Introduction
Uric acid (UA) is one of the most important circulating antioxidants. Although high UA levels might reflect an inherent response to oxidative stress, and serve as a protective mechanism to combat the generation of free radicals, UA might also function as a pro-oxidant, as the reaction of UA with oxidants may also produce other free radicals that might propagate a radical chain reaction and oxidative damage to cells.Reference Sautin and Johnson1 High UA levels are associated with various systemic disorders, including cardiovascular disease, hypertension, dyslipidemia, obesity, impaired glucose metabolism, and metabolic syndrome.Reference Wu, Gladden, Ahmed, Ahmed and Filippatos2 High levels of UA are also associated with peripheral neuropathies,Reference Abraham, Breiner and Barnett3 including diabetic sensorimotor polyneuropathy,Reference Papanas, Katsiki and Papatheodorou4, Reference Lin, Xu, Zhao, Luo and Pan5 and chronic inflammatory demyelinating polyneuropathy (CIDP).Reference Abraham, Albulaihe and Alabdali6 Furthermore, uric acid levels have been found to correlate with both clinical and electrophysiological severity of diabetic sensorimotor polyneuropathy, mainly with sensory functions.Reference Abraham, Breiner and Barnett7 In contrast, low UA levels have been found in various other neurologic disorders, such as amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis.Reference Abraham and Drory8 In this study, we aimed to determine whether higher UA levels are associated negatively with nerve function in healthy subjects. As high UA levels are found in various neuropathiesReference Abraham, Breiner and Barnett3 and show negative correlation with nerve function in diabetic polyneuropathy,Reference Abraham, Breiner and Barnett7 we hypothesized that a similar negative correlation will be found in healthy subjects, although to a lesser degree, and without clinical nerve function impairment.
Materials and Methods
Healthy subjects included controls recruited prospectively as part of two studies, the first aimed to identify the concurrent and predictive validity of corneal confocal microscopy to detect and predict diabetic sensorimotor polyneuropathy, and included 72 controls,Reference Wu, Ahmed and Bril9 while the second was part of the Canadian Study of Longevity in Type 1 DM, and included 75 controls.Reference Lovshin, Boulet and Lytvyn10 The Research Ethics Board of the University Health Network approved the study protocol and all patients provided informed consent.
Out of 72 controls in the first cohort, 19 were missing UA and 1 had HbA1c >6.4%, and these subjects were therefore excluded. All controls in the second cohort had UA measured; one had high HbA1c and was therefore excluded.
We extracted demographic data, body mass index (BMI), blood pressure, Toronto Clinical Neuropathy Score (TCNS), electrophysiological findings, vibration perception thresholds (VPT), and laboratory test results including UA, hemoglobin A1c (HbA1c), estimated glomerular filtration rate (eGFR), and lipid levels.
The TCNS is a valid and reliable scale for diagnosing and staging of diabetic sensorimotor polyneuropathy, as well as for a wide spectrum of other polyneuropathies. The scale incorporates sensory and motor symptoms, as well as lower limb sensory and reflex findings. The score ranges from a minimum of 0 to a maximum of 19 points, and a score of 5 or below indicates no or minimal neuropathy.Reference Abraham, Barnett, Katzberg, Lovblom, Perkins and Bril11 Nerve conduction studies (NCS) were performed using the Sierra Wave instrument (Cadwell Laboratories Inc., Kennewick, WA, USA) according to the standards of the Toronto General Hospital (University Health Network) electromyography laboratory. Leg temperature was measured prior to the study, and in case needed, warming was performed to ensure a surface temperature of ≥31.0°C. Peroneal and sural NCS were performed using surface stimulating and recording techniques according to the standards of the Canadian Society of Clinical Neurophysiology and the American Association of Neuromuscular and Electrodiagnostic Medicine.Reference Bolton, Benstead, Grand’Maison, Tardif and Weston12 Latencies, amplitudes, and conduction velocities were automatically calculated by the electromyography Instrument. Peroneal compound motor action potential (CMAP) amplitudes were measured from baseline to peak, and sural sensory nerve action potential (SNAP) amplitudes were measured from baseline to negative peak, or from the positive peak (if present) to the negative peak.Reference Abraham, Alabdali and Qrimli13 VPT were determined using a Neurothesiometer (Horwell Scientific, London, UK) at the fingers and toes, and are described elsewhere.Reference Abraham, Barnett, Katzberg, Lovblom, Perkins and Bril11
Statistical Analysis
Statistical analysis was performed using SAS 9.4 (SAS Institute). Data are reported as means ± standard deviations (SD) or as frequencies. The distribution of UA was assessed for normality (Shapiro--Wilk and review of histogram). Correlations between UA and demographic, clinical, and electrophysiological parameters were explored using linear regression: for each parameter, separate univariable and multivariable regression models were constructed, with UA as the dependent variable and the parameter as an independent variable. The multivariable models included age, gender, and eGFR as covariates, which were chosen a priori due to their strong associations with AU. Regression coefficients β are reported. Selected univariable models are presented graphically. VPT were dichotomized as normal or abnormal, with β representing the unadjusted or adjusted difference between these groups (β > 0 implying higher UA in the abnormal group). P values of <0.05 were considered significant; as we consider this analysis hypothesis generating, no adjustments for multiple comparisons were done.
Results
The mean age of the cohort was 56 ± 17 years with 56% females. Electrophysiological and laboratory results and VPT were within normal limits in all subjects except six, all older than 70 years with abnormal sural and/or VPT values, which we consider normal for age. Males had higher UA values compared with females (347 ± 68 vs. 277 ± 76 μmol/L, P < 0.001) (Table 1).
Table 1: Demographic, clinical, and electrophysiological findings in 126 healthy subjects

BMI = body mass index; BP = blood Pressure; TCNS = Toronto Clinical Neuropathy Score; NCS = nerve conduction studies; CV = conduction velocity; VPT = vibration perception threshold; eGFR = estimated glomerular filtration rate.
* Males had significantly higher uric acid levels compared with females (P < 0.001). Data presented as mean ± standard deviation, or number (percent).
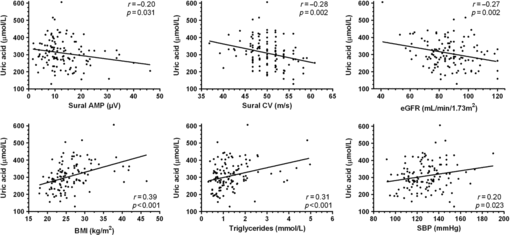
Univariate beta regression coefficient analysis between UA levels and demographic, clinical, electrophysiological and laboratory results are shown in Table 1. We observed significant positive correlations with male gender, components of the metabolic syndrome including BMI, systolic blood pressure, and triglycerides, and VPT. An inverse correlation was found with eGFR and with electrophysiological sensory parameters, including sural nerve amplitude and conduction velocity, but not with motor parameters. A Multivariate regression model, adjusted for age, gender, and eGFR, showed positive correlations with BMI, finger VPT, and triglycerides only (Table 2 and Figure 1).
Table 2: Univariate and multivariate beta regression coefficient analyses between uric acid levels and demographic, clinical, and electrophysiological findings in 126 healthy subjects

BMI = body mass index; BP = blood Pressure; TCNS = Toronto Clinical Neuropathy Score; NCS = nerve conduction studies; CV = conduction velocity; VPT = vibration perception threshold; eGFR = estimated glomerular filtration rate.
β values represent the unadjusted (left columns) and adjusted (right columns) change in uric acid per unit change in the indicated independent variable.
* Adjusted for age, gender, and eGFR. Statistically significant P values are shown in bold font.

Figure 1: Relationship between uric acid and indicated variables with linear regression lines.
Discussion
Previous studies have shown a high frequency of elevated UA levels in various peripheral neuropathies.Reference Abraham, Breiner and Barnett3, Reference Lin, Xu, Zhao, Luo and Pan5–Reference Abraham, Breiner and Barnett7 Furthermore, UA levels were found to correlate with the severity of diabetic polyneuropathy, mainly with sensory function.Reference Abraham, Breiner and Barnett7 Our study results show that UA levels have an inverse correlation with sensory nerve function in healthy controls also, without clinical or electrophysiological evidence of polyneuropathy, broadening the currently known association between UA and nerve impairment to the subclinical state. Although the degree of correlation is weak, many confounders, such as genetic, metabolic and environmental factors, which were not addressed in this study might influence this complex relationship. This might also explain why UA levels did not correlate with electrophysiological measures using a multivariate model, which did not account for all potential confounders, and also requires a higher number of subjects in order to reach statistical significance. However, UA might merely serve as a marker for other conditions that inversely correlate with sensory nerve function, such as obesity.Reference Buschbacher14
In line with a previous study in patients with diabetic polyneuropathy,Reference Abraham, Breiner and Barnett7 UA showed an inverse correlation with sural nerve amplitude and conduction velocity, and a positive correlation with VPT at the fingers and toes, but not with peroneal nerve amplitude or conduction velocity. An additional similarity was the correlation with finger VPT, but not with toe VPT using a multivariate regression model. As finger VPT was measured at the pulp of the first finger, which lies within the median nerve distribution, this finding might reflect increased susceptibility to UA levels of the median nerve at the carpel tunnel, which has been described previously in patients with polyneuropathy.Reference Abraham, Breiner and Barnett3 However, as median nerve conduction studies were not included in this study, we cannot confirm this hypothesis.
Similar to the previous report,Reference Lee, Kim, Cho, Oh, Choi and Suh15 our study has also shown a correlation between UA levels and components of the metabolic syndrome, including BMI, systolic blood pressure, and triglycerides. Interestingly, in contrast to the association between high UA levels and polyneuropathy, low UA levels have been reported in various other neurologic diseases, such as multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis.Reference Abraham and Drory8 A possible explanation for this discrepancy might stem from different pathophysiology of polyneuropathy and mainly degenerative neurological diseases. UA is a major antioxidant in the blood, and higher levels may reflect an inherent response to oxidative stress. However, a paradoxical rise in UA levels has been observed in cardiovascular diseases. A possible explanation is that UA functions as a pro-oxidant, as the reaction of UA with other oxidants may produce free radicals leading to oxidative damage to various tissues.Reference Sautin and Johnson1 The growing evidence for the deleterious effects of UA in cardiovascular and nervous system might suggest that studies aimed at lowering UA levels, which showed positive results in systemic disorders,Reference Bomalaski and Clark16–Reference Takir, Kostek and Ozkok18 might need to include nerve function studies as an additional outcome measure.
This study has several limitations. The lack of significant correlations of UA levels and most sensory nerve functions in the multivariate model suggests that a higher number of subjects is required to confirm this relationship. However, it should be indicated that similar associations between UA levels and nerve function have been observed in prior studies.Reference Abraham, Breiner and Barnett3, Reference Abraham, Albulaihe and Alabdali6, Reference Abraham, Breiner and Barnett7 Furthermore, as this study has a cross-sectional design, and not a longitudinal evaluation, we cannot address the possible risk of accelerated nerve function deterioration in subjects with high UA levels over time. However, considering the observed modest effect of UA on nerve function, and the expected slow electrophysiological progression, a large-scale study with long-term follow-up of several years would be required to address this question.
In conclusion, the current study results show that UA levels correlate with lesser, subclinical sensory nerve function in healthy subjects, expanding evidence of possible negative impact of UA on peripheral nerves, although a causative role has not yet established. Larger epidemiological studies are required in order to confirm these findings and possible therapeutic implications.
Funding
Bruce A. Perkins was supported by JDRF (operating grants 17-2008-715 and 17-2013-312).
Disclosures
Hans D. Katzberg reports grants, personal fees and non-financial support from Grifols, personal fees and non-financial support from Genzyme, grants and personal fees from CSL Behring, grants, personal fees and non-financial support from Octapharma, personal fees from Terumo, personal fees from Akcea, personal fees from Alexion, from Pfizer, personal fees and non-financial support from Flexpharma, personal fees from Amazentis, outside the submitted work.
Bruce A. Perkins reports grants from NIH, Diabetes Canada, CIHR, personal fees from Speaker honoraria from Medtronic, Novo Nordisk, Dexcom, Abbott, Astra Zeneca, Insulet; has received research grant support from Boehringer Ingelheim and BMO (Bank of Montreal); and serves as a consultant for Insulet, Abbott, Novo Nordisk, Boehringer Ingelheim., outside the submitted work.
Vera Bril reports grants from Bionevia, grants and personal fees from CSL Behring, grants from Dainippon Sumitomo, grants from Eisai, grants and personal fees from Grifols, grants from Lilly, personal fees from Pfizer, personal fees from Alexion, personal fees from Alnylam, grants and personal fees from Shire, grants and personal fees from Argenx, outside the submitted work.
Alon Abraham and Leif E. Lovblom have nothing to disclose.
Author Contributions
Alon Abraham contributed to the study concept and design and analysis and interpretation of data; Hans D. Katzberg to the study concept and design and acquisition of data; Leif E. Lovblom to the study concept and design and analysis and interpretation of data; Bruce A. Perkins to the study concept and design and acquisition of data; Vera Bril to the study concept and design, analysis and interpretation of data, study supervision, and critical revision of manuscript for intellectual content.