High-yielding dairy cows are subjected to several challenges during the transition period, with metabolic stress and inflammatory condition contributing to the increased incidence and severity of diseases (Sordillo et al., Reference Sordillo, Contreras and Aitken2009; Trevisi et al., Reference Trevisi, Amadori, Cogrossi, Razzuoli and Bertoni2012). This period is characterized by 5 critical events: reduction of immune competences; negative energy balance resulting in mobilization of adipose and muscle tissue; hypocalcemia due to demand of the mammary gland for milk synthesis; an overt systemic inflammatory response around calving and oxidative stress due to increased production of pro-oxidant molecules (Trevisi and Minuti, Reference Trevisi and Minuti2018).
Holstein is a breed selected for high production efficiency and in general, is today recognized as the breed with the highest milk production in the world. Selection for high production efficiency has often been associated with decreased longevity, increased culling rate and a sharp decrease in fertility in Holstein cows (Hare et al., Reference Hare, Norman and Wright2006; Miller et al., Reference Miller, Kuhn, Norman and Wright2008; Norman et al., Reference Norman, Wright, Hubbard, Miller and Hutchison2009) together with undesired side effects on animal welfare (Oltenacu and Broom, Reference Oltenacu and Broom2010). A recent study of genome-wide association between longevity traits and single nucleotide polymorphisms identified the presence of two suggestive quantitative trait loci (QTLs) which included genes with biological functions related to fertility, reproductive disorders, heat stress and cow welfare (Steri et al., Reference Steri, Moioli, Catillo, Galli and Buttazzoni2019). Furthermore, increased inbreeding has been associated with fitness problems including enhanced risk of disease occurrence and reduced survival (Sorensen et al., Reference Sørensen, Madsen, Sorensen and Berg2006; McParland et al., Reference McParland, Kearney, Rath and Berry2007). Crossbreeding is a practice that may help to overcome these problems. This breeding strategy in dairy production is used to increase the economic profit by improving health, fertility, longevity or milk components, through the benefits due to the effect of heterosis, which reduces the likelihood of inbreeding depression by increasing heterozygosity. The main contribution of crossbreeding in livestock is the increase of efficiency of the production system due to breeds having their genetic merits in different traits so obtaining benefits of heterosis. Crossbred animals are more robust and economically efficient compared to the parental breeds (Sørensen et al., Reference Sørensen, Norberg, Pedersen and Christensen2008).
Heterosis occurs when unrelated breeds are crossed. Its effect is opposite to the inbreeding depression. In animal breeding this is usually expressed as mid-parent heterosis or the superiority of the F1 cross over the mean performance of the two parents (Falconer, Reference Falconer1960). Deviations from the mid-parent value can be positive or negative but are mostly found to be beneficial.
Crossbreeding between Simmental (dairy type) and Holstein has been performed for a long time, mostly in Europe. Several studies have demonstrated that relative crossbreds had higher reproductive performance and a better ability to restore immune homeostasis afther calving than Holstein cows (Knob et al., Reference Knob, Alessio, Thaler Neto and Mozzaquatro2016, Reference Knob, Scholz, Alessio, Bergamaschi Mendes, Perazzoli, Kappes and Thaler Neto2020; Puppel et al., Reference Puppel, Bogusz, Golebiewski, Nalecz-Tarwacka, Kuczynska, Slosarz, Budzinski, Solarczyk, Kunowska-Slosarz and Przysucha2018; Scatà et al., Reference Scatà, Grandoni, Barile, Catillo and De Matteis2020). Knob et al. (Reference Knob, Alessio, Thaler Neto and Mozzaquatro2016) highlighted improved fertility traits (lower calving interval, higher conception rate and shorter calving to first service interval) and improved longevity in Holstein × Simmental F1 population respect to Holstein purebred. Moreover, Lopreiato and coworkers showed different metabolic adaptation during the transition period and different expression of genes involved in immune adaptation and inflammatory response, between Simmental and Holstein breeds (Lopreiato et al., Reference Lopreiato, Minuti, Trimboli, Britti, Morittu, Piccioli Cappelli, Loor and Trevisi2019, Reference Lopreiato, Minuti, Morittu, Britti, Piccioli Cappelli, Loor and Trevisi2020). Recently, Knob et al. (Reference Knob, Thaler Neto, Schweizer, Weigand, Kappes and Scholz2021) published a study on a combination of metabolic traits and some body condition traits with early performance characteristics during the transition period in a crisscross breeding program of Holstein and Simmental. The research showed that after calving, Simmental and R1-Simmental cows were able to deal with a negative energy balance to a better extent than purebred Holstein and the other crossbred lines.
With this study we wanted to investigate the changes in energy metabolism, oxidative stress and inflammation biomarkers during the peripartal and early lactation periods in Italian Simmental (SI), Italian Holstein (HO) and their cross (CR) in order to evaluate the response of the F1 crossbred population in comparison to the parental breeds. To the best of our knowledge it is the first time that, beside the energy metabolism parameters, oxidative status and inflammatory response in Simmental × Holstein crossbred cows are reported.
Materials and methods
Animal management
Thirty-three cows, from 30 d before the expected calving date until 60 d post actual calving, were included in this study: 8 Italian Simmental (SI), 9 Italian Holstein (HO) and 16 Italian Simmental (sire) × Italian Holstein (dam) crossbred (CR). The animals were housed at the Council for Agricultural Research and Economics (CREA) – Research Centre for Animal Production and Aquaculture. Cows were mixed parity including primiparous, all kept under the same management conditions and fed ad libitum once a day with a total mixed ration based on sorghum silage, hay and concentrates (Table 1). Cows were milked twice a day in a milking parlor with automatic milk recorders and data were acquired and stored by the ALPRO™ herd management system (DeLaval International AB). The Body Condition Score (BCS) was evaluated monthly by a trained operator using a 5-point scale (1 = emaciated, 5 = obese) according to the method described by Edmonson et al. (Reference Edmonson, Lean, Weaver, Farver and Webster1989). Cows were subjected to routine veterinary examinations to exclude animals with clinical signs of diseases such as endometritis, mastitis or metabolic disorders. Only healthy animals were included in the data analysis. The health status was also evaluated trough the inflammatory markers: interleukin-6 (IL-6), haptoglobin (Hp) and serum amyloid A protein (SAA).
Table 1. Ingredient and nutrient composition of dry and lactation diets.
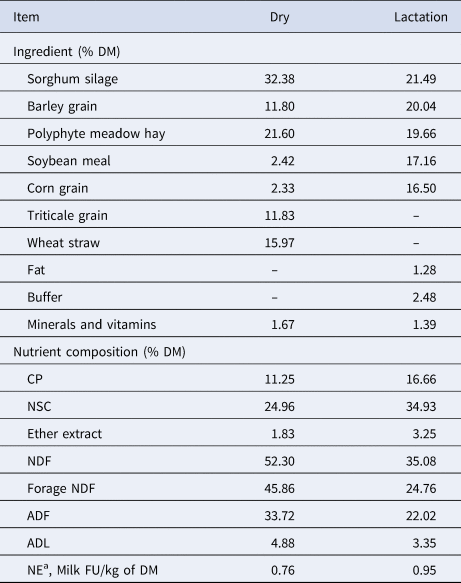
NSC, non-structural carbohydrates.
a The net energy of each feedstuff, expressed as milk FU/kg DM, was determined using the chemical composition and digestibility of the organic matter (INRA, 1988).
Blood sample collection and biomarker analysis
The experimental procedure was carried out in compliance with the European Directive 2010/63/UE and the Italian regulation D. Lgs n. 26/2014 (Health Ministry authorization n 529/2017-PR). Blood samples were collected from the jugular vein into lithium-heparin BD Vacutainer® (Beckton Dickinson, Plymouth, UK) before the morning feeding. Six different time points were chosen: 30 ± 3 and 15 ± 3 d before the expected calving date, calving day or if not possible the day after, 15, 30, and 60 d after calving. Samples were centrifuged at 3000 g for 15 min at 4 °C and plasma collected and divided into aliquots for storage at −20°C until analysis.
Plasma samples were assayed by a clinical chemistry analyzer (ILAB 650, Instrumentation Laboratory, Lexington, MA, USA) using commercial kits for albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, creatine kinase (CK), creatinine, alkaline phosphatase (ALP), glucose, total protein, and urea (all Instrumentation Laboratory spa, Milan, Italy) as well as β-hydroxybutyrate (BHB) and non-esterified fatty acids (NEFA) (FUJIFILM Wako Chemicals Europe GmbH, Germany).
The amount of free oxygen radicals and the concentration of antioxidants in plasma samples were determined using the derived reactive oxygen metabolites (d-ROMs), biological antioxidant potential (BAP), d-ROMs and BAP tests respectively (Diacron, Grosseto, Italy), modified for a microplate procedure using the same proportion of reagents as the standard method. Briefly, for the d-ROM test, the end-point mode was used and a mix solution of 300 μl of R2 (acidic buffer), 3 μl of R1 (chromogenic mixture) and 1.5 μl of plasma sample was incubated for 90 min at 37°C. Finally, the absorbance at 546 nm was registered by the Multiskan™ FC Microplate Photometer (Thermo Fisher Scientific, Waltham, MA, USA). The results were expressed in arbitrary Carratelli Units (U CARR), where 1 (U CARR) is equivalent to the oxidizing power of 0.8 mg H2O2/l. For the BAP test, a mix solution of 300 μl of R1 (chromogenic mixture), 15 μl of R2 (ferric ions solutions, Fe3+) and 3 μl of plasma sample was incubated for 5 min at 37 °C. Then, the absorbance at 505 nm was registered by the same microplate photometer. Results of the test were expressed as μmol of ferric ions reducing antioxidants per litre of sample. The degree of oxidative stress was expressed as an Oxidative Status index (OSi) where d-ROMs/BAP × 100 = OSi (Celi, Reference Celi, Mandelker and Vajdovich2011; Fiore et al., Reference Fiore, Spissu, Sechi and Cocco2019).
IL-6 was determined in plasma samples using a bovine ELISA kit (OKEH03748-Aviva Systems Biology, Corp., San Diego, CA, USA) according to the manufacturer's instruction. Hp and SAA concentrations were determined in plasma samples using the commercial tests PHASE™ RANGE Haptoglobin kit (Tridelta Development Ltd., Ireland) and PHASE™ RANGE Multispecies SAA ELISA kit (Tridelta Development Ltd., Ireland) according to manufacturer's instructions. All the samples were analyzed in duplicate. The O.D. absorbance was read at 450 nm with the same microplate photometer.
Statistical analysis
The statistical analysis of the metabolic, oxidative and inflammatory parameters was carried out by the PROC MIXED procedure (SAS Inst. Inc., Cary, NC, USA, release 9.4) using a linear model that included genetic group, parity and interaction time × genetic group and time × parity as factor:
where y is the observation vector for each trait; M is the total average; B is the breed (3 levels): HO, SI, CR; P is the parity (2 levels): primiparous, multiparous; T is the time of sampling (6 levels): −30, −15, 0, 15, 30, 60 d; error represents random effects of the residuals.
The traits of the milk production at 15, 30 and 60 d after the calving, in correspondence with blood sampling, were analyzed using the same model described above with the fixed effect time of sampling (T) with 3 levels (15, 30 and 60 d). The traits related to the production during lactation, such as 305-d milk yeld, % protein, % fat and the BCS parameter were analyzed using the linear model whit two fixed effect: breed (B) and parity (P).
The statistical significance of all traits and least-squares means were determined using Tukey's test available in the MIXED procedure (LSMEANS/ADJUST = TUKEY), with a probability level of P < 0.05. Tukey's test was used due to unbalanced data in the different groups, as described by Kramer (Reference Kramer1956). In order to verify whether there were any relationships between the productive traits (milk production at 15, 30 and 60 d after the calving) and the metabolic, oxidative and inflammatory parameters, the correlation coefficients were estimated using the PROC CORR procedure of the SAS software.
The estimation of the effect due to heterosis was carried out as the difference between the estimated average values of each single trait of the crossbreed and the half-sum of the estimated average values of the two pure parental breeds:
Results
Milk production over the first two months of lactation is shown in Table 2. Even though no significant differences in daily milk yield were detected at the experimental time points among the three genetic groups, the 305-d milk yield of CR was intermediate between HO and SI (Table 2). For milk components, the fat percentage was significantly higher in SI than in HO (P < 0.05) and the protein percentage was higher in SI than in CR or HO (P < 0.05; P = 0.01, respectively). The average BCS was 3.2, 3.2 and 2.9 for CR, SI and HO groups, respectively. SI and CR had higher BCS values than HO (P < 0.05; P = 0.01).
Table 2. Least-squares means (LSM) and standard errors of estimation (see) of the productive traits

CR, Italian Simmental × Italian Holstein crossbred; SI, Italian Simmental; HO, Italian Holstein; BCS, body condition score
a, b Within the same row indicate significant differences between groups (P < 0.05).
Significant differences were found in the mean values of plasma concentration of metabolites, oxidative status and inflammatory response between the three genetic groups, considering all the sampling times (Table 3). In particular, the CR group showed lower average values of glucose (P = 0.001) and NEFA (P = 0.05) compared to HO group, lower values of urea (P < 0.01) than SI group and an intermediate and signficantly different value of creatinine when compared to HO (P < 0.001). The Hp value was lower in CR (P < 0.001) as well as in HO group (P < 0.001) compared to SI group. The most relevant differences between the three groups were found for the oxidative status: CR cows showed the lowest average value of d-ROMs respect to SI and HO (both P < 0.001). The oxidative status index (OSi) was lower in the CR group than in HO group (P < 0.001).
Table 3. Least-squares means (LSM) and standard errors of estimation (see) of biochemical, oxidative and inflammatory parameters of the three genetic groups

CR, Italian Simmental × Italian Holstein crossbred; SI, Italian Simmental; HO, Italian Holstein; NEFA, non-esterified fatty acid; BHB, β-Hydroxybutyrate; Total bil., total bilirubin; ALT, alanine transaminase; AST, aspartame aminotransferase; ALP, alkaline phosphatase; CK, creatine kinase; d-ROMs, derivates of reactive oxygen metabolites; BAP, Biological Antioxidant Potential; OSi, Oxidative Status index; IL-6, interleukin-6; Hp, haptoglobin; SAA, serum amyloid A.
The differences between the three genetic groups at different time points are shownd in Figs. 1 and 2 and online Supplementary Tables S1–S3. Significantly lower glucose concentrations were found at calving in CR (P < 0.001) compared to HO (Fig. 1a). Interestingly, CR and SI groups showed higher and statistically different creatinine values respect to HO group at −15 d (both P < 0.01), while at calving the only significant difference was between CR vs. HO (P < 0.05) (Fig. 1b). The urea was statisticaly lower in HO than SI group, but only at + 15 d (Fig. 1c). The trend of other metabolic parameters was similar and no significant differences were found at any time points (online Supplementary Table S1).
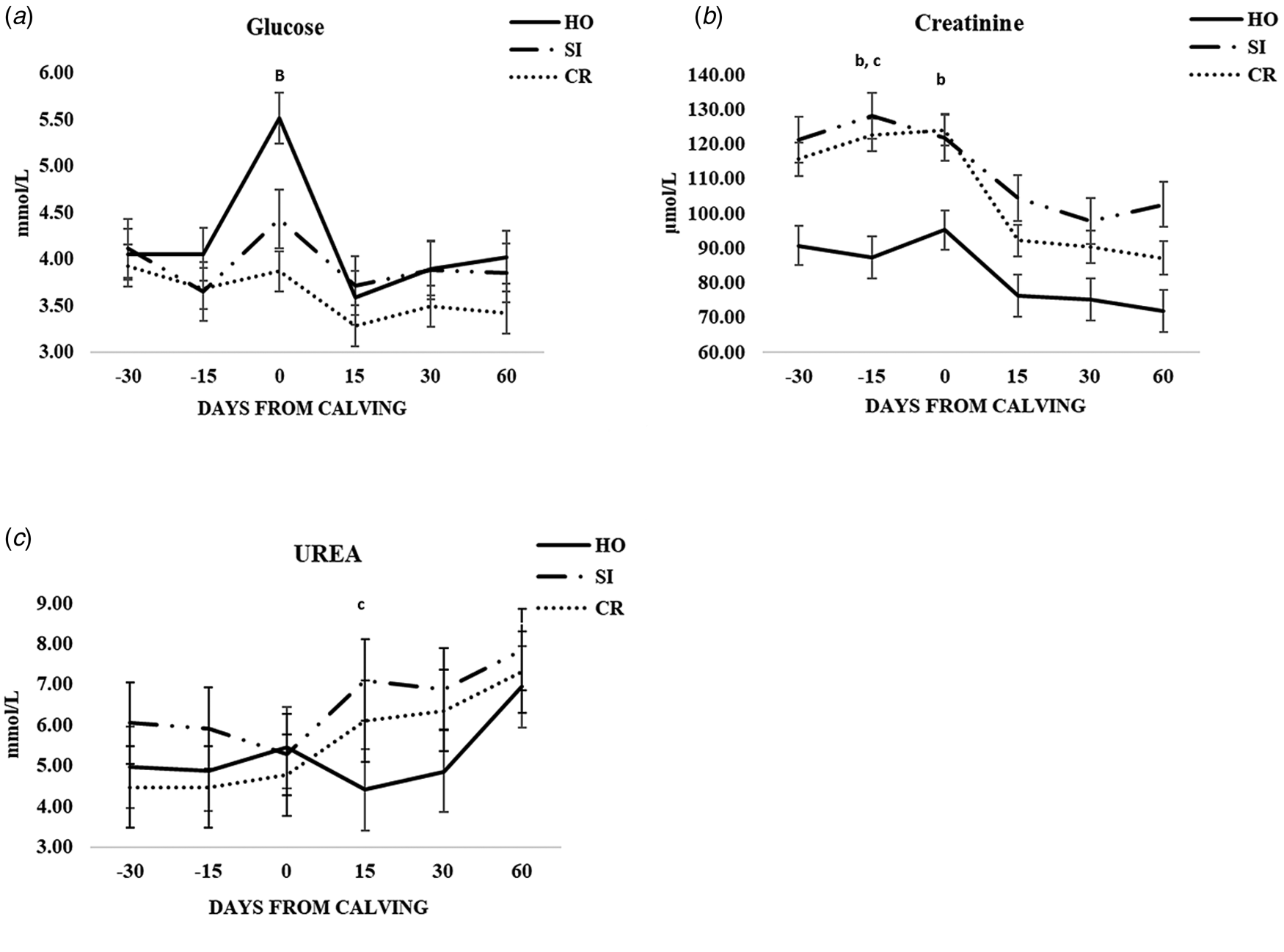
Fig. 1. Patterns of variation of glucose (a), creatinine (b) and urea (c) in the three groups of cows (CR, Italian Simmental × Italian Holstein; SI, Italian Simmental; HO, Italian Holstein), at different days from calving (time point) during the observation period. Significant differences (P < 0.05) are indicated at each time point as: a = CR vs. SI; b = CR vs. HO; c = SI vs. HO. Significant differences (P < 0.001) are indicated at each time point as A = CR vs. SI; B = CR vs. HO; C = SI vs. HO.
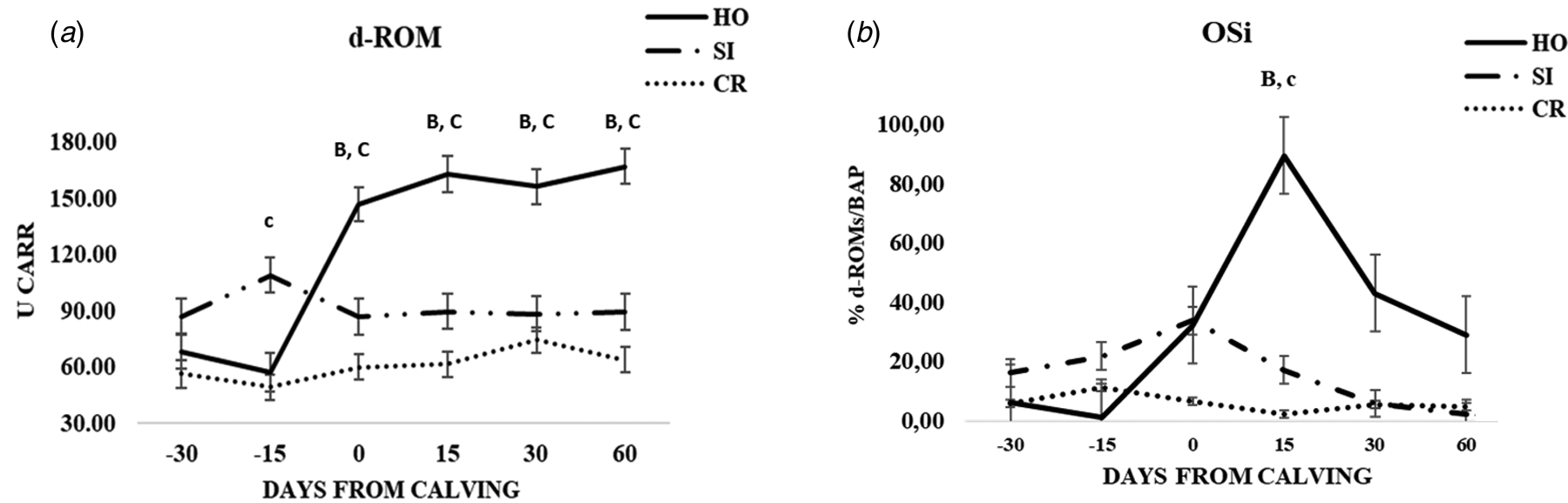
Fig. 2. Patterns of variation of d-ROM (a), OSi (b) in the three groups of cows (CR, Italian Simmental × Italian Holstein; SI, Italian Simmental; HO, Italian Holstein), at different days from calving (time point) during the observation period. Significant differences (P < 0.05) are indicated at each time point as: a = CR vs. SI; b = CR vs. HO; c = SI vs. HO. Significant differences (P < 0.001) are indicated at each time point as A = CR vs. SI; B = CR vs. HO; C = SI vs. HO.
A lower concentration of oxidative stress biomarkers was found in CR and SI cows compared to HO group that showed an increase of d-ROMs at calving (online Supplementary Table S2; Fig. 2a). Significantly lower OSi values were obtained for CR and SI at + 15 d (P < 0.001 and P < 0.01, respectively) (online Supplementary Table S2; Fig. 2b). No meaningful differences were observed for the inflammatory markers IL-6, Hp and SAA at any time point (online Supplementary Table S3).
Online Supplementary Table S4 shows the correlations between biochemical, inflammatory and oxidative parameters and milk yield at 15, 30 and 60 d after calving. Haptoglobin and SAA were negatively correlated with milk yield at 60 d (r = −0.74, r = −0.73, P < 0.001), when cows typically reach peak production. A weaker but significant negative correlation was obtained with total bilirubin in the same lactation period. BHB is positively correlated (r = 0.63) to production in the first lactation period at 15 d.
As for the effect of heterosis, positive percentage was found for ALT (5%), AST (14%), ALP (8%), CK (98%), creatinine (7%), BAP (47%) and SAA (6%). On the other hand, negative percentage was found for glucose (−11%), NEFA (−20%), total bilirubin (−17%), urea (−4%), d-ROMs (−39%), OSi (−75%) and Hp (−17%). Values close to zero were found for total protein (−2%), albumin (1%), BHB (0%) and IL-6 (−2%), (online Supplementary Table S5).
Discussion
The transition period (3 weeks before to 3 weeks after parturition) is notoriously a critical period for lactating cows as it is characterized by physiological and metabolic changes that affect immune function and increase susceptibility to postpartum diseases (Goff and Horst, Reference Goff and Horst1997). High-yielding dairy cows are subjected to many challenges during this period and, in addition, parturition leads to a systemic inflammatory state (Trevisi et al., Reference Trevisi, Amadori, Cogrossi, Razzuoli and Bertoni2012; Bradford et al., Reference Bradford, Yuan, Farney, Mamedova and Carpenter2015). In the first third of the lactation period, high-producing cows enter a state of negative energy balance during which they mobilize body reserves and lose muscle body mass (Weber et al., Reference Weber, Hametner, Tuchscherer, Losand, Kanitz, Otten, Singh, Bruckmaier, Becker, Kanitz and Hammon2013). This phenomenon could be related to the milk production and to some genetic characteristics selected for high milk yield in Holstein cows. As reported by Curone et al. (Reference Curone, Filipe, Cremonesi, Trevisi, Amadori, Pollera, Castiglioni, Turin, Tedde, Vigo, Moroni, Minuti, Bronzo, Addis and Riva2018), Holstein Friesian cows showed a more severe fat mobilization and systemic inflammatory response postpartum in comparison with Rendena cows (a breed adapted to hard climatic and pasture conditions of the Italian alpine mountain range). Furthermore, Petrera et al. (Reference Petrera, Napolitano, Dal Prà and Abeni2015) showed different glucose, NEFA and BHB concentration values between Italian Friesian and Modenese cows (a minor Italian breed cows selected for milk yield in the area of Parmesan cheese) during the peripartum period. These studies indicate a different ability of cattle breeds to cope with metabolic stress around the calving period and suggest that the differences might depend on the different genetic merit for milk production.
Previous studies that compared the performance of Holstein purebred with its respective crossbred with Normande, Montbéliarde, Scandinavian Red or Jersey, demonstrated reduced somatic cell score, enhanced first-service conception rate, increased solids in milk and shorter calving interval in crossbreds (Heins and Hansen, Reference Heins and Hansen2012; Heins et al., Reference Heins, Hansen, Hazel, Seykora, Johnson and Linn2012; Coffey et al., Reference Coffey, Horan, Evans and Berry2016). Moreover, Knob et al. (Reference Knob, Alessio, Thaler Neto and Mozzaquatro2016) highlighted better reproductive performance and higher survival rate in Holstein × Simmental crossbred cows than in Holstein cows.
Few studies have investigated the metabolic profile in crossbred cows. Mendonça et al. (Reference Mendonça, Abade, da Silva E, Litherland, Hansen, Hansen and Chebel2014) found no differences in peripartum metabolic status between Holstein pure breed and Montbéliarde-sired crossbred. Our study has shown significant differences for energy profile between HO and CR cows. We found lower levels of NEFA and glucose (mainly at calving time) in CR and in SI when compared to HO cows. Different level of BHB were found between the two pure breeds in agreement with Lopreiato et al. (Reference Lopreiato, Minuti, Trimboli, Britti, Morittu, Piccioli Cappelli, Loor and Trevisi2019). Recently, Knob et al. (Reference Knob, Thaler Neto, Schweizer, Weigand, Kappes and Scholz2021) compared the crossbred generations following the F1 in a two-breed rotational system with the parental dairy cattle breeds Holstein and Simmental. They showed that Simmental cows had lower NEFA values while BHBA and glucose did not differ among genetic groups. Generally, Simmental and R1-Simmental cows seemed to deal better with a negative energy balance after calving than purebred Holstein and the other crossbred lines.
In the weeks immediately after calving, the energy demand for synthesis and secretion of milk is not fully covered by feed intake, particularly in high-yielding dairy cows, thus resulting in a negative energy balance and mobilization of adipose depots (mainly sub-cutaneous). The development of a negative energy balance increases lipid mobilization as NEFA and the subsequent accumulation of BHB in the blood, as a result of the incomplete NEFA oxidation in the β-oxidation pathway (Wankhade et al., Reference Wankhade, Manimaran, Kumaresan, Jeyakumar, Ramesha, Sejian, Rajendran and Varghese2017). In this context it is noteworthy to highlight that, although similar between SI and CR cows at d + 15 after calving, NEFA were much higher in HO cows, that showed average values near to the threshold of ketosis (around 1.2 mmol/l) for such a milk production (about 26–27 kg/d). Moreover, SI cows showed lower values of BHB leading to the hypothesis that the liver was able to pack NEFA into VLDL (very low-density lipoprotein) to a greater extent and to lower the level of BHB as a consequence of its higher oxidation (Radostits et al., Reference Radostits, Gay, Hinchcliff and Constable2000). This mechanism could also explain the higher milk fat content in early lactation stage, in spite of a lower NEFA mobilization. This speculation could be further supported by the fact that there was no difference in energy requirements after calving, as indirectly shown by NEFA levels, between SI and CR cows, at least in the first 15 d of lactation. Indeed, BHB is the final biomarker of NEFA catabolism in liver and it is not directly linked to lipolysis of fat depots for the energy requirements for milk production, while levels of NEFA, the first product of the lipolysis, are. In order to meet the energy demand for lactation, in the first period after parturition, production of glucose increases through the stimulation of hepatic gluconeogenesis but, despite this mechanism, the greater glucose demand after parturition for milk production leads to a decrease in plasma concentrations, particularly in high yielding cows (Weber et al., Reference Weber, Hametner, Tuchscherer, Losand, Kanitz, Otten, Singh, Bruckmaier, Becker, Kanitz and Hammon2013). In our study the trend of glucose reflected the data reported in literature (Trevisi et al., Reference Trevisi, Amadori, Cogrossi, Razzuoli and Bertoni2012; Knob et al., Reference Knob, Thaler Neto, Schweizer, Weigand, Kappes and Scholz2021) showing an increase just before calving and a decrease in the first weeks of lactation. Differently from Knob et al. (Reference Knob, Thaler Neto, Schweizer, Weigand, Kappes and Scholz2021), who found glucose levels not significantly different among Holstein, Simmental and their crosses, in our study the CR group showed a lower average value. At the time of calving we found a particular increase of glucose in HO with respect to the other breeds. This finding could be related to the higher value of granulocyte, neutrophil and monocyte proportions at calving found in the HO compared to SI and CR in our previous work (Scatà et al., Reference Scatà, Grandoni, Barile, Catillo and De Matteis2020). Indeed, glucose is required by phagocytic cells for proliferation, survival and differentiation, and has been shown to be the preferred metabolic fuel during inflammation for activated PMN, macrophages and lymphocytes (Ingvartsen and Moyen, Reference Ingvartsen and Moyes2013). Furthermore, Holstein cows were shown to display an overt systemic inflammatory response related to the transition period, even without signs of microbial infections and/or otherwise determined pathology (Sordillo et al., Reference Sordillo, Contreras and Aitken2009; Trevisi et al., Reference Trevisi, Amadori, Cogrossi, Razzuoli and Bertoni2012).
The elevated NEFA level in plasma as observed in HO cows has previously been correlated with risk factor for proinflammatory periparturient diseases such as mastitis and metritis (Bernabucci et al., Reference Bernabucci, Ronchi, Lacetera and Nardone2005; Trevisi et al., Reference Trevisi, Amadori, Cogrossi, Razzuoli and Bertoni2012) and has been linked with early lactation diseases and altered immune competence (Erdmann et al., Reference Erdmann, Mohr, Derno, Tuchscherer, Schäff, Börner, Kautzsch, Kuhla, Hammon and Röntgen2018).
In our previous study involving the same groups of cows, we found that CR and SI showed a better ability to restore immune homeostasis after calving with respect to HO. This last group showed altered levels of cellular immunological traits at calving and in early lactation (Scatà et al., Reference Scatà, Grandoni, Barile, Catillo and De Matteis2020). Furthermore, in the same study, after the evaluation of heterosis for cellular immunological traits we identified an improved activation of humoral immune response and a better ability to restore immune homeostasis after calving in SI × HO crossbred cows with respect to parental breeds. In the present study, the F1 derived population seems to follow the Simmental parental breed as shown by heterosis values for the glucose (−11%), NEFA (−20%) and BHB (5.5%). The heterosis related to these parameters could indicate a possible ameliorative effect in F1 group. Based on these results, CR and SI showed lower energy requirement (lower energy waste) and more efficient mobilization of body reserves and oxidation of fatty acids in the liver.
In agreement with Lopreiato et al. (Reference Lopreiato, Minuti, Trimboli, Britti, Morittu, Piccioli Cappelli, Loor and Trevisi2019) we observed higher values of creatinine and urea in SI as well as in CR compared with HO group. The higher urea production in SI and CR cows could be supported by mobilization of muscle protein confirmed by the higher levels of creatinine throughout the whole observation period. Creatinine is an important indicator of body muscle mass and, supporting observations by Pires et al. (Reference Pires, Delavaud, Faulconnier, Pomiès and Chilliard2013) and Osorio et al. (Reference Osorio, Trevisi, Ji, Drackley, Luchini, Bertoni and Loor2014), we found a decrease in concentration around and beyond parturition in all groups. In our experimental population SI and CR cows had higher BCS compared to HO cows, in agreement with Knob et al. (Reference Knob, Alessio, Thaler Neto and Mozzaquatro2016) who found a higher BCS in HO × SI crossbred than HO and similarly to Mendonça et al. (Reference Mendonça, Abade, da Silva E, Litherland, Hansen, Hansen and Chebel2014) who reported lower values in HO than in Montbéliarde-sired crossbred cows.
In contrast to a previous study of Lopreiato et al. (Reference Lopreiato, Minuti, Trimboli, Britti, Morittu, Piccioli Cappelli, Loor and Trevisi2019), all cows used in the present study had access to the same diet. We hypothesized that a greater flux of amino acids from muscle catabolism to the liver, which are in turn catabolised, may occur in SI or CR cows rather than in HO cows. This condition could also explain the better postpartum energy balance in SI and CR cows. However, a different efficiency in the rumen utilization of protein and energy between these two breeds and their crossbred cannot be excluded.
The oxidant capacity of plasma measured with the d-ROMs test was significantly lower in CR group compared to each parental breeds. Changes in d-ROMs in HO cows during the study were in agreement with previous reports that showed an increase in oxidant species after parturition (Bionaz et al., Reference Bionaz, Trevisi, Calamari, Librandi, Ferrari and Bertoni2007; Trevisi et al., Reference Trevisi, Amadori, Bakudila and Bertoni2009; Abuelo et al., Reference Abuelo, Hernández, Benedito and Castillo2013). Furthermore, the oxidative status was assessed using an arbitrary index (OSi) obtained from the ratio between d-ROMs and BAP. Recently, Invernizzi et al. (Reference Invernizzi, Koutsouli, Savoini, Mariani, Rebucci, Baldi and Politis2019) observed significant differences in d-ROMs and OSi parameters across the periparturient period, meaning that they may be considered useful biomarkers for oxidative stress.
Like them, we found increased values of d-ROMs and OSi in HO group at calving. These results indicate that HO group underwent a greater oxidative stress condition from calving to early lactation compared to SI and CR groups and that the increased d-ROMs concentration is not, or not fully, justified by a higher milk production. Furthermore, the absence of differences within the groups in SAA, an acute phase proteins (APP) indirect marker of infections and/or inflammation (Ceciliani et al., Reference Ceciliani, Ceron, Eckersall and Sauerwein2012), suggest that the differences in oxidative markers in HO cows could be linked more to stress related to calving than to subclinical conditions.
The evaluation of d-ROMs and OSi values and the results suggested a better balance between oxidants and antioxidants during the peripartal and early lactation periods for CR and SI and a better reaction to calving stress. Moreover, we found a high positive percentage of heterosis for BAP value (47%) and markedly negative values for OSi (−75%) and d-ROMs (−39%) in F1 derived population toward SI parental breed. In general, these findings shed light on the different metabolic adaptation during peripartum period and early lactation by the three genetic groups.
Haptoglobin, one of the APPs, has been used as a marker of systemic inflammation in transition dairy cows, as it is elevated immediately after parturition (Bionaz et al., Reference Bionaz, Trevisi, Calamari, Librandi, Ferrari and Bertoni2007; Huzzey et al., Reference Huzzey, Duffield, LeBlanc, Veira, Weary and von Keyserlingk2009). Lopreiato et al. (Reference Lopreiato, Minuti, Trimboli, Britti, Morittu, Piccioli Cappelli, Loor and Trevisi2019) found higher concentration of Hp after parturition both in SI and HO cows. We observed higher mean average of Hp in SI compared to HO and CR cows with higher levels at calving. It remains unknown why SI has a higher Hp level than the other two groups. Perhaps this increment could be a specific response of SI cows to the applied managing and feeding conditions that were not tailored for that breed or due to the acute trauma of tissue at calving (Trevisi and Minuti, Reference Trevisi and Minuti2018). Moreover, we found a negative correlation of Hp and SAA with milk yield at 60 d of lactation. Since these two parameters are indicators of an inflammatory state, when their value is lower the cow shows a better health condition which is expressed with a higher production.
In conclusion, our data suggest that CR and SI cows are less sensitive to peripartal and early lactation stresses, although this conclusion needs to be validated in higher numbers of animals. These more resilient animals face more efficiently the negative energy metabolism and the inflammatory and oxidative status giving a better adaptive response. Furthermore, this study showed that the use of crossbreeding programs focusing on Holstein cows bred with Simmental sire, does not significantly impair milk production and produces animals that are more resistant to early lactation stressors.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029921000650
Acknowledgments
This research was funded by the Italian Ministry of Agriculture – MiPAAF – Directorate General for Rural Development (DISR IV) within the Research Project IMMA ‘Identification of immunological markers for disease resistance’- (Grant number: D.M. 16850/7303/2016). The authors gratefully acknowledge: Nicolò Pazzaglia, DVM and Mr. Carlo Cesare Riccioni for blood samples collection and for skillful technical assistance with animals.







