Introduction
Late-life mental health is becoming an increasingly important issue. It is estimated that 37%–43% of older adults have symptoms of anxiety or depression (Braam et al., Reference Braam2014; Rodda et al., Reference Rodda, Walker and Carter2011), while 9%–14% have a diagnosed anxiety or major depressive disorder (Rodda et al., Reference Rodda, Walker and Carter2011; Wolitzky-Taylor et al., Reference Wolitzky-Taylor, Castriotta, Lenze, Stanley and Craske2010). Anxiety or depression in later life is associated with an increased risk of cognitive decline, functional decline, and increased use of healthcare services (Meeks et al., Reference Meeks, Vahia, Lavretsky, Kulkarni and Jeste2011; Wolitzky-Taylor et al., Reference Wolitzky-Taylor, Castriotta, Lenze, Stanley and Craske2010). Frailer older adults, commonly experiencing physical or cognitive comorbidities affecting functioning (i.e. difficulties carrying out activities of daily living due to physical health conditions, in addition to depression), have a four-fold increase in the risk of clinically significant anxiety or depression (Ni Mhaolain et al., Reference Ni Mhaolain2012). Comorbid physical and mental health disorders increases the risk of greater frailty, mortality, and primary and secondary healthcare service use (Djernes et al., Reference Djernes, Gulmann, Foldager, Olesen and Munk-Jørgensen2011; Vaughan et al., Reference Vaughan, Corbin and Goveas2015). In an ageing population, demand for mental health services specific to later life is likely to increase.
There is an abundance of evidence assessing the effectiveness of treatments for late-life depression. However, frailer older adults represent an understudied subgroup of this population, and there is evidence that physical illness, older age, and impaired executive functioning can negatively impact pharmacological and non-pharmacological treatment outcomes (Tunvirachaisakul et al., Reference Tunvirachaisakul2017). Antidepressants only appear to be effective when studied as a class, rather than individual drug types, and when reviews use an “older adult” threshold of 55+ years rather than 65+ (Jonsson et al., Reference Jonsson2016; Kok et al., Reference Kok, Nolen and Heeren2012). Antidepressant Randomized Controlled Trials (RCTs) do not account for frailty or malnutrition and the “older old (75+ years)” populations are underrepresented (Benraad et al., Reference Benraad, Kamerman-Celie, van Munster, Oude Voshaar, Spijker and Olde Rikkert2016), despite greater concerns about falls and polypharmacy in these populations. Collaborative care interventions and home care interventions have some evidence of effectiveness for depression, but the evidence base is very limited (Dham et al., Reference Dham2017; Simning and Simons, Reference Simning and Simons2017). Exercise interventions for depression have not been found to be effective in those with physical comorbidities (Schuch et al., Reference Schuch2016). Additionally, many older adults state a preference for psychological interventions (Gum et al., Reference Gum2006; Mohlman, Reference Mohlman2011).
However, secondary care services (e.g. community psychiatric services) focus mainly on severe mental illness, while center-based and intensive approaches may be unsuited to community-dwelling older adults with limited mobility and frailty. Cognitive behavioural therapy (CBT) shows smaller effect sizes for older rather than younger adults and has mostly been evaluated in samples with a mean age of 60–70 years old in people who are otherwise healthy (Gould et al. Reference Gould, Coulson and Howard2012a; Reference Gould, Coulson and Howard2012b). Existing psychological therapies offered in community settings may not fully account for issues such as increasing dependency, social isolation, reduced functional abilities and cognitive decline that are key for older people. However, some non-pharmacological therapies such as problem-solving therapy have shown promise in reducing disability, in addition to depression, in older adults with major depressive disorder and may offer promise for further research (Kirkham et al., Reference Kirkham, Choi and Seitz2016). Although some reviews have looked at frailer subgroup analyses of older adults (Jonsson et al., Reference Jonsson2016), these have studied psychological therapies alone and documented “frailty indicators,” rather than clearly defining an impaired population.
Consequently, the only review of this subpopulation with impairments as its main focus appears to have been carried out two decades previously (Landreville and Gervais, Reference Landreville and Gervais1997). We, therefore, aimed to review the effectiveness of non-pharmacological interventions to reduce depression and anxiety in older adults with comorbidities affecting functioning.
Methods
We systematically reviewed Randomized Controlled Trials (RCTs) (Prospero registration CRD42017068441).
Search strategy and inclusion criteria
We developed a comprehensive search strategy (see Appendix A1) with terms based on age, impairments, depression/anxiety, and study type. We searched the following databases (inception-July 2017): MEDLINE, MEDLINE in Process & Other Non-Indexed Citations, EMBASE, AMED, Web of Science: Social Science Citation Index, Cochrane Central Register of Controlled Trials and NHS Health Economic Evaluation Database, PsycINFO, Cumulative Index to Nursing and Allied Health Literature, Sociological Abstracts, Social Care Online, and Applied Social Sciences Index and Abstracts.
We performed additional searches of clinicaltrials.gov, UK Clinical Trials Gateway, and World Health Organization International Clinical Trials Registry Platform (inception-Sep 2017) to identify ongoing studies. We screened the reference lists of included studies/relevant systematic reviews and used forward citation tracking of all included studies. We used author searches to follow up conference abstracts, trials register entries, and protocols where available and necessary.
Our inclusion criteria were as follows:
Participants: Older adults (aged 60+ years); functional difficulties [including difficulties in activities of daily living (ADLs) or instrumental ADLs (IADLs), housebound, frail, low functioning scores on a validated scale, recipient of relevant support services (e.g. social care services)]; symptoms/diagnosis of depression and/or anxiety
Interventions: Home- or community-based interventions delivered by any health, social, lay, or voluntary provider; single or multicomponent intervention aimed primarily at addressing depression or anxiety
Comparator: Any
Outcomes: Depressive and anxiety symptoms using validated questionnaires, other depression or anxiety outcomes (e.g. recovery), well-being, quality of life, functioning, service use
Study type: Parallel-group, cluster, or crossover randomized controlled trials, economic evaluations of RCTs
We lowered our original age inclusion criterion from 75+ to 60+ years, as this allowed inclusion of a number of additional relevant studies of functionally impaired older adults with mean ages 60–75.
We excluded interventions targeted at caregivers or targeted at specific health conditions (e.g. dementia, arthritis) in order to be more applicable to the wider frailer population, such as people described as generically “at risk” or “multimorbid” (unless there were documented associated difficulties in functioning); care-home interventions (recently reviewed by Simning and Simons [Reference Simning and Simons2017]); studies in which participants were not experiencing at least mild depression/anxiety; inpatient interventions; medication-only interventions (interventions containing a medication component were included); interventions focused primarily on another issue (e.g. frailty, falls prevention) in which depression/anxiety is a secondary target as it would be difficult to be sure of the effective components; reviews, qualitative studies, quasi-experimental, and uncontrolled studies.
RF screened titles and abstracts, and YB independently checked 10% of these. We took an inclusive approach and screened the full texts of all studies assessing interventions in samples of depressed or anxious older adults, as we anticipated that difficulties in functioning may not be clearly reported in the abstract. RF and YB independently screened 10% of the full texts to ensure consistency in applying the inclusion criteria, with disagreements resolved through discussion, then each screened 50% of the remaining full texts. Dual review was undertaken for a further 7% of studies where there was uncertainty, with input from KW where needed. We contacted five authors for additional data to further inform about the inclusion criteria, and three replied. We sought further data regarding ten included studies from nine authors and eight replied, six of whom were able to provide further data.
Data extraction and quality assessment
We extracted data on participants, study type, intervention description (according to the TIDIER checklist [Hoffmann et al., Reference Hoffmann2014]), outcomes assessed, and main findings. If a study had multiple publications, we considered the main results paper as the primary paper and included information from related papers (e.g. protocols) where relevant. RF and YB independently assessed risk of bias using the Cochrane Risk of Bias tool (Higgins et al., Reference Higgins2011) and resolved disagreements through discussion. Overall ratings were judged on the least score counts; however, as non-pharmacological therapies are often compared to usual care and a placebo control is not always desirable or practical, we did not include participant blinding in the overall trial rating. Within “other bias,” we assessed whether studies documented or controlled for use of antidepressants. Risk of bias ratings informed our narrative synthesis. We intended to assess publication bias using funnel plots but had insufficient data for this (Sterne et al., Reference Sterne2011).
Synthesis
We grouped studies according to intervention type: problem-solving therapy, other psychological therapies, and collaborative care (defined as complex interventions involving a multi-professional approach to care, a structured management plan, scheduled follow-ups, and enhanced interprofessional communication [Archer et al., Reference Archer2012]). We conducted meta-analysis of similar outcomes post-intervention, the most relevant, and widely available timepoint (follow-up timepoint meta-analysis was precluded by differing outcome types and timepoints). Self-reported and clinician-rated outcomes were not combined as these produced different effect estimates in a previous meta-analysis (Cuijpers et al., Reference Cuijpers, Li, Hofmann and Andersson2010). We summarized effects for continuous data using mean difference (MD) or standardized mean difference (SMD), where appropriate, with weighting by inverse variance (Higgins and Green, Reference Higgins and Green2011). For dichotomous data, we combined effects using odds ratios, weighted by the Mantel-Haenszel method (Higgins and Green, Reference Higgins and Green2011). All analyses used a random effects model as we anticipated high clinical heterogeneity, quantified using the I2 statistic. Similar interventions within a single trial were aggregated into one intervention group for meta-analysis using Higgins and Green’s (Reference Higgins and Green2011) formulae. Where necessary, we used p values and test statistics to compute SDs, standardized mean difference, and 95% confidence intervals, then we combined studies using the generic inverse variance method (Higgins and Green, Reference Higgins and Green2011).
RF performed meta-analysis using Revman 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration 2014). YB checked all data used in meta-analysis with the original papers or the authors’ communications and independently verified calculations (e.g. calculation of standard deviations from p values). We narratively summarized all other outcomes, including longer term follow-up timepoints.
Results
We identified 7708 unique references and screened 698 full texts (see Figure 1). Full texts were largely excluded due to their not targeting depression/anxiety (in participants or intervention [n = 181]), study type (n = 164), or not targeting an impaired older population (n = 154). We found five relevant ongoing trials as protocols, or trial register entries, which had not previously been located (findings not currently reported, summarized in Appendix A3).
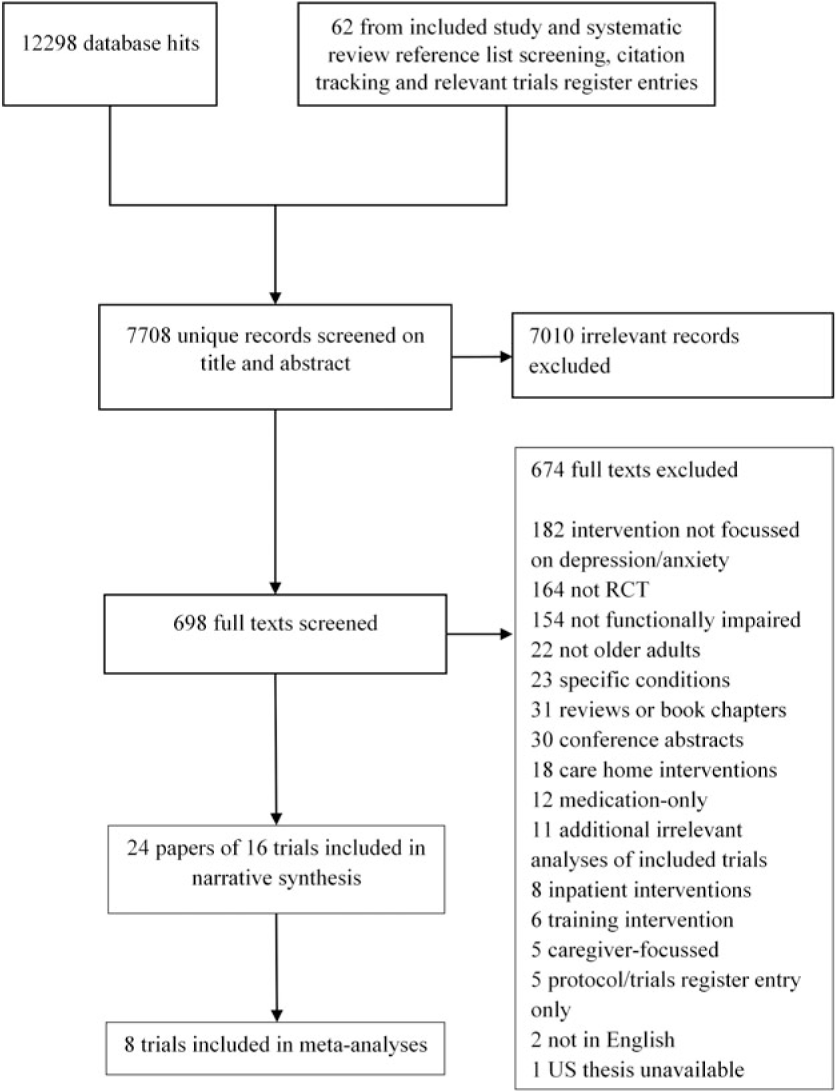
Figure 1. Flow diagram of studies throughout the review.
Description of included studies
We included 14 trials with a total of n = 2099 randomized participants, comprising 11 RCTs (Alexopoulos et al., Reference Alexopoulos2016; Banerjee et al., Reference Banerjee, Shamash, Macdonald and Mann1996; Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014; Ciechanowski et al., Reference Ciechanowski2004; Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007; Enguidanos et al., Reference Enguidanos, Davis and Katz2005; Gellis et al., Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008; Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010; Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015; Nyunt Reference Nyunt2010; Serrano et al., Reference Serrano, Latorre, Gatz and Montanes2004), one cluster RCT (Bruce et al., Reference Bruce2015), one pilot RCT (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007), and one RCT with a non-concurrent control group (Llewellyn-Jones et al., Reference Llewellyn-Jones1999) (see Appendix A2 for a detailed summary of study characteristics). We also included two RCT subgroup analyses (Blanchard et al., Reference Blanchard, Waterreus and Mann1995; Landreville and Bissonnette, Reference Landreville and Bissonnette1997) (one n not reported, one n = 23 randomized). Most studies were carried out in the US (n = 10), with two UK studies (Banerjee et al., Reference Banerjee, Shamash, Macdonald and Mann1996; Blanchard et al., Reference Blanchard, Waterreus and Mann1995), and one each in Australia (Llewellyn-Jones et al., Reference Llewellyn-Jones1999), Singapore (Nyunt Reference Nyunt2010), Spain (Serrano et al., Reference Serrano, Latorre, Gatz and Montanes2004), and Canada (Landreville and Bissonnette, Reference Landreville and Bissonnette1997). Sample sizes varied from 23 to 311.
Participant mean ages ranged from 64.8 to 84.9 years, with higher proportions of women in all trials (range from 69.6% to 87.5%). Many studies captured a diverse population, including substantial proportions of ethnic minorities in studies reporting this data (see Table 1). Four studies focused on or included a considerable proportion of those with low incomes (Alexopoulos et al., Reference Alexopoulos2016; Bruce et al., Reference Bruce2015; Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014; Landreville and Bissonnette, Reference Landreville and Bissonnette1997) and a range of educational levels were reported. Many studies used a screening instrument to define depression criteria, with or without the Structured Clinical Interview for DSM-IV (SCID-IV) (see Table 1).
Table 1. Characteristics of participants in each study

ADL = Activities of Daily Living; DRS IP = Dementia Rating Scale Initiation/Preservation subscale; IADL = Instrumental Activities of Daily Living; MMSE = Mini-Mental State Examination; N = Number; NR = Not Reported; QoL = Quality of Life; RCT = Randomized Control Trial; SI = Screening Instrument; SCID = Structured Clinical Interview for DSM; SPMSQ = Short Portable Mental Status Questionnaire; Stroop CW = Stroop Color-Word test.
The majority of studies excluded people below a certain cognition threshold (n = 8) or those with dementia (n = 3) (see Table 1). Consequently, many studies, even if using a lower cognition threshold, reported mean baseline cognition scores within a normal range (i.e. Mini-Mental State Examination (MMSE) scores between 26.8 and 29.3 across studies (Alexopoulos et al., Reference Alexopoulos2016; Bruce et al., Reference Bruce2015; Llewellyn-Jones et al., Reference Llewellyn-Jones1999), 1.58 on the Short Portable Mental Status Questionnaire (Enguidanos et al., Reference Enguidanos, Davis and Katz2005), and 5.5 on the 6-item MMSE [Ciechanowski et al., Reference Ciechanowski2004]). Only two studies included people with greater cognitive impairment (Kiosses et al., Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015) or executive dysfunction (Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010) resulting in a wider range of cognition scores, but still normal mean cognitive function in Kiosses et al (2010). Two studies did not report any cognition data (Banerjee et al., Reference Banerjee, Shamash, Macdonald and Mann1996; Blanchard et al., Reference Blanchard, Waterreus and Mann1995).
Risk of bias in included studies
Overall, most studies were at an unclear risk of bias (see Figure 2), largely due to limited reporting of random sequence generation (n = 8), allocation concealment (n = 13), and lack of an online or published protocol to assess selective reporting (n = 11). Studies were most commonly at a low risk of bias for outcome assessment (n = 12), incomplete outcome data (n = 10), and reporting or controlling for antidepressant use (other bias, n = 9). Studies were most commonly at a high risk of bias for participant blinding (n = 7), although this was expected as blinding participants can be difficult in trials of complex interventions and so these ratings were not included in the overall score. Individual risk of bias ratings are discussed throughout the synthesis for each outcome.

Figure 2. Risk of bias in included studies (NB selective reporting judgment refers to depressive symptoms and is highlighted where different for other outcomes in the text).
Modified problem-solving therapy (n = 7 trials, including n = 688 randomized participants)
Problem-solving therapy (PST) aims to systematically identify and address daily life problems to reduce depression and improve future coping skills (Ciechanowski et al., Reference Ciechanowski2004) through standard steps including: identifying problems, establishing achievable goals, brainstorming solutions, using decision-making guidelines, evaluating and contrasting solutions, developing action plans, and evaluating outcomes. PST was modified in some studies to suit a more impaired population:
Integration into existing home care services, with six sessions to fit home care’s fast pace and limited resources and individuals’ frailty (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008)
Emphasizing social activation, increasing outdoor interactions, and developing an exercise program (Ciechanowski et al., Reference Ciechanowski2004)
Problem Adaptation Therapy with environmental adaptations to circumvent behavioral and/or functional limitations and involving willing/available caregivers over 12 weeks (Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010; Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015)
Delivery over videoconferencing software (tele-PST) to enable home-based therapy (Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014)
Increasing pleasant events (Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014; Ciechanowski et al., Reference Ciechanowski2004; Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008; Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010)
PST was most commonly delivered by social workers (usually Masters-level) (Alexopoulos et al., Reference Alexopoulos2016; Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014; Ciechanowski et al., Reference Ciechanowski2004; Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010), registered nurses (Ciechanowski et al., Reference Ciechanowski2004) or clinical psychologists, and clinical doctorate candidates (Kiosses et al., Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015). All delivered PST face-to-face at participants’ homes over 6–12 sessions, apart from one trial comparing delivery over Skype to face-to-face and to-care calls (Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014) (collapsed to a single PST group for meta-analysis as per Higgins and Green [Reference Higgins and Green2011]). Other trial comparators included usual care (Ciechanowski et al., Reference Ciechanowski2004), enhanced usual care (e.g. with basic education) (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008) or attention controls (e.g. supportive therapy) (Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010; Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015). One compared case management plus PST to case management alone (Alexopoulos et al., Reference Alexopoulos2016).
Depressive symptoms
Modified PST significantly reduced clinician-rated Hamilton Depression Rating Scale (HAM-D) scores post-intervention (see Figure 3[a], n = 5, MD −4.94 [95% CI −7.90 to −1.98], p = 0.001). Two further studies found significantly lower scores on other measures, including the Montgomery–Åsberg Depression Rating Scale (MADRS), compared to supportive therapy (Kiosses et al., Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015) and 20-item Hopkins Symptom Checklist Depression Scale (HCSL-20) compared to usual care (Ciechanowski et al., Reference Ciechanowski2004). Reduced depressive symptoms were maintained 6–12 months from baseline in studies assessing this outcome (Alexopoulos et al., Reference Alexopoulos2016; Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014; Ciechanowski et al., Reference Ciechanowski2004; Gellis et al., Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008). All studies were at an overall unclear risk of bias (with some low-risk domains), apart from Choi et al. (Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014), who used non-blinded outcome assessors. However, PST did not significantly increase the post-intervention clinician-rated odds of response (≥50% symptom reduction from baseline, OR 3.24 [95% CI 0.52 to 20.27], Figure 3[b]) or remission (OR 2.62 [95%CI 0.57 to 12.16], Figure 3[c]) using the HAM-D (Alexopoulos et al., Reference Alexopoulos2016), HSCL-20 (Ciechanowski et al., Reference Ciechanowski2004), and MADRS (Kiosses et al., Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015). Similarly, no effects were found 12 weeks post-intervention (Alexopoulos et al., Reference Alexopoulos2016) or at the 36-week (Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014) or 12-month follow-up (Ciechanowski et al., Reference Ciechanowski2004). Kiosses et al. (Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015) did not assess longer term outcomes.

Figure 3. Forest plots of meta-analyses: (a) meta-analysis of the effectiveness of problem-solving therapy upon clinician-rated depressive symptoms; (b) meta-analysis of the effectiveness of problem-solving therapy upon response (≥50% reduction in symptoms from baseline); (c) meta-analysis of the effectiveness of problem-solving therapy upon remission (not depressed according to threshold); (d) meta-analysis of the effectiveness of problem-solving therapy upon self-reported disability; and (e) meta-analysis of the effects of collaborative care upon self-reported depressive symptoms.
We could not meta-analyze self-reported depressive symptoms, as two papers reported identical mean and SD 15-item Geriatric Depression Scale (GDS-15) scores at all timepoints, suggesting a reporting error (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008). These figures did however, indicate significantly lower symptoms at post-intervention, 3 months, and 6 months (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008). One also found significantly lower Beck Depression Inventory scores post-intervention (PST vs. usual care, 10.20 vs 27.4, p < 0.001), maintained at 6 months (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007).
Functioning and disability
PST did not significantly reduce self-reported disability post-intervention (Figure 3[d], n = 4, SMD −1.35 [95% CI −2.96 to 0.25], p = 0.10) when assessed using the World Health Organization Disability Assessment Schedule and the Sheehan Disability Scale. Within one study, significant effects upon disability were maintained at 24 weeks for tele-PST and face-to-face PST and at 36 weeks for tele-PST (Choi et al., Reference Choi, Nathan Marti, Bruce, Hegel, Wilson and Kunik2014). Another study, similarly to post-intervention, found no effects at the 12-week follow-up (Alexopoulos et al., Reference Alexopoulos2016).
Quality of life
Evidence about the impact of PST upon the quality of life was mixed across two studies using the Quality of Life Inventory. Meta-analysis was not undertaken as the SDs (but not means) reported were identical in both papers at all timepoints (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008), suggesting a potential reporting error. Significantly higher quality of life was found post-intervention and at 3 and 6 months in Gellis et al. (Reference Gellis, McGinty, Horowitz, Bruce and Misener2007) but no effects were found in Gellis et al. (Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008). Within Ciechanowski et al. (Reference Ciechanowski2004) there were improvements in emotional and functional but not social or physical well-being over 12 months on the Functional Assessment of Cancer Therapy Scale: General.
Service use and costs
None of the three studies reporting service use found PST to significantly affect primary, secondary, or home care service use over the treatment period or longer follow up, although this was considered beneficial where PST was delivered as part of home care (Ciechanowski et al., Reference Ciechanowski2004; Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008). One study reported intervention costs ($630 per patient) (Ciechanowski et al., Reference Ciechanowski2004), and two noted that social work home visits were reimbursable by Medicare (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008). No other studies reported this data.
Collaborative care interventions (n = 7 studies including n = 1351 participants)
Seven studies evaluated collaborative care interventions, provided in the community or in care services (components listed in Table 2). Key intervention providers were usually nurses (Blanchard et al., Reference Blanchard, Waterreus and Mann1995; Bruce et al., Reference Bruce2015; Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007; Nyunt Reference Nyunt2010), but could include social workers, psychologists (Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007), or medical staff (Banerjee et al., Reference Banerjee, Shamash, Macdonald and Mann1996).
Table 2. Collaborative care intervention content

* aka key worker, case worker, Clinical Depression Specialist etc.
MDT = Multidisciplinary Team.
Baseline depressive symptoms levels were mainly mixed (Blanchard et al., Reference Blanchard, Waterreus and Mann1995; Bruce et al., Reference Bruce2015; Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007; Enguidanos et al., Reference Enguidanos, Davis and Katz2005), with two studies focusing on major depression (Banerjee et al., Reference Banerjee, Shamash, Macdonald and Mann1996; Llewellyn-Jones et al., Reference Llewellyn-Jones1999) and one on minor depression (Nyunt Reference Nyunt2010). Comparators included “routine care” (further details not reported) (Llewellyn-Jones et al., Reference Llewellyn-Jones1999); GP management with or without notification of depression severity (Banerjee et al., Reference Banerjee, Shamash, Macdonald and Mann1996; Blanchard et al., Reference Blanchard, Waterreus and Mann1995; Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007; Nyunt Reference Nyunt2010); usual geriatric case management with care plan (Enguidanos et al., Reference Enguidanos, Davis and Katz2005); and usual care enhanced by screening, notifying GPs (Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007), or following agency procedures (Bruce et al., Reference Bruce2015) if the patient did not improve.
Potential meta-analysis for collaborative care interventions relied mainly on data from an unpublished thesis, Nyunt (Reference Nyunt2010), whose analysis was at high risk of bias as they did not use an intention-to-treat analysis. We, therefore, only undertook meta-analysis where higher quality data were available (self-reported depressive symptoms).
Effects upon depressive symptoms (n = 7)
There were mixed effects on continuous depressive symptom scores. Two large studies found no effects on clinician-rated HAM-D scores post-intervention or at 12 months (Bruce et al., Reference Bruce2015; Nyunt Reference Nyunt2010). A subgroup with major depression and one smaller study (n = 69) found significant reductions in depressive symptoms (Banerjee et al., Reference Banerjee, Shamash, Macdonald and Mann1996; Bruce et al., Reference Bruce2015), while Blanchard et al.’s (Reference Blanchard, Waterreus and Mann1995) subgroup analysis found greater symptom improvements in those with physical capacity than those without, although this did not interact with the treatment group. There were similarly no effects upon post-intervention self-reported depressive symptoms (n = 2, SMD −0.09 [95%CI −0.29 to 0.12], p = 0.41, Figure 3[e]). A further unpublished study (at high risk of bias due to higher intervention arm dropout rates) found no differences in 9-item Patient Health Questionnaire (PHQ-9) scores post-intervention or at 12 months (Enguidanos et al., Reference Enguidanos, Davis and Katz2005). Nyunt (Reference Nyunt2010) (at high risk of bias) found some differences in GDS and BDI scores post-intervention but not at 12 months.
Odds of depression remission were mixed. Banerjee et al. (Reference Banerjee, Shamash, Macdonald and Mann1996) found significantly higher odds of clinician-rated remission using Geriatric Mental State AGECAT categories, while Nyunt (Reference Nyunt2010) found no differences in remission using HAM-D at any timepoint. Odds of self-reported remission was significantly higher using the GDS-15 (Nyunt Reference Nyunt2010) with significant positive movement into “less depressed” categories using the GDS-30 cutoffs (Llewellyn-Jones et al., Reference Llewellyn-Jones1999); but not using the BDI (Nyunt Reference Nyunt2010) or in the odds of response using the PHQ-9 at 4, 8, or 12 months (Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007).
Functioning (n = 2)
There was no evidence that collaborative care affected 12-item Short Form (SF-12) physical functioning subscale scores, Mahoney and Barthel Activities of Daily Living (ADL) scores, Lawton Instrumental Activities of Daily Living (IADL) scores (Nyunt Reference Nyunt2010), or SF-20 scores (Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007) at any timepoint up to 12 months.
Quality of life (n = 2)
Mental functioning scores (SF-12) were significantly higher post-intervention in the collaborative care group compared to usual care but not at 12 months in Nyunt (Reference Nyunt2010), and Ell et al. (Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007) found no differences in odds of an improvement in mental, social, or role functioning scores.
Service use and costs (n = 5)
One study found significant effects for collaborative care on the number of days in hospital (3.59 vs. 6.31, p = 0.04, unpublished data supplied by authors) (Enguidanos et al., Reference Enguidanos, Davis and Katz2005). However, one large cluster trial found no significant reductions in risk of 30- or 60-day hospitalization from home health services (except when including service-level data from all people in all clusters, rather than those agreeing to full-study participation) (Bruce et al., Reference Bruce, Lohman, Greenberg, Bao and Raue2016). Others similarly found no differences in the 12-month odds of hospitalization (meta-analysis not possible due to insufficient data) (Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007; Nyunt Reference Nyunt2010), home care visits (Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007; Enguidanos et al., Reference Enguidanos, Davis and Katz2005), primary care or physician visits (Enguidanos et al., Reference Enguidanos, Davis and Katz2005; Nyunt Reference Nyunt2010), home care readmissions (Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007), moves to long-term care (Enguidanos et al., Reference Enguidanos, Davis and Katz2005), or emergency department visits (Ell et al., Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007; Enguidanos et al., Reference Enguidanos, Davis and Katz2005; Llewellyn-Jones et al., Reference Llewellyn-Jones1999). Only Enguidanos et al. (Reference Enguidanos, Davis and Katz2005) calculated healthcare costs (unpublished), which were significantly lower at four months ($3295 vs. $5417, p = 0.04) and 12 months ($8403 vs. $11,242, p = 0.05). Banerjee et al. (Reference Banerjee, Shamash, Macdonald and Mann1996) reported extra costs of a part time doctor as a depression case manager but did not cost this.
Other psychological therapies
The evidence base available for other psychological therapies in this population consisted of small single studies at an unclear risk of bias. Four sessions of life review therapy that focused on different life periods had significant post-intervention effects on self-reported depressive symptoms (20-item Center for Epidemiological Studies—Depression Scale) and life satisfaction (Life Satisfaction Index A), compared to social visits in 50 social care services clients (Serrano et al., Reference Serrano, Latorre, Gatz and Montanes2004). Bibliotherapy (i.e. reading a CBT-based book Feeling Good over 4 weeks) reduced depressive symptoms in two out of three scales (BDI, GDS-30, Inventory to Diagnose Depression) compared to weekly supportive phone calls in a subgroup analysis of 23 people with disability in at ≥1 ADL, IADL or mobility, with no effect upon functioning (Landreville and Bissonnette, Reference Landreville and Bissonnette1997). Neither study reported service use or cost data.
Discussion
Within this review, we included 14 RCTs including 2099 randomized individuals, plus two subgroup analyses, primarily assessing modified problem-solving therapy (n = 7) and collaborative care interventions (n = 7). For older adults with depression and physical comorbidities affecting functioning, we found that home-based PST significantly reduced depressive symptoms, but did not affect remission, functioning, or service use, and showed mixed effects on quality of life. The evidence for collaborative care was heterogeneous, with no effects on depressive symptoms in meta-analysis and narrative synthesis showed little effect upon quality of life, service use or functioning, and mixed effects upon remission. The evidence base for bibliotherapy and life review was too limited to draw conclusions. No treatments for anxiety were identified in this population.
Similar effect sizes for PST on depressive symptoms have been noted in the general older adult population (Jonsson et al., Reference Jonsson2016; Kirkham et al., Reference Kirkham, Choi and Seitz2016). However, Kirkham et al. (Reference Kirkham, Choi and Seitz2016) also found effects on disability. We included a greater number of trials than Kirkham et al, and outcomes appeared to be broadly consistent between different measures used. It is, therefore, likely that either trials were underpowered to detect differences (as functioning was never a primary outcome), or the results were mainly affected by a single study in which both study arms also received case management (Alexopoulos et al., Reference Alexopoulos2016). The latter study may also be the reason for the lack of effect on remission despite a strong effect on symptoms or this may be due to the high baseline depressive symptom scores (e.g. HAM-D scores of 21–24). Heterogeneity was also very high (≥90%) for some PST outcomes, which could relate to differences in timepoints (e.g. immediately post-intervention or some weeks after) or intervention components. There were too few studies reporting quality of life outcomes to draw firm conclusions, and quality of life appears to get little attention in trials assessing late-life depression treatments (Jonsson et al., Reference Jonsson2016; Kirkham et al., Reference Kirkham, Choi and Seitz2016). While a few larger studies have shown positive effects upon late-life depression for primary care-based collaborative care in the general older adult populations, smaller studies in other settings indicate mixed effects (Dham et al., Reference Dham2017) similarly to our review. However, an individual patient meta-analysis has reported similar collaborative care effects regardless of the number or type of chronic physical conditions across all ages (Panagioti et al., Reference Panagioti2016).
Although executive dysfunction has been linked to worse treatment outcomes (Tunvirachaisakul et al., Reference Tunvirachaisakul2017), the two studies including participants with a wider range of cognition scores (Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010; Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015) found results consistent with those restricted to normal scores, suggesting that PST may be equally effective in a cognitively impaired population. Mixed reporting of cognition and mixed results in collaborative care studies mean that conclusions cannot be drawn for these interventions.
Strengths and limitations of the evidence base
The evidence base was largely at an unclear risk of bias due to poor reporting. Studies at high risk of bias were rare, apart from a few key collaborative care trials. Regarding generalizability, the included studies recruited ethnically and socioeconomically diverse participants; however, most were carried out in the US and all had a higher proportion of female participants. Although within most studies participants fell within a “normal” cognition range, some studies included people with a range of cognition levels that included those with cognitive impairment. Within this review, PST appears to have consistent effects on depression in studies with participants who have greater cognitive impairment (Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010; Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015) and those with executive dysfunction but no physical disability (Alexopoulos et al., Reference Alexopoulos, Raue and Areán2003; Areán et al., Reference Areán, Raue, Mackin, Kanellopoulos, McCulloch and Alexopoulos2010).
Post-intervention effectiveness was well-documented, but follow-up periods of over one year were rare. Previous research suggests that depression is associated with faster physical decline and greater health service use (Djernes et al., Reference Djernes, Gulmann, Foldager, Olesen and Munk-Jørgensen2011; Vaughan et al., Reference Vaughan, Corbin and Goveas2015). Reducing depression may maintain physical functioning, rather than improve it, which may only be apparent in larger samples over longer follow up. Quality of life information was limited, while adverse event data were omitted by all but two studies (Banerjee et al., Reference Banerjee, Shamash, Macdonald and Mann1996; Kiosses et al., Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015). We found no trials of CBT or any therapies for anxiety targeted to our population. Attrition in many studies was similar across control and treatment arms, suggesting that dropout was not an issue likely to affect review results. However, collaborative care studies had mixed fidelity and adherence in those measuring it, which may be a source of heterogeneity in outcomes (e.g. in Ell et al. [Reference Ell, Unützer, Aranda, Gibbs, Lee and Xie2007] 30% received no intervention care). Within PST, generally high fidelity scores were reported and where assessed, participant attendance was fairly high, even for studies with greater numbers of sessions. Book-based CBT had very low adherence (on average only half of the book was read [Landreville and Bissonnette, Reference Landreville and Bissonnette1997]).
Strengths and limitations of the review
We searched a wide range of databases using comprehensive search terms, and additional methods located only one extra subgroup analysis and one small study. One reviewer assessed titles, abstracts, and full texts. However, they took an inclusive approach and a proportion were screened independently by a second reviewer with good agreement. We did not include trials of interventions for depression in older people as a general population. Although these may include some participants with difficulties in functioning arising from physical comorbidities, they are only likely to be a small proportion as the mean age within many of these trials is 65–70 (Gould et al., Reference Gould, Coulson and Howard2012b; Reference Gould, Coulson and Howard2012a). However, we took an inclusive approach to screening to identify larger trials that included a subgroup analysis in a frailer population. Two independent reviewers assessed risk of bias and extracted meta-analysis data. Meta-analysis was limited by inconsistencies in outcome timepoints (e.g. immediately post-intervention to six weeks after), differing intervention lengths and poor study quality. We received a good response from authors to our requests for further data.
Implications for practice
Currently, UK psychological therapy services commonly focus primarily on delivering CBT for depression and anxiety, with low referral to and uptake of these services in older people (Walters et al., Reference Walters, Falcaro, Freemantle, King and Ben-Shlomo2018; Wang et al., Reference Wang2005). In our review, we found no evidence regarding the use of CBT in older people with physical comorbidities affecting functioning. It may be that CBT is more cognitively demanding than other therapies and so has not been trialed in those who are frailer and more likely to have mild cognitive impairment. Although there is some evidence for its effectiveness in the general older adult population, effect sizes are smaller than for younger people (Gould et al., Reference Gould, Coulson and Howard2012a; Reference Gould, Coulson and Howard2012b). Also, frailer people may find CBT difficult or even detrimental to apply when their problems or negative thoughts may be realistic and valid (Isaacowitz and Seligman, Reference Isaacowitz and Seligman2002).
Home-based problem-solving therapy, delivered by non-mental health specialists, in perhaps as few as six sessions, may be effective in this population with moderate-severe depressive symptoms, at least short-term and should be considered in practice. The effect size is slightly higher for modified PST (1.79 [3.39, 0.20], converting the MD from Figure 3[a] to SMD) than that reported for SSRIs in general older adult populations (SMD 1.2 [0.3–2.1]) (Kok et al., Reference Kok, Nolen and Heeren2012). PST also has a high level of acceptability (Gellis et al., Reference Gellis, McGinty, Horowitz, Bruce and Misener2007; Reference Gellis, McGinty, Tierney, Jordan, Burton and Misener2008; Kiosses et al., Reference Kiosses, Arean, Teri and Alexopoulos2010; Reference Kiosses, Ravdin, Gross, Raue, Kotbi and Alexopoulos2015) and may address the importance placed by older people with complex problems upon feeling a sense of mastery about solving issues and helping a combination of issues that older people with complex problems attribute depression to (Von Faber et al., Reference Von Faber2016). Provision of this alternative therapy in routine care may support increasing the access to mental health support for frail older people. The evidence for collaborative care is more conflicting and insufficient to make recommendations. Bibliotherapy and ‘life review’ (a therapy including the recall, evaluation and integration of life experiences to achieve a positive sense of self in the later stages of life) (Lan et al., Reference Lan, Xiao and Chen2017) lacked sufficient evidence to make recommendations for this group.
Implications for research
PST showed positive short-term effects for reducing depressive symptoms, although it did not show effects upon other outcomes. Large scale RCTs, therefore, need to be powered to assess the effects of PST upon a more comprehensive set of outcomes more relevant to older people, particularly functioning (a key frailty outcome) (Ferrucci et al., Reference Ferrucci2004) and quality of life (Lenze et al., Reference Lenze2016), especially in the long term. Evaluation of hospitalizations, social care use, and cost-effectiveness is also vital to assist commissioning decisions, as home-based services are potentially costly but may deliver long-term cost-savings in health or social care use. Other non-pharmacological interventions for depression that have shown to be effective in the general older adult populations, such as CBT, behavioral activation, and life review (Gould et al., Reference Gould, Coulson and Howard2012a; Reference Gould, Coulson and Howard2012b; Lan et al., Reference Lan, Xiao and Chen2017; Orgeta et al., Reference Orgeta, Brede and Livingston2017), may also offer promise, but could be optimized through working with frail older people to understand how these treatments could be better tailored to their needs and abilities. Book-based CBT had low adherence in this population and exercise interventions do not appear to be effective when people have a medical comorbidity (Schuch et al., Reference Schuch2016), so these may be less useful avenues for investigation. Research into interventions for anxiety (and comorbid anxiety and depression, which negatively impact upon late-life depression treatment outcomes [Tunvirachaisakul et al., Reference Tunvirachaisakul2017]) in this population is also needed, as the evidence base for older adults tends to focus on depression to the detriment of other psychiatric disorders (Dham et al., Reference Dham2017).
Conclusions
Home-based problem-solving therapy may significantly reduce depressive symptoms in older adults with physical comorbidities affecting functioning in the short term. The evidence for collaborative care is mixed in this population, and effects on functioning, quality of life, and service use are currently unknown. Other therapies and therapies for anxiety lack an evidence base in this population and require further investigation.
Conflict of interest declaration
RF was funded by the National Institute of Health Research School for Primary Care Research. This paper presents independent research funded by the National Institute for Health Research School for Primary Care Research (NIHR SPCR). The views expressed are those of the author(s) and not necessarily those of the NIHR, the NHS or the Department of Health.
The other authors declare no conflicts of interest.
Description of authors’ roles
RF and KW designed the review protocol. RF ran the searches, screened references, assessed risk of bias, conducted meta-analysis and drafted the manuscript. YB conducted trials register searches, screened references, assessed risk of bias and provided feedback on manuscript drafts. KW provided feedback throughout the review process, was a third reviewer for resolving any inclusion disagreements and provided feedback on manuscript drafts. All authors have read and approved the final manuscript.
Acknowledgments
We would like to thank all authors who kindly responded to our requests for further data.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1041610218001564.







