Pregnancy loss associated with miscarriage or stillbirth is common, affecting more than an estimated 1 million women in the USA and 70 000–90 000 women in the UK each year. Reference Wong, Crawford, Gask and Grinyer1 Between 14 and 20% of clinically recognised pregnancies end in miscarriage, Reference Farquharson, Jauniaux and Exalto2 defined as the loss of an intrauterine pregnancy from natural causes before the 24th (in the UK) or 20th (in the USA) week of pregnancy. Stillbirth, or the loss of a pregnancy prior to delivery after the 20th week (USA) or 24th week (UK) of gestation due to natural causes, is estimated to occur in nearly 1 in 200 pregnancies. 3,Reference Martin, Kochanek, Strobino, Guyer and MacDorman4 There is significant psychological/psychiatric morbidity associated with prenatal loss. Women exhibit significantly elevated levels of depression and anxiety in the weeks and months following the loss, compared with samples of pregnant, community or postpartum women. Reference Janssen, Cuisinier, Hoogduin and de Graauw5–Reference Lok, Yip, Lee, Sahota and Chung10 Between 50 and 80% of women who experience prenatal loss become pregnant again. Reference Cordle and Prettyman11–Reference Hughes, Turton and Evans14 That is significant because women with a history of prenatal loss are consistently reported to exhibit significantly elevated rates of anxiety and depressive symptoms during a subsequent pregnancy. Reference Hughes, Turton and Evans14–Reference Armstrong, Hutti and Myers18 What is not clear from existing studies of the impact of prenatal loss is whether or not symptoms associated with a previous loss persist beyond the birth of a subsequent (healthy) child (see also Hughes et al, Reference Hughes, Turton and Evans14 Armstrong et al, Reference Armstrong, Hutti and Myers18 Hunfeld et al Reference Hunfeld, Taselaar-Kloos, Agterberg, Wladimiroff and Passchier19 ). That is, does the birth of a healthy baby resolve the affective symptoms associated with previous loss or do the symptoms persist? Prior research has been unable to answer this important question because of limited follow-up periods, small sample sizes, selection biases, lack of control groups and lack of data on subsequent birth outcomes that may alter postnatal adjustment. The current study capitalised on a large community sample (Avon Longitudinal Study of Parents and Children (ALSPAC)) that had several research design advantages that allowed us to differentiate between two competing hypotheses with substantial clinical and public health significance: a history of prenatal loss would be associated with elevated depressive and anxiety symptoms that would be limited to the prenatal period, i.e. the birth of a healthy child would resolve the impact of the previous loss; and a history of prenatal loss would have a persisting effect on depressive and anxiety symptoms that extended beyond the birth of a subsequent healthy child.
Method
Data
Data for this study were obtained as part of ALSPAC, an ongoing population-based study designed to investigate the effects of a wide range of influences on the health and development of children.
Study population
Pregnant women residing in the Avon area of south-west England who had an estimated date of delivery between 1 April 1991 and 31 December 1992, were invited to participate in the study. It was estimated that 85–90% of the eligible population participated. Reference Golding, Pembrey and Jones20 All data used for this study were collected via postal questionnaires. The study cohort consisted of 14 541 pregnancies and 13 998 children who were still alive at 12 months of age. The study protocol has been published previously, Reference Golding, Pembrey and Jones20 and further details can be found at: www.bris.ac.uk/alspac. Ethical approval for all measures was obtained from the ALSPAC Ethics and Law Committee and from local research ethics committees. The current study focuses on the 13 133 women for whom we had data on previous prenatal loss. The data for the current study are based on six assessments, two during pregnancy at 18 and 32 weeks’ gestation, and four in the postpartum period, at 8 weeks and 8, 21 and 33 months.
Measures
Prenatal loss
At the assessment at 18 weeks’ gestation, respondents were asked to report the number of previous miscarriages and the number of previous stillbirths that they had experienced. Although the terms ‘miscarriage’ and ‘stillbirth’ were not explicitly defined, in the UK a stillbirth certificate is issued where there was a prenatal death after 24 weeks’ gestation; accordingly, respondents who did experience a stillbirth would have had that document to designate formally a stillbirth. We also collected data on the number of previous elective terminations; these were counted separately.
Anxiety
Maternal anxiety at each occasion was measured using the anxiety items from the Crown–Crisp Experiential Index (CCEI), a validated self-rating inventory. Reference Birtchnell, Evans and Kennard21 It has been shown to correlate with the State (0.70) and Trait (0.76) subscales of the Spielberger State–Trait Anxiety Inventory. Reference Spielberger22 There is no established clinical cut-off, and so for categorical analyses we defined a mother as anxious if she scored in the top 15% of the sample.
Depression
Depression was assessed using the Edinburgh Postnatal Depression Scale (EPDS), a 10-item self-report questionnaire that has been extensively used and shown to be valid in and outside the postnatal period. Reference Cox, Holden and Sagovsky23 A cut-off score of ≥12 is recommended to identify cases of probable major depression. Reference Murray and Cox24
Covariates
A series of covariates were chosen because of their known links with depression and/or anxiety or because they were thought a priori to be a possible confound linking prenatal loss and depressive and anxiety symptoms. Specific covariates included maternal age at initial interview, currently living with husband or partner, number of living children, education level, ethnicity and use of tobacco and alcohol during the first 3 months of the pregnancy. Respondents were also asked ‘Have you ever had a severe depression?’ Those who answered ‘yes, in the past not now’ were classified as having a previous depressive episode. Birth weight was used as an indicator of healthy birth outcome; we dichotomised weight into ≤2500 g or >2500 g representing low or normal birth weight. Gestational age at birth was dichotomised into <32 weeks or >32 weeks. A household crowding index was ascertained, which represents the number of residents per room. A high crowding index score is well established as an indicator of low socioeconomic status, a highly stressful situation, and is associated with high morbidity and mortality risks in a range of health outcomes. Reference Melki, Beydoun, Khogali, Tamim and Yunis25
Statistical analysis
Generalised estimating equations (or GEE) is a method used for longitudinal data modelling. This method is semi-parametric and therefore does not require normal distribution assumptions to be met. Given that depression and anxiety can be treated as discrete or continuous data they do not meet normality assumptions for parametric data analysis. The GEE was performed to model the change of depression and anxiety over time. A backward elimination procedure was applied to control covariates and interactions. It should be noted that there were a significant number of missing values for depression and anxiety, especially for the last two visits. Specifically, sample sizes at the six visits were 12 121, 12 096, 11 710, 11 195, 10 259 and 9683. The impact of missing data was characterised by model estimates through two well-established missing data mechanisms: the missing completely at random assumption and the missing at random assumption. Reference Robins, Rotnitzky and Zhao26 The missing completely at random assumption was tested by modelling the missingness as a function of observed responses and baseline covariates using logistic regression. It was found that depression at previous visit was a strong predictor, thus the missing completely at random assumption is inappropriate and weighted generalised estimating equations (WGEE) were used with weights estimated from the logistic model for missing data. Reference Robins, Rotnitzky and Zhao26
Results
Online Table DS1 provides descriptive data on the sample. The majority (n = 10 310, 79%) of women reported no miscarriages. Rates of previous stillbirths were low, with n = 106 (0.8%) reporting one and just three women reporting two prior stillbirths.
The first analysis examined whether stillbirths predicted subsequent depressive and anxiety symptoms more strongly than miscarriage. The non-parametric Wilcoxon rank sum test was applied to each visit to check whether there was any difference in depression and anxiety symptom scores between mothers who experienced a previous miscarriage and mothers who experienced a previous stillbirth. Results indicated that the difference between stillbirth and miscarriage was not significant (P = 0.27). Thus, stillbirth and miscarriage were combined in the analyses below.
Figure 1(a) and (b) present the mean (95% CI) scores of depressive and anxiety symptoms across the assessment period according to the number of previous losses (miscarriages and stillbirths).
Table 1 presents results from the GEE model. Results indicate that, as expected, many of the psychosocial and sociodemographic covariates were associated with depressive and anxiety symptom scores. In addition, there was a significant prediction from the number of prenatal losses for both depressive and anxiety symptom scores. The magnitude of the effect was moderate: for each additional prenatal loss there was a corresponding increase of approximately one-quarter of a standard deviation in mood symptoms. Analyses to test the hypothesis that previous prenatal loss was a stronger predictor for pre- than postnatal assessments was carried out using an interaction between time of assessment and prenatal loss. For neither depressive nor anxiety symptoms was there an interaction between time of assessment and prenatal loss; that is, the association between prenatal loss and depressive and anxiety symptoms was not significantly different across the pre- and postnatal assessments (the interactions are not included in the final models inTable 1).
Table 1 Regression analyses predicting depression and anxiety symptoms from previous prenatal losses and covariates
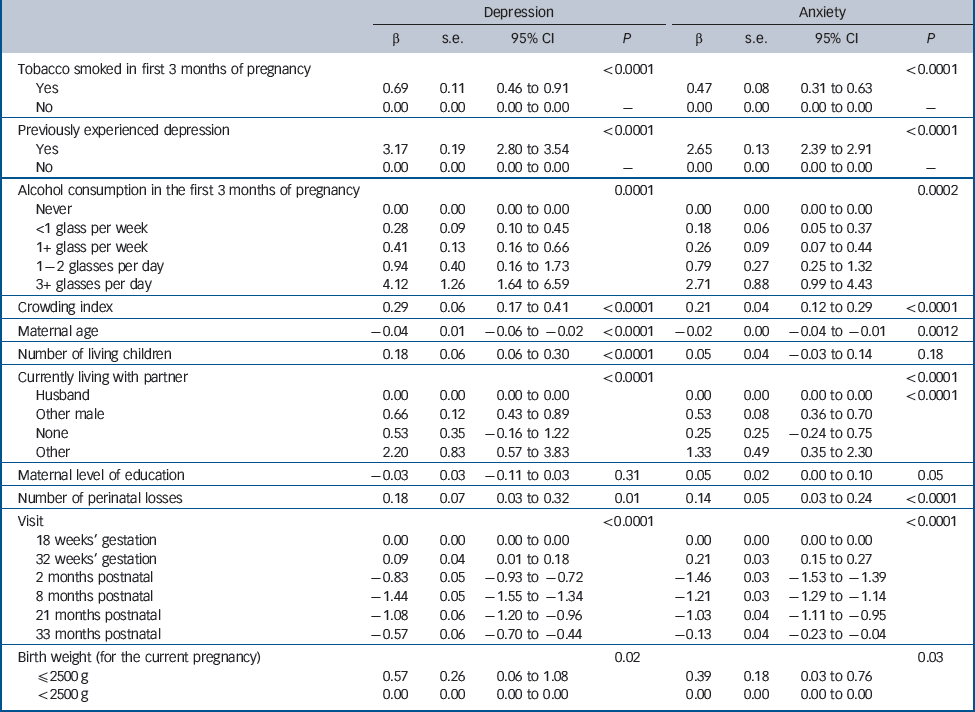
| Depression | Anxiety | |||||||
|---|---|---|---|---|---|---|---|---|
| β | s.e. | 95% CI | P | β | s.e. | 95% CI | P | |
| Tobacco smoked in first 3 months of pregnancy | < 0.0001 | < 0.0001 | ||||||
| Yes | 0.69 | 0.11 | 0.46 to 0.91 | 0.47 | 0.08 | 0.31 to 0.63 | ||
| No | 0.00 | 0.00 | 0.00 to 0.00 | – | 0.00 | 0.00 | 0.00 to 0.00 | – |
| Previously experienced depression | <0.0001 | <0.0001 | ||||||
| Yes | 3.17 | 0.19 | 2.80 to 3.54 | 2.65 | 0.13 | 2.39 to 2.91 | ||
| No | 0.00 | 0.00 | 0.00 to 0.00 | – | 0.00 | 0.00 | 0.00 to 0.00 | – |
| Alcohol consumption in the first 3 months of pregnancy | 0.0001 | 0.0002 | ||||||
| Never | 0.00 | 0.00 | 0.00 to 0.00 | 0.00 | 0.00 | 0.00 to 0.00 | ||
| <1 glass per week | 0.28 | 0.09 | 0.10 to 0.45 | 0.18 | 0.06 | 0.05 to 0.37 | ||
| 1+ glass per week | 0.41 | 0.13 | 0.16 to 0.66 | 0.26 | 0.09 | 0.07 to 0.44 | ||
| 1–2 glasses per day | 0.94 | 0.40 | 0.16 to 1.73 | 0.79 | 0.27 | 0.25 to 1.32 | ||
| 3+ glasses per day | 4.12 | 1.26 | 1.64 to 6.59 | 2.71 | 0.88 | 0.99 to 4.43 | ||
| Crowding index | 0.29 | 0.06 | 0.17 to 0.41 | <0.0001 | 0.21 | 0.04 | 0.12 to 0.29 | <0.0001 |
| Maternal age | – 0.04 | 0.01 | – 0.06 to –0.02 | <0.0001 | – 0.02 | 0.00 | – 0.04 to –0.01 | 0.0012 |
| Number of living children | 0.18 | 0.06 | 0.06 to 0.30 | <0.0001 | 0.05 | 0.04 | – 0.03 to 0.14 | 0.18 |
| Currently living with partner | <0.0001 | <0.0001 | ||||||
| Husband | 0.00 | 0.00 | 0.00 to 0.00 | 0.00 | 0.00 | 0.00 to 0.00 | <0.0001 | |
| Other male | 0.66 | 0.12 | 0.43 to 0.89 | 0.53 | 0.08 | 0.36 to 0.70 | ||
| None | 0.53 | 0.35 | – 0.16 to 1.22 | 0.25 | 0.25 | – 0.24 to 0.75 | ||
| Other | 2.20 | 0.83 | 0.57 to 3.83 | 1.33 | 0.49 | 0.35 to 2.30 | ||
| Maternal level of education | – 0.03 | 0.03 | – 0.11 to 0.03 | 0.31 | 0.05 | 0.02 | 0.00 to 0.10 | 0.05 |
| Number of perinatal losses | 0.18 | 0.07 | 0.03 to 0.32 | 0.01 | 0.14 | 0.05 | 0.03 to 0.24 | <0.0001 |
| Visit | <0.0001 | <0.0001 | ||||||
| 18 weeks’ gestation | 0.00 | 0.00 | 0.00 to 0.00 | 0.00 | 0.00 | 0.00 to 0.00 | ||
| 32 weeks’ gestation | 0.09 | 0.04 | 0.01 to 0.18 | 0.21 | 0.03 | 0.15 to 0.27 | ||
| 2 months postnatal | – 0.83 | 0.05 | – 0.93 to –0.72 | – 1.46 | 0.03 | – 1.53 to –1.39 | ||
| 8 months postnatal | – 1.44 | 0.05 | – 1.55 to –1.34 | – 1.21 | 0.03 | – 1.29 to –1.14 | ||
| 21 months postnatal | – 1.08 | 0.06 | – 1.20 to –0.96 | – 1.03 | 0.04 | – 1.11 to –0.95 | ||
| 33 months postnatal | – 0.57 | 0.06 | – 0.70 to –0.44 | – 0.13 | 0.04 | – 0.23 to –0.04 | ||
| Birth weight (for the current pregnancy) | 0.02 | 0.03 | ||||||
| ≤ 2500 g | 0.57 | 0.26 | 0.06 to 1.08 | 0.39 | 0.18 | 0.03 to 0.76 | ||
| <2500 g | 0.00 | 0.00 | 0.00 to 0.00 | 0.00 | 0.00 | 0.00 to 0.00 | ||
Table 2 shows the percentage and number of women who scored greater than 12 on the EPDS, which is indicative of a case of probable major depression, grouped by the number of losses.
Table 2 Participants with an Edinburgh Postnatal Depression Scale score >12 grouped by number of losses and assessment point
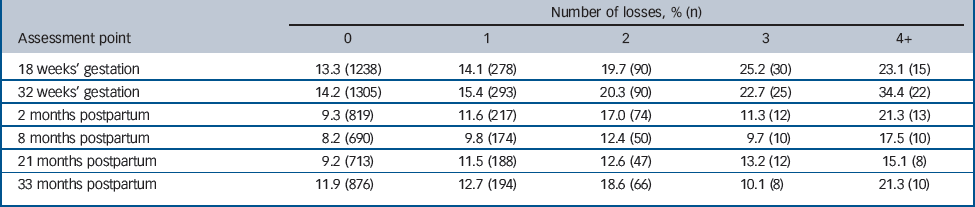
| Number of losses, % (n) | |||||
|---|---|---|---|---|---|
| Assessment point | 0 | 1 | 2 | 3 | 4+ |
| 18 weeks’ gestation | 13.3 (1238) | 14.1 (278) | 19.7 (90) | 25.2 (30) | 23.1 (15) |
| 32 weeks’ gestation | 14.2 (1305) | 15.4 (293) | 20.3 (90) | 22.7 (25) | 34.4 (22) |
| 2 months postpartum | 9.3 (819) | 11.6 (217) | 17.0 (74) | 11.3 (12) | 21.3 (13) |
| 8 months postpartum | 8.2 (690) | 9.8 (174) | 12.4 (50) | 9.7 (10) | 17.5 (10) |
| 21 months postpartum | 9.2 (713) | 11.5 (188) | 12.6 (47) | 13.2 (12) | 15.1 (8) |
| 33 months postpartum | 11.9 (876) | 12.7 (194) | 18.6 (66) | 10.1 (8) | 21.3 (10) |
Supplementary analyses
Analyses were re-run using categorical cut-off scores for depression and anxiety rather than a continuous scale. We found substantively comparable results using this alternate scaling. Given the overlap between depressive and anxious symptoms across the reproductive period, a final set of regression analyses (not reported; details available from the author) was carried out to investigate whether the effect of prenatal loss on anxious symptoms was distinguishable from that for depressive symptoms and vice versa. It was not. The association between prenatal loss and anxious symptoms was confounded by the association between prenatal loss and depressive symptoms and the high degree of covariation between anxious and depressive symptoms (>r = 0.70 at each assessment).

Fig. 1 Mean (95% CI) of (a) depression and (b) anxiety symptom scores throughout the perinatal period according to number of previous prenatal losses (miscarriages + stillbirths).
Numbers of participants are as follows: zero losses, n = 10 250; one, n = 2158; two, n = 515; three, n = 131; and four or more, n = 79.
Discussion
Main findings
We found no evidence that affective symptoms associated with previous prenatal loss resolve with the birth of a healthy child. Rather, previous prenatal loss showed a persisting prediction of depressive and anxiety symptoms well after what would conventionally be defined as the postnatal period. There were changes over time in the perinatal period in depression and anxiety, but these did not vary significantly for women with different histories of prenatal loss. The predictions of anxious and depressive symptoms were similar and inseparable, the result of comparable effect sizes and the high degree of overlap between the two dimensions. Previous studies had documented that women who had experienced miscarriage or stillbirth had significantly higher levels of anxiety and depression in a subsequent pregnancy. Reference Janssen, Cuisinier, Hoogduin and de Graauw5,Reference Thapar and Thapar9,Reference Beutel, Deckardt, Von and Weiner6,Reference Armstrong and Hutti27 The current study extends this work by showing that the impact persists well past the subsequent pregnancy and despite the birth of a healthy child (indexed here by birth weight and gestational age of the subsequent child).
Findings from other studies
Our findings of prolonged and elevated depressive and anxious symptoms in women with a prior prenatal loss nearly 3 years after the birth of a subsequent child contrast with some previous studies. Hughes et al Reference Hughes, Turton and Evans14 found that, compared with controls, women who were pregnant subsequent to a stillbirth had significantly higher levels of depression and state anxiety during pregnancy, but did not differ from controls at 6 and 26 weeks postpartum. Interestingly, the subset of women who conceived less than a year following the stillbirth had significantly higher depression and anxiety scores across all time points than women who conceived after a year. The possibility that time since previous prenatal loss moderates the persisting impact of distress could not be examined in this study because we did not have reliable information on the timing of the previous loss. It may be that a more recent loss is associated with higher levels of affective symptoms that continue in the postpartum period, perhaps as a function of bereavement. Reference Beutel, Deckardt, Von and Weiner6,Reference Hughes, Turton and Evans14
Although Armstrong et al Reference Armstrong, Hutti and Myers18 also reported that depressive and anxious symptoms during pregnancy decreased following the birth of a healthy child, they noted that mothers with higher levels of depression and anxiety in the postpartum period reported increased concerns about their investment in and health concerns about their infant. This raises the important issue of how and whether previous perinatal loss and associated mood symptoms may alter child outcomes. Limited available data suggest that mothers may have more concerns about and greater difficulty managing the needs of a child born after a prenatal loss; Reference Hunfeld, Taselaar-Kloos, Agterberg, Wladimiroff and Passchier19,Reference Theut, Moss, Zaslow, Rabinovich, Levin and Bartko28 also, 12-month-old infants born following prenatal loss were reported to show higher rates of disorganised attachment patterns to their mothers than children born into families without a loss history. Thus, even if there is no persistence of mood disturbance into the postnatal period, there may still be adverse effects of a previous prenatal loss on the parent–child relationship and child outcomes. This possibility requires further attention.
Brockington Reference Brockington31 has argued that pregnancy and childbirth should be seen as a general stressor, like any other life event that can potentially trigger an affective illness episode. Reference Brown, Harris and Hepworth32 The current findings underscore the view that pregnancy and childbirth are major life events, a careful assessment of which may reveal information of value in understanding psychiatric morbidity.
Clinical implications
There are important clinical implications of this work. Currently, prenatal loss is not routinely considered a risk factor for antenatal or postpartum depression in the same way as, for instance, personal or family history of depression, exposure to stressful life events or lack of social support. Our findings suggest that routinely assessing loss history, which could be accomplished briefly and without some of the report bias that accompanies other assessments, would be valuable as a predictor of current and postpartum risk and as a possible marker for intervention. Approximately 15% of women experience clinically significant antenatal depression and anxiety Reference Heron, O'Connor, Evans, Golding and Glover33 and so recognition of and effective treatment for perinatal mood disturbance are of the utmost importance. Both prenatal depression and anxiety are among the biggest predictors of postpartum depression, Reference Robertson, Grace, Wallington and Stewart34,Reference O'Hara and Swain35 which in turn has deleterious effects on maternal–child attachment, child behaviour, and cognitive and neuroendocrine outcomes that persist into adolescence. Reference Halligan, Herbert, Goodyer and Murray36–Reference O'Connor, Heron, Golding, Beveridge and Glover40 Given the adverse outcomes of persistent maternal depression on both child and family outcomes, early recognition of symptoms can lead to preventive interventions to reduce the burden of illness, provide coping strategies to reduce anxiety and depression and promote healthy adjustment of the mother, family and child.
Strengths and limitations
Strengths of the study include the large community sample and detailed and repeated assessments of depressive and anxiety symptoms in the prenatal and postnatal period; the follow-up well past the postnatal period is a particular novelty in this area of study. There are, however, limitations. Participants were asked about the number of miscarriages and stillbirths experienced retrospectively, which could be subject to recall bias. However, the objective nature of the event provides some protection against this possibility, and the persistence of effect years after the enquiry about perinatal loss makes a simple recall bias account unlikely. Self-reports of prenatal loss may be underestimated insofar as mothers are often unaware of spontaneous early miscarriages. Reference Martin, Kochanek, Strobino, Guyer and MacDorman4 Accordingly, the current study is able to assess the psychological impact of prenatal loss, but is not positioned to examine biological hypotheses that there may be risk underlying both prenatal loss and the experience of depressive and anxiety symptoms. Finally, as noted, we were unable to assess the impact of time since loss as a potential predictor of postpartum mood.
Funding
The UK Medical Research Council, the Wellcome Trust and the University of Bristol currently provide core support for ALSPAC. This particular project was funded in part by National Institutes of Health (NIH) grants and .
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.






eLetters
No eLetters have been published for this article.