, Agnes Pockels performed similar experiments in Brunswick, Lower Saxony, Germany, calculating a thickness of 1.3 nm. As a woman of those days, Agnes Pockels was declined university education, and so she self-educated herself, with the help of textbooks provided to her by her younger brother Friedrich, who was a professor in physics, and with the help of experiments that she performed in her kitchen (Derrick Reference Derrick1982). As she did not succeed in publishing her results in Germany, she wrote to Lord Rayleigh (in German), who made sure that Pockels's results could be published as a Letter in Nature (Pockels Reference Pockels1891). That Letter also contained a description of her surface film balance technique with which she could precisely measure the surface tension and which was very similar to the technique which now is called Langmuir's trough (Derrick Reference Derrick1982).
Agnes Pockels was the first to realize that the damping of the capillary waves by the oil layer could not solely be due to the reduction of the surface tension, but that there must be some extra viscous resistance and dissipation to account for the observations. Her seminal achievements were broadly recognized only much later and in 1932 she received the honorary doctoral degree of the University of Brunswick (Derrick Reference Derrick1982).
3. Carlo Marangoni, surface elasticity and emerging gradients in surfactant concentration
The main origin of the extra dissipation became clear only many decades later, namely the surface-dilational elasticity, also known as Gibbs surface elasticity (Lucassen-Reynders & Lucassen Reference Lucassen-Reynders and Lucassen1970; Manikantan & Squires Reference Manikantan and Squires2020). Waves locally stretch or compress the surface, leading to locally lower or higher surfactant concentration and thus to locally higher or lower surface tension. The emerging surface tension gradients induce tangential shear forces (Marangoni & Stefanelli Reference Marangoni and Stefanelli1872; Scriven & Sternling Reference Scriven and Sternling1960), which are now called Marangoni forces and which lead to extra flow and extra dissipation in the upper water layers, thus to some extra damping effect. As first realized by Carlo Marangoni himself, the consequences of this effect on the flow are much stronger than that of the reduction of the surface tension by the surfactants (Marangoni & Stefanelli Reference Marangoni and Stefanelli1872; Behroozi et al. Reference Behroozi, Cordray, Griffin and Behroozi2007).
If the oil is nearly immiscible with water, the oil molecules act as surfactants and accumulate on the surface. However, surface tension gradients can also emerge due to more miscible molecules, even on large scales. Stefan & Szeri (Reference Stefan and Szeri1999) worked out how rising microbubbles, which are generated by the passage of a ship, scavenge surfactants from below and transport them up to the surface, where they can stay for a very long time. The resulting gradient in surface tension makes the wake of the ship visible for many hours and up to a length scale of 100 km, so that these traces of the ships are even visible from space (Griffin et al. Reference Griffin, Peltzer, Reed and Beck1992).
4. The effect of surfactants on plunging breakers
Though the ocean surface conditions Franklin will have experienced on his journey across the Atlantic from America to England will have been anything else but exclusively smooth, surprisingly, the focus of the studies on the effect of surfactants on waves until recently has been on relatively smooth gravity and capillary waves. After Liu & Duncan (Reference Liu and Duncan2006) had studied the effect of surfactants on gentle spilling breakers, Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) have now studied their effect on plunging breakers – exactly the rough flow situation Franklin will have met on his passage. But Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) did not do so in the ocean, but under very controlled conditions, namely in a laboratory wave tank which allows for the creation of plunging breakers with a dispersively focused wave packet. They performed the experiments with two sets of surfactants: first, with different concentrations of the soluble surfactant Triton X-100 dissolved in clean water; second, less controlled, with originally clean water exposed to the environment for increasing times up to 21 hr, before the plunging breaker experiments were done. Remarkably, the results of these two series of experiments were qualitatively quite similar: accumulating surfactant from the surrounding for some time had nearly the same effect as contaminating the water with Triton X-100. Low surfactant concentration would lead to a strongly modified dynamics of the plunging breakers and resulting air bubble entrainment processes, see figure 1(b,c). The origin of the effect is again surface elasticity, see figure 1(d): Compression and dilatation of the surface lead to concentration gradients of the surfactant and thus to Marangoni flows, which strongly affect the dynamics of the plunging breaker.
One may wonder why this effect also works for soluble surfactants as applied here, and not only for nearly immiscible surfactants as in the case of oil. The reason is that the time scales of the plunging breaker dynamics are much faster than the time scales of surfactant exchange between surface and bulk, so that by dilation a fresh and relative clean surface is created and vice versa a relatively ‘dirty’ surface by compression. This implies that for surfactants in abundance the effect is expected to cease, as then the plunging breaker surface is fully covered with surfactant even in the stretched state so that no Marangoni forces emerge any longer. This is exactly what Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) observe: for large enough Triton X-100 concentration the dynamics is quite similar to the clean case. This again shows that it is not the surface tension itself which mainly matters but the surface tension gradient.
Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) went a step further and measured the concentration-dependent surface tension with a Langmuir trough, building on the ideas and design of Agnes Pockels. This information was then combined with their (two-dimensional) direct numerical volume-of-fluid simulations of a plunging breaker, which employed Popinet's open-source Basilisk software (Popinet Reference Popinet2018), in order to quantitatively reveal the degree of compression and dilation on the surface and the resulting Marangoni forces. This is a very creative way to combine the strengths of the experiments and those of simulations, in order to reveal the physics of this phenomenon.
5. Relevance and outlook
The work by Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) beautifully shows how the physicochemical hydrodynamics on the nanoscale of the ocean surface strongly affects the macroscopic outcome of the plunging breakers. In models for the coupling between the ocean and the atmosphere this effect clearly matters, as the plunging breaker dynamics is key to the entrainment of air and thus CO $_2$ from the atmosphere into the ocean and vice versa for the injection of droplets and aerosols from the ocean into the atmosphere and thus for the formation of clouds. Future climate models clearly should take notice of this effect and embody and parametrize its consequences – a full inclusion of the effect is obviously out of reach and will be so for many decades to come.
$_2$ from the atmosphere into the ocean and vice versa for the injection of droplets and aerosols from the ocean into the atmosphere and thus for the formation of clouds. Future climate models clearly should take notice of this effect and embody and parametrize its consequences – a full inclusion of the effect is obviously out of reach and will be so for many decades to come.
What in the near future may be achievable, however, are improved numerical volume-of-fluids methods and packages which do combine the effect of surfactants of various types on the surface and in the bulk with that of the stretching and compressing of a free interface, fully resolving both the time scale of the interface and that of the surfactant exchange between bulk and surface. This is not only key for ocean waves and plunging breakers, but also on a much smaller length scale. An example for this is inkjet printing of complex inks, which contain many different liquids, surfactants and pigments, and where the physicochemical hydrodynamics at the surface and the exchange of ingredients between bulk and interface are key, both during the droplet jetting and detachment from the nozzle and during the impact of the droplet on the substrate and its sidewards spreading (Lohse Reference Lohse2022).

 $\mathrm {\mu }$mol L
$\mathrm {\mu }$mol L $^{-1}$ Triton-X-100 contamination. In (d) the mechanism of compression of the surfactants at the crest of the waves and dilation elsewhere is illustrated. Panels (b–d) are taken from Erinin et al. (2023).
$^{-1}$ Triton-X-100 contamination. In (d) the mechanism of compression of the surfactants at the crest of the waves and dilation elsewhere is illustrated. Panels (b–d) are taken from Erinin et al. (2023).
1. Benjamin Franklin and the use of ‘storm oil’
In 1757, Benjamin Franklin was sent by the Pennsylvania Assembly to England, to negotiate on taxation. On the passage with a fleet of sailing vessels, when watching the wind-ruffled and troubled sea around him, he observed that the wakes of some ships were remarkably smooth. He asked the captain about this phenomenon, who apparently considered this to be a very stupid question, as everybody would know that the smoothening is due to oil poured into the water; in this case presumably as the cooks had just emptied their greasy water into the ocean.
Franklin was fascinated by the observation and, after having arrived in England, he was the first to do controlled experiments on it on various English ponds and lakes and to report on it in a scientific publication (Franklin Reference Franklin1774). He wrote: ‘… The oil, though not more than a teaspoonful, produced an instant calm of a space over several yards square, which spread amazingly and extended itself gradually … till is reached half an acre, as smooth as a looking glass’. That paper also included an attempt to explain the phenomenon as being connected to the ‘friction’ between the wind blowing over the water and the water itself, viewing the oil as kind of a thin lubrication layer in between (Tanford Reference Tanford1989; Mertens Reference Mertens2006; Behroozi et al. Reference Behroozi, Cordray, Griffin and Behroozi2007). Though this explanation has major shortcomings, Franklin did realize that it is key for the effect that the oil layer is extremely thin. In the publication he also reported in detail his communication with the sea captain on this matter and that the effect had long been known by seamen in various cultures, who called the oil used in this way ‘storm oil’. The earliest mention of the wave-stilling properties of oil can be found in Pliny's Natural History (Mertens Reference Mertens2006).
Through his exchange with sea captains Franklin also became aware of a letter of the Dutch colonial official Mr. Tengnagel, in which he relates his and the ship's survival in a storm in the Indian Ocean to the captain's action of pouring oil into the sea, ‘to prevent the waves breaking over her, which had an excellent effect’ (Mertens Reference Mertens2006). Based on this, Franklin suggested pouring oil into the rough sea to ease landing on an island, and did corresponding experiments.
In late 1774, first orally and then by reading Franklin's publication, the Dutch merchant and cloth manufacturer Frans van Lelyveld became aware of Franklin's experiments and his exchange with seamen. He repeated the oil pouring experiments in the Oude Vest canal in Leiden in 1775 and spoke to various Dutch seamen and fishermen, being intrigued by the fact that an effect so well known to them had not been known to the learned. To share his knowledge, and to learn more about the effect, Lelyveld wrote the essay ‘Reports and Prize Questions as to the pouring of oil, whale oil, tar, or other floating substances to reduce shipping dangers’ (Lelyveld Reference Lelyveld1776), see figure 1(a) for an illustration out of that publication. The 17 prize questions were on the history of the practice, on what oil would be best, on how to pour the oil into the sea, on how long the smoothening effect would last, etc. The prize money for the best essay was 30 ducats (Mertens Reference Mertens2006). Lelyveld's publication led to the interesting discussion on whether a scientific approach to the problem would be necessary, or whether the practitioner's knowledge would be sufficient. Joannes le Francq van Berkhey, a lecturer of natural history at Leiden University, considered it to be an offence to the honour of Dutch fishermen and seamen that Lelyveld had asked for more scientific work on the problem and he wanted ‘to uphold the honour of the fishermen and seamen of the Low Countries … who deserve this honour for their ancestral knowledge’ (Mertens Reference Mertens2006).
Figure 1. (a) The effect of storm oil in the North Sea. The drawn experiment was conducted by the Dutch captain Isak Kalisvaar in 1776. He reported about it in a letter to Frans van Lelyveld in 1776. In the illustration, A is KalisvaarÕs ship, B is a flag indicating the wind direction, C shows the current direction, and the arc EDF identifies a circle of whale oil spreading behind the anchored ship. (Adapted from Lelyveld (Reference Lelyveld1776), p. 197, according to Mertens (Reference Mertens2006), from where the figure is taken with permission.) (b,c) Breaker profile images from high-speed movies of the wave crest for the case of clean water (a) and for the case of water with 7.5 $\mathrm {\mu }$mol L
$\mathrm {\mu }$mol L $^{-1}$ Triton-X-100 contamination. In (d) the mechanism of compression of the surfactants at the crest of the waves and dilation elsewhere is illustrated. Panels (b–d) are taken from Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023).
$^{-1}$ Triton-X-100 contamination. In (d) the mechanism of compression of the surfactants at the crest of the waves and dilation elsewhere is illustrated. Panels (b–d) are taken from Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023).
2. Lord Rayleigh, Agnes Pockels and her quantitative measurements of surface films
The next major step in understanding the effect of ‘storm oil’ was done by Strutt (Reference Strutt1890), the later Lord Rayleigh. He dropped 0.81 mg of olive oil on water and found that it spread towards 555 cm $^2$, from which he deduced that the layer thickness was 1.6 nm. This corresponds to a monolayer of oil molecules, though he would not have realized this in those days.
$^2$, from which he deduced that the layer thickness was 1.6 nm. This corresponds to a monolayer of oil molecules, though he would not have realized this in those days.
In parallel, Agnes Pockels performed similar experiments in Brunswick, Lower Saxony, Germany, calculating a thickness of 1.3 nm. As a woman of those days, Agnes Pockels was declined university education, and so she self-educated herself, with the help of textbooks provided to her by her younger brother Friedrich, who was a professor in physics, and with the help of experiments that she performed in her kitchen (Derrick Reference Derrick1982). As she did not succeed in publishing her results in Germany, she wrote to Lord Rayleigh (in German), who made sure that Pockels's results could be published as a Letter in Nature (Pockels Reference Pockels1891). That Letter also contained a description of her surface film balance technique with which she could precisely measure the surface tension and which was very similar to the technique which now is called Langmuir's trough (Derrick Reference Derrick1982).
Agnes Pockels was the first to realize that the damping of the capillary waves by the oil layer could not solely be due to the reduction of the surface tension, but that there must be some extra viscous resistance and dissipation to account for the observations. Her seminal achievements were broadly recognized only much later and in 1932 she received the honorary doctoral degree of the University of Brunswick (Derrick Reference Derrick1982).
3. Carlo Marangoni, surface elasticity and emerging gradients in surfactant concentration
The main origin of the extra dissipation became clear only many decades later, namely the surface-dilational elasticity, also known as Gibbs surface elasticity (Lucassen-Reynders & Lucassen Reference Lucassen-Reynders and Lucassen1970; Manikantan & Squires Reference Manikantan and Squires2020). Waves locally stretch or compress the surface, leading to locally lower or higher surfactant concentration and thus to locally higher or lower surface tension. The emerging surface tension gradients induce tangential shear forces (Marangoni & Stefanelli Reference Marangoni and Stefanelli1872; Scriven & Sternling Reference Scriven and Sternling1960), which are now called Marangoni forces and which lead to extra flow and extra dissipation in the upper water layers, thus to some extra damping effect. As first realized by Carlo Marangoni himself, the consequences of this effect on the flow are much stronger than that of the reduction of the surface tension by the surfactants (Marangoni & Stefanelli Reference Marangoni and Stefanelli1872; Behroozi et al. Reference Behroozi, Cordray, Griffin and Behroozi2007).
If the oil is nearly immiscible with water, the oil molecules act as surfactants and accumulate on the surface. However, surface tension gradients can also emerge due to more miscible molecules, even on large scales. Stefan & Szeri (Reference Stefan and Szeri1999) worked out how rising microbubbles, which are generated by the passage of a ship, scavenge surfactants from below and transport them up to the surface, where they can stay for a very long time. The resulting gradient in surface tension makes the wake of the ship visible for many hours and up to a length scale of 100 km, so that these traces of the ships are even visible from space (Griffin et al. Reference Griffin, Peltzer, Reed and Beck1992).
4. The effect of surfactants on plunging breakers
Though the ocean surface conditions Franklin will have experienced on his journey across the Atlantic from America to England will have been anything else but exclusively smooth, surprisingly, the focus of the studies on the effect of surfactants on waves until recently has been on relatively smooth gravity and capillary waves. After Liu & Duncan (Reference Liu and Duncan2006) had studied the effect of surfactants on gentle spilling breakers, Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) have now studied their effect on plunging breakers – exactly the rough flow situation Franklin will have met on his passage. But Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) did not do so in the ocean, but under very controlled conditions, namely in a laboratory wave tank which allows for the creation of plunging breakers with a dispersively focused wave packet. They performed the experiments with two sets of surfactants: first, with different concentrations of the soluble surfactant Triton X-100 dissolved in clean water; second, less controlled, with originally clean water exposed to the environment for increasing times up to 21 hr, before the plunging breaker experiments were done. Remarkably, the results of these two series of experiments were qualitatively quite similar: accumulating surfactant from the surrounding for some time had nearly the same effect as contaminating the water with Triton X-100. Low surfactant concentration would lead to a strongly modified dynamics of the plunging breakers and resulting air bubble entrainment processes, see figure 1(b,c). The origin of the effect is again surface elasticity, see figure 1(d): Compression and dilatation of the surface lead to concentration gradients of the surfactant and thus to Marangoni flows, which strongly affect the dynamics of the plunging breaker.
One may wonder why this effect also works for soluble surfactants as applied here, and not only for nearly immiscible surfactants as in the case of oil. The reason is that the time scales of the plunging breaker dynamics are much faster than the time scales of surfactant exchange between surface and bulk, so that by dilation a fresh and relative clean surface is created and vice versa a relatively ‘dirty’ surface by compression. This implies that for surfactants in abundance the effect is expected to cease, as then the plunging breaker surface is fully covered with surfactant even in the stretched state so that no Marangoni forces emerge any longer. This is exactly what Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) observe: for large enough Triton X-100 concentration the dynamics is quite similar to the clean case. This again shows that it is not the surface tension itself which mainly matters but the surface tension gradient.
Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) went a step further and measured the concentration-dependent surface tension with a Langmuir trough, building on the ideas and design of Agnes Pockels. This information was then combined with their (two-dimensional) direct numerical volume-of-fluid simulations of a plunging breaker, which employed Popinet's open-source Basilisk software (Popinet Reference Popinet2018), in order to quantitatively reveal the degree of compression and dilation on the surface and the resulting Marangoni forces. This is a very creative way to combine the strengths of the experiments and those of simulations, in order to reveal the physics of this phenomenon.
5. Relevance and outlook
The work by Erinin et al. (Reference Erinin, Lius, Liu, Mostert, Deike and Duncan2023) beautifully shows how the physicochemical hydrodynamics on the nanoscale of the ocean surface strongly affects the macroscopic outcome of the plunging breakers. In models for the coupling between the ocean and the atmosphere this effect clearly matters, as the plunging breaker dynamics is key to the entrainment of air and thus CO $_2$ from the atmosphere into the ocean and vice versa for the injection of droplets and aerosols from the ocean into the atmosphere and thus for the formation of clouds. Future climate models clearly should take notice of this effect and embody and parametrize its consequences – a full inclusion of the effect is obviously out of reach and will be so for many decades to come.
$_2$ from the atmosphere into the ocean and vice versa for the injection of droplets and aerosols from the ocean into the atmosphere and thus for the formation of clouds. Future climate models clearly should take notice of this effect and embody and parametrize its consequences – a full inclusion of the effect is obviously out of reach and will be so for many decades to come.
What in the near future may be achievable, however, are improved numerical volume-of-fluids methods and packages which do combine the effect of surfactants of various types on the surface and in the bulk with that of the stretching and compressing of a free interface, fully resolving both the time scale of the interface and that of the surfactant exchange between bulk and surface. This is not only key for ocean waves and plunging breakers, but also on a much smaller length scale. An example for this is inkjet printing of complex inks, which contain many different liquids, surfactants and pigments, and where the physicochemical hydrodynamics at the surface and the exchange of ingredients between bulk and interface are key, both during the droplet jetting and detachment from the nozzle and during the impact of the droplet on the substrate and its sidewards spreading (Lohse Reference Lohse2022).
Acknowledgements
D.L. thanks E. Villermaux, L. Deike, J. Duncan, M. Erinin and V. Sanjay for comments on the manuscript, and A. Soldati, who last year made him aware of A. Pockels.
Declaration of interests
The author reports no conflict of interest.