INTRODUCTION
Blastocystis is a common, single-celled, enteric endosymbiont of man and animals [Reference Stenzel and Boreham1–Reference Parkar5]. Often, Blastocystis is the only potential disease-causing agent found in faecal specimens from patients with diarrhoea or other gastrointestinal (GI) symptoms; however, Blastocystis is also common in asymptomatic individuals [Reference Böhm-Gloning, Knobloch and Walderich6–Reference Özyurt11]. For eradication of Blastocystis infections, metronidazole is considered the first-line therapy [Reference Stenzel and Boreham1, Reference Tan12].
Based on molecular analysis of the small subunit (SSU) rRNA gene, Blastocystis isolates from man, mammals and birds can be assigned to one of at least ten subtypes [Reference Stensvold2, Reference Stensvold13], the genetic distance between some of which is >7% [Reference Clark14, Reference Arisue, Hashimoto and Yoshikawa15], which is more than that seen between homologous genes of pathogenic and non-pathogenic species of Entamoeba [Reference Clark14].
So far, studies seeking to unveil subtype-specific pathogenicity have produced conflicting conclusions [Reference Böhm-Gloning, Knobloch and Walderich6–Reference Özyurt11, Reference Stensvold16]. However, such studies have not consistently included testing for other enteropathogens, e.g. Dientamoeba fragilis which is often a co-infection with Blastocystis [Reference Windsor17, Reference Stensvold18]. Moreover, the identification of Blastocystis carriers and non-carriers has been unconvincing due to the use of insensitive diagnostic methods [Reference Stensvold19].
Taamasri et al. [Reference Taamasri20] and Li et al. [Reference Li21] investigated risk factors associated with Blastocystis carriage; however, neither study provided data on the health status of the study population.
Aims of the present investigation included (i) molecular characterization of Blastocystis isolates from Danish symptomatic and asymptomatic individuals, (ii) identification of potential risk factors for infections due to Blastocystis, and (iii) description of symptoms and identification of potential differences in pathogenicity in the distinct subtypes.
MATERIALS AND METHODS
Study types, study populations, specimen collection and parasitological examination
Two independent studies were included in the investigation. Both studies involved the collection of stool samples for the testing of pathogens and administration of a structured questionnaire to capture demographic and clinical data, as well as information on possible risk factors for GI pathogens.
NORMAT study
The NORMAT study was initiated in 2002 as part of DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme) to monitor antimicrobial drug resistance in commensal bacteria obtained from volunteers in the general population without acute gastroenteritis. Participants were identified through the Danish Civil Registry, a continuously updated registry of all residents in Denmark. A selection algorithm was developed to generate a sample of residents, representative of the age and gender distribution of the Danish population which took into account the differential participation rates of various demographic groups. Individuals who consented to participate were mailed a questionnaire and a faecal sampling test tube. Faecal samples were analysed for Salmonella, Campylobacter, Yersinia enterocolitica, Shigella, Vibrio, Aeromonas, Plesiomonas shigelloides, Clostridium difficile and diarrhoeagenic Escherichia coli using previously published methods including multiplex PCR [Reference Persson22]. Specimens were processed by the formol ethyl acetate concentration technique (FECT) and examined for parasites. Faecal concentrates were also stained by the modified Ziehl–Neelsen technique and evaluated for oocysts of sporozoa. From each fresh specimen about 200 mg was submitted to DNA extraction. Samples analysed in the present study were collected until the end of 2005.
Follow-up (FU) study
The FU study was a follow-up of patients, referred by general practitioners (GPs), diagnosed with Blastocystis between January and October 2005 by the use of FECT. The patients were contacted 3–6 weeks following diagnosis, after informed consent from their respective GPs. Patients were asked to complete a questionnaire, which was different from the questionnaire used in the NORMAT study, and to submit stool samples for follow-up investigation. Stools were cultured and examined for Blastocystis after 48–72 h as described previously [Reference Stensvold23]. The remainder of the specimen was submitted for DNA extraction, stool concentration and microscopy for parasites as above.
DNA extraction, Blastocystis PCR, (pyro)sequencing and subtype identification
DNA was extracted from faecal samples as described previously [Reference Stensvold23]. All study individuals were screened for Blastocystis by PCR. Three Blastocystis PCR protocols were applied to detect and characterize patient isolates: Since the nested PCR protocol described in previous papers [Reference Parkar5, Reference Stensvold24], based on the RD3/RD5 primers [Reference Clark14] and the F1/R1 primers [Reference Böhm-Gloning, Knobloch and Walderich6], was insensitive regarding detection of Blastocystis sp. ST3, patients' samples were also analysed by the PCR method described previously [Reference Stensvold23]. Dideoxy sequencing was as described by Stensvold et al. [Reference Stensvold24]. Finally, 48 samples (15 NORMAT samples and 33 FU samples) were submitted to PCR and pyrosequencing as described previously [Reference Stensvold24]. Table 1 displays the primers used in the study for PCR amplification of Blastocystis-specific DNA.
Table 1. Primers targeting the small subunit rRNA gene of Blastocystis used in the study

Blastocystis sp. subtype identification
Sequence chromatograms and pyrograms were manually interpreted, nucleotide sequences edited, aligned and phylogenetically analysed as described previously [Reference Stensvold24] to identify subtypes. Blastocystis sp. subtype terminology according to Stensvold et al. [Reference Stensvold13] was used.
PCR for D. fragilis
Faecal, genomic DNA from all study individuals was also screened for D. fragilis by PCR using the primers D. fragilisF 5′-CGGAGGTGGTAATGACCAGTTAT-3′ and D. fragilisR 5′-TTGCAGAGCTGGAATTACCG-3′, with standard PCR reagents and conditions [Reference Stensvold25]. Internal process controls were incorporated to identify cases of inhibition.
Questionnaires – data collection
The NORMAT questionnaire covered information on gender, age and employment, travel in the 3 months before diagnosis, medications, GI diseases and symptoms during the most recent month, other symptoms, food consumption in the month before diagnosis, and contact to animals 7 days prior to diagnosis. Participants were asked to complete the questionnaire as soon as possible after collecting the faecal sample. Upon receipt of the sample in the laboratory, investigators contacted participants for a telephone interview based on their written responses.
Individuals in the FU study were invited to complete a questionnaire which included information on demographic factors, travel activity, regular contact with animals, dietary preferences, symptoms at the time of diagnosis and at follow-up, chronic conditions, hospital admission and chemotherapeutic treatment.
For both studies, individuals were excluded from the study if a potential or known enteric pathogen other than Blastocystis or D. fragilis was isolated from the sample.
Statistical analysis
Questionnaire data from both studies were entered into Excel spread sheets and imported into the Stata 9.0/SE software program (Stata Corporation, USA) using StatTransfer 7.0 (Circle Systems, USA).
NORMAT study
As the dataset consisted of an unselected group from the general population with individuals both positive and negative for Blastocystis and D. fragilis, it was analysed retrospectively as a cohort study. Separate analyses were undertaken for Blastocystis and D. fragilis. Relative risks (RR) of infection associated with potential risk factors, and 95% confidence intervals (CI) were calculated using Fisher's exact test. In addition, the RR of various outcomes (symptoms and conditions) potentially associated with infection and 95% CI were calculated using Fisher's exact test.
Multivariable logistic regression was used to model potential risk factors for Blastocystis and D. fragilis selecting variables based on a P value of <0·2 from the univariate analysis. Stepwise exclusion was used in the reduction of the model using the likelihood ratio test.
FU study
As all individuals in the dataset were positive for Blastocystis, we examined for association between risk factors or symptoms and distinct subtypes by Fisher's exact test. Multivariable analysis included variables with a P value <0·2 and possible confounders where relevant. A non-parametric test, Kruskal–Wallis one-way ANOVA, was used to examine the difference in age in the subtypes.
Ethical considerations
The Scientific Ethics Committee for the Copenhagen and Frederiksberg municipalities approved the protocols for the NORMAT study (KF 01-006/02) and the FU study (KF 01-188/04). Written consent was obtained from all participants who were ensured confidentiality.
RESULTS
NORMAT study
Microbiological and demographic data
The study comprised 134 study individuals. Seven individuals were excluded due to the presence of Clostridium difficile, enteroaggregative- or verocytotoxin-producing Escherichia coli and one was excluded due to Giardia duodenalis infection, which left 126 individuals in the dataset. By PCR, 24 (19%) individuals were shown to host Blastocystis, 14 (58%) of whom had been negative for Blastocystis by microscopy of faecal concentrates.
Co-infection with D. fragilis was seen in four cases, and in total, 16 (13%) persons had D. fragilis. Ninety individuals were negative for all pathogens tested for. The median ages of solely Blastocystis- and Dientamoeba-infected individuals were 42 years (range 4–76 years) and 41 years (range 2–71 years), respectively. The median age of pathogen-free individuals was 46 years (range 1–81 years). In total, 16 individuals, four of whom had Blastocystis, one D. fragilis, and one dual infection, reported having experienced GI symptoms in the month before stool submission. The subtypes of Blastocystis cases with and without GI symptoms are given in Table 2. Mixed Blastocystis subtype infection (MSI) was seen in three individuals. The most common subtypes (ST) were ST3 and ST4, isolated from 75% of the persons, most of whom were asymptomatic (78%) (Table 2).
Table 2. Subtypes of Blastocystis cases from the NORMAT group with and without a history of gastrointestinal (GI) symptoms
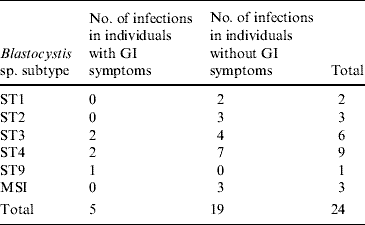
MSI, Mixed subtype infections: ST2 and ST3, ST2 and ST4, ST2 and ST4 and ST6.
Risk analysis of separate variables
When comparing the 24 Blastocystis-positive individuals to 102 individuals negative for Blastocystis, a significant association was seen between irritable bowel syndrome (IBS) and Blastocystis infection (Table 3). However, due to the small number of Blastocystis-positive individuals (n=5), IBS could not be correlated to any particular subtype. Blastocystis carriage was not associated with reports on GI symptoms (Table 3), and neither Blastocystis nor D. fragilis infections were associated with chronic diseases such as gastric ulcer (n=0), enteric cancer (n=0), intestinal radiation damage (n=0), inflammatory bowel disease (n=0), Crohn's disease (n=0), other chronic intestinal inflammation (n=0) or other enteric disease (n=1).
Table 3. Univariate analysis of Blastocystis infection, gastrointestinal (GI) symptoms and irritable bowel syndrome (IBS) in the NORMAT group
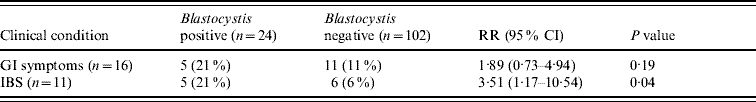
RR, Relative risk; CI, confidence interval.
Fifty-five persons were asked to provide information on conditions considered to be sequelae of infection, and joint pain was associated with Blastocystis infection (RR 3·20, 95% CI 1·03–9·97, P=0·07). All Blastocystis-infected individuals complaining of joint pains (n=4) reported pain in multiple joints, each describing pain in the knees.
Contact to pigs or poultry was strongly associated with Blastocystis infection (Table 4). Three Blastocystis-positive individuals reporting contact with pigs hosted ST2 and ST3 (one case had a mixed ST2/3 infection), and those five reporting contact with poultry had ST1, ST2, ST3 and ST4 (one case had ST2 and ST3). Drinking unpasteurized milk (RR 2·11, 95% CI 0·79–5·59, P=0·18) and contact with rabbits (Table 4) had P values <0·2. Due to co-linearity and small numbers, a multivariable logistic regression model could not be built.
Table 4. Univariate analysis of Blastocystis infection and animal contact 7 days before diagnosis in the NORMAT group
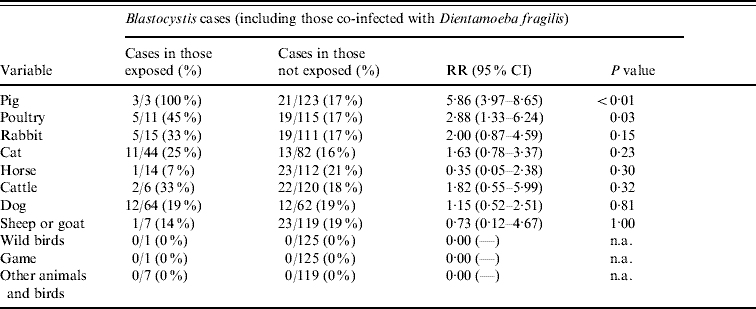
RR, Relative risk; CI, confidence interval; n.a., not applicable.
Travel 3 months prior to diagnosis was found not to be a risk factor for Blastocystis infection in the univariate analysis (RR 1·00, 95% CI 0·47–2·15, P=1·00).
Fifteen individuals reported having rabbits as pets, and five of these were positive for D. fragilis (data not shown). Contact with poultry and game had P values <0·2 and were therefore also included in a logistic regression model; contact with rabbits remained as the only independent variable (RR 3·60, 95% CI 1·42–9·10, P=0·02).
FU study
Microbiological and demographic data
The study included 111 patients. The average number of stool specimens submitted per patient for follow-up examination was 2·8; no patient submitted more than three stool samples. A total of 93 (83·8%) patients were Blastocystis-positive at the time of follow-up by PCR. The remaining 18 were negative by microscopy, culture and PCR and were therefore excluded as was one patient positive for Cryptosporidium; this left 92 cases in the dataset. Fifty-six patients had pure Blastocystis infections, whereas 32 (34·8%) were co-infected with D. fragilis. In four cases, D. fragilis PCR analysis was inconclusive. The subtype distribution of Blastocystis isolates related to symptoms is listed in Table 5. Overall, taking single and MSI together, ST3 was the most common subtype detected in 35 (38%) of the patients, followed by ST2 and ST1 in 33 (36%) and 29 (32%), respectively. Blastocystis sp. subtype was not associated with D. fragilis co-infection (P=0·26, Fisher's exact test). Age was significantly associated with discrete subtypes (P=0·02, Kruskal–Wallis test), those having ST3, ST4 and ST7 being older (median ages 60, 54 and 46 years, respectively) compared to ST1 (27 years).
Table 5. Blastocystis sp. subtype distribution related to symptoms in the Follow-up group (n=92)
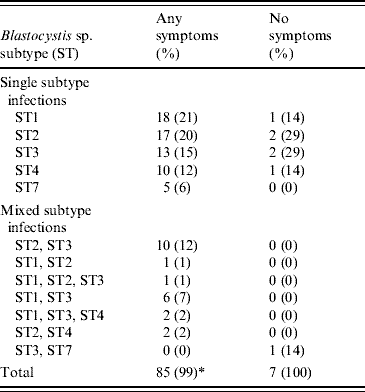
* Percentages only add up to 99% due to rounded figures.
Analysis of Blastocystis infection outcomes
Reported symptoms of FU study individuals are listed in Table 6, with diarrhoea being the most common symptom (70%). At follow-up, 74% still had symptoms. Eighteen (20%) reported having one or more chronic disease(s): One had Crohn's disease, one had diverticulosis, seven had IBS, one had celiac disease, five suffered from allergic diseases, three had gastric ulcer, one had urticaria and four reported of arthritis. The subtype distribution of Blastocystis in IBS patients was independent of symptoms of IBS (P=0·97, Fisher's exact test) and patients with chronic diseases did not harbour specific subtypes (data not shown).
Table 6. Reported gastrointestinal (GI) symptoms in Blastocystis cases with (+) or without (−) Dientamoeba fragilis (DF) in the Follow-up group

* Diarrhoea, nausea/vomiting or abdominal pain.
The most common reported symptoms lasting for ⩾1 month were diarrhoea (25/92, 27%) and abdominal pain (21/92, 23%). ST1 or ST2 were hosted by >50% of patients with long-term diarrhoea (n=14) or abdominal pain (n=11). Of six patients infected with ST7, five (83%) complained of multiple GI symptoms, all describing diarrhoea and abdominal pain.
Reports on bloating at follow-up were associated with subtype (P=0·02, Fisher's exact test), with most case-patients having ST2 (n=12, P<0·01). Seven patients reported blood in stool, four of whom had MSI (P=0·06, Fisher's exact test).
A total of 68 (74%) of the patients had been travelling in the year before diagnosis, which left very few cases for an analysis of risk factors in Denmark. Analysis of all cases, irrespective of whether they travelled or not, could not establish any significant risk factor associated with a particular subtype (data not shown).
Of those with any symptoms (n=85), 40 (47·0%) received medical treatment because of their symptoms. Of those treated with standard drugs 23/31 continued to have symptoms or experienced the return of symptoms after treatment. A total of 17 took metronidazole treatment as prescribed, 10 (59%) of whom reported reoccurrence of symptoms at the time of follow-up.
DISCUSSION
The present investigation is among the most comprehensive with regard to subtype analysis of Blastocystis from human hosts, and the first to generate data from risk-factor analysis of Blastocystis in symptomatic and asymptomatic individuals. Moreover, it is the first study to identify potential risk factors for D. fragilis infection.
The subtype distribution detected in the studies reflects the distribution reported in studies from Eurasian countries [Reference Özyurt11], the most frequent subtypes found in humans being ST1–ST4. Various studies identified ST3 as the most common subtype [Reference Böhm-Gloning, Knobloch and Walderich6, Reference Özyurt11, Reference Stensvold19, Reference Li21, Reference Stensvold23, Reference Wong26] accounting for up to 75·9% and 70·5% of subtypes detected in Turkey [Reference Özyurt11] and China [Reference Li21], respectively.
Whereas ST1 was rare in NORMAT individuals, it was present in 32% of the patients in the FU study. Conversely, the share of single subtype infections due to ST3 and ST4 was much larger (63%) in the NORMAT group than in the FU group (28%). ST7 isolates were found only in FU individuals. These findings may be central in the understanding of the transmission and relative pathogenicity of separate subtypes and partly reinforce trends from other studies in which ST1 has been identified as ‘pathogenic’ and ST3 ‘non-pathogenic’ [Reference Kaneda8, Reference Yan10].
ST5–ST9, occurring infrequently in humans, could be mainly zoonotically transmitted [Reference Stensvold2]. In the present study, ST7 and ST9 isolates were seen in seven of the individuals, six (86%) of whom were symptomatic. It should be investigated to what extent such potentially zoonotic subtypes are correlated with disease, and clearly, more studies should aim at clarifying issues related to transmission routes and host specificity.
MSI were more prevalent in the FU individuals (26% vs. 12% in the NORMAT group) and most patients reporting blood in stool had MSI. Therefore, the data might indicate that MSI are more prevalent in individuals seeking medical advice due to GI symptoms, and this should be explored in future studies. Taking both studies together, the proportion of MSI was remarkably high compared to previous reports, but this may be due to the fact that 2–3 PCR assays were used in concert to characterize each isolate, and preferential amplification of discrete subtypes by different primers has previously been accounted for [Reference Stensvold24, Reference Wong26]. Hitherto, MSI has been detected in up to 14·3% of analysed cases [Reference Yan10]. A high prevalence of MSI could indicate that super-infection occurs frequently and/or that infections persist for a long time if left untreated.
The NORMAT study saw a strong association between IBS and Blastocystis infection. IBS could result in an increased susceptibility of Blastocystis, or a tendency for longer carriage. It has also been speculated whether Blastocystis could be (partly) responsible for the development of IBS [Reference Hussain27–Reference Yakoob30], and it should be investigated whether certain subtypes of Blastocystis could be linked to IBS. It is suggested, that the focus on resolving differential diagnostic aspects of IBS is increased, and therefore patients suspected of IBS should be examined for Blastocystis. Moreover, future studies should include large-scale molecular analyses of Blastocystis isolates from IBS patients and controls.
Reports on joint pain were associated with Blastocystis infection. A few studies have reported Blastocystis-related reactive arthritis [Reference Lee31–Reference Krüger, Kamilli and Schattenkirchner33], suggesting that also this topic requires further research. In the NORMAT study, travel was found not to be a risk factor for Blastocystis infection, thus endorsing previous data indicating that Blastocystis infection is of endemic occurrence in Denmark [Reference Stensvold18].
A recent study [Reference Li21] showed that pig ownership was a risk factor for Blastocystis infection. Similarly, in the NORMAT study, risk factors included contact with pigs or poultry within 7 days prior to stool submission. This is not surprising considering accumulating data from molecular studies of Blastocystis from potential reservoir hosts [Reference Noël3–Reference Parkar5, Reference Abe, Wu and Yoshikawa34–Reference Yan37]. No particular subtype was associated with pig or poultry contact, and individuals reporting such contact were shown to host ST1, ST2, ST3 and/or ST4, which are the subtypes usually isolated from humans. However, pigs have been shown to host ST1, ST2, ST3 and ST5 (Stensvold et al., unpublished observations). Contact with rabbits was a risk factor for D. fragilis infection. To date there have been no reports on D. fragilis in animals, except for pigs [Reference Crotti38] and gorillas [Reference Stark39].
The overall prevalence of Blastocystis and D. fragilis in the NORMAT group was surprisingly high, almost comparable to the prevalence in symptomatic individuals reported in a previous study [Reference Stensvold18], which might be explained by the fact that the response rate in the NORMAT study was little more than 10%. This could indicate that individuals particularly predisposed for activities related to this study participated, e.g. persons with suspected, current or previous (intestinal) disease.
In the FU study, 92 (84%) of the patients were still positive for Blastocystis at the time of follow-up. Unless these patients resolved infection spontaneously or by treatment and then became re-infected before follow-up, this suggests that Blastocystis infections typically persist for at least several weeks.
Although considered the drug of choice for eradication of Blastocystis, there have been several reports on the failure of metronidazole treatment [Reference Stensvold16, Reference Tungtrongchitr29, Reference Nigro40]. In the FU study, 59% of patients taking metronidazole treatment as prescribed by their GP experienced a recurrence of symptoms, and all patients were still positive for Blastocystis at follow-up. It is possible that parasite load is reduced during metronidazole treatment below the detection limit of microscopy methods [Reference Stensvold16]. While these data are strongly indicative of limited efficacy of metronidazole treatment, randomized controlled treatment studies are needed to further elucidate this.
ACKNOWLEDGEMENTS
The authors thank Dr Andrew Thompson and his group at Murdoch University, Murdoch, Western Australia for invaluable help and inspiration. Thanks are also due to Lis Lykke Wassmann and her colleagues at the Laboratory of Parasitology, Statens Serum Institut, Copenhagen, for excellent technical assistance. Part of the work was funded by the University of Copenhagen, Denmark.
DECLARATION OF INTEREST
None.








