The global decline of amphibian populations is an enigmatic problem. Although understanding of losses due to habitat fragmentation has improved (Collins & Storfer, Reference Collins and Storfer2003) amphibians have also declined in environments where habitat modification and fragmentation were not readily apparent (Crump et al., Reference Crump, Hensley and Clark1992). Climate change (Carey & Alexander, Reference Carey and Alexander2003), increased UV-B radiation, chemical contaminants (Blaustein et al., Reference Blaustein, Romansic, Kiesecker and Hatch2003), introduced species and emerging infectious diseases (Daszak et al., Reference Daszak, Cunningham and Hyatt2003) may also be influencing declines. The effects of these factors are complex and they may be working synergistically with habitat destruction or each other.
In the West Indies large-scale extinctions of frogs and other forest dwelling species due to habitat loss may not occur until forest cover reaches low levels (Hedges, Reference Blair Hedges1993) but epidemic infections could confound this. Two diseases in particular could influence amphibian conservation in this region: cutaneous chytridiomycosis, caused by the chytrid fungus Batrachochytrium dendrobatidis, which results in die offs followed by extinctions, and ranavirus infection (Daszak et al., Reference Daszak, Cunningham and Hyatt2003), caused by DNA viruses of the genus Ranavirus, in which populations may recovery following mortality.
Chytrid fungus is the first taxon of the Phylum Chytridiomycota to be recognized as a vertebrate parasite, and was first described as the cause of death of adult frogs, in Australia and Panama, in 1993 (Berger et al., Reference Berger, Speare, Daszak, Earl Green, Cunningham, Goggin, Slocombe, Ragan, Hyatt, McDonald, Hines, Lips, Marantelli and Parkes1998). In the Caribbean B. dendrobatidis has been linked to amphibian declines in Puerto Rico (Burrowes et al., Reference Burrowes, Joglar and Green2004) and to unusually high mortality of the mountain chicken Leptodactylus fallax in Dominica (McIntyre, Reference McIntyre2003).
Ranavirus epidemics, which have been reported from Australia, North America and the UK, are usually characterized by extremely high mortality rates (Green et al., Reference Green, Converse and Schrader2002). Although ranaviruses have been isolated from amphibians in South America (Zupanovic et al., Reference Zupanovic, Lopez, Hyatt, Shiell and Robinson1998a,Reference Zupanovic, Musso, Lopez, Louriero, Hyatt, Hengstberger and Robinsonb) no disease or mortality there have so far been associated with ranavirus infection, and the ranavirus status of amphibians in the Caribbean is unknown.
The mountain chicken (Family Leptodactylidae), the largest Caribbean amphibian (>1 kg), is categorized as Critically Endangered on the IUCN Red List (IUCN et al., 2005; IUCN, 2006). Its historical range included seven eastern Caribbean islands but it is now confined to Dominica and Montserrat (Schwartz & Henderson, Reference Schwartz and Henderson1991). On Montserrat the species occurs in an area of <17 km2 in the Centre Hills. Here we present results of a study of the health status of the mountain chicken and sympatric amphibian species in the Centre Hills. We draw tentative conclusions about disease threats to the Montserrat mountain chicken population and make recommendations for safeguarding the species.
Montserrat has three distinct volcanic massifs along its north-south axis: Silver Hills (maximum altitude 403 m), Centre Hills (740 m) and Soufriere Hills (915 m). Mountainous terrain covers 102 km2, of which only 33 km2 are habitable. The Centre Hills are covered with montane rainforest and characterized by deep valleys with a radial rainage (Fig. 1). During November–December 2003 and March–April 2005 we walked established line transects in the Centre Hills. These transects, used for regular monitoring of long-term changes in amphibian populations, were originally placed in areas where frogs were known to occur and to facilitate regular visits by Montserrat Agriculture Department staff (Daltry, Reference Daltry1998). We caught frogs by hand, recorded locations before release, and took blood samples and skin swabs. For ranavirus serology testing we extracted 1 ml of blood using cardiocentesis from 40 mountain chickens in 2003. Each sample was centrifuged within 2 h of collection to separate the serum from the blood cells; serum was removed and stored frozen for later testing. For detection of B. dendrobatidis, skin swabs were taken from 100 mountain chickens in 2003, and 219 in 2005. Cotton tipped swabs were used to gently but firmly swab the skin of the ventral abdomen, drink patch, and all legs and feet. The swabs were either stored dry or in 70% ethanol. In addition, swabs from 45 cane toads Bufo marinus and toe clips from 46 Johnstone's whistling frogs Eleutherodactylus johnstonei, the only other amphibians on the island, were collected in 2005. To minimize the risk of transmitting disease between sites and contamination of samples, we followed an appropriate code of practice for fieldwork (DAPTF, 1998).
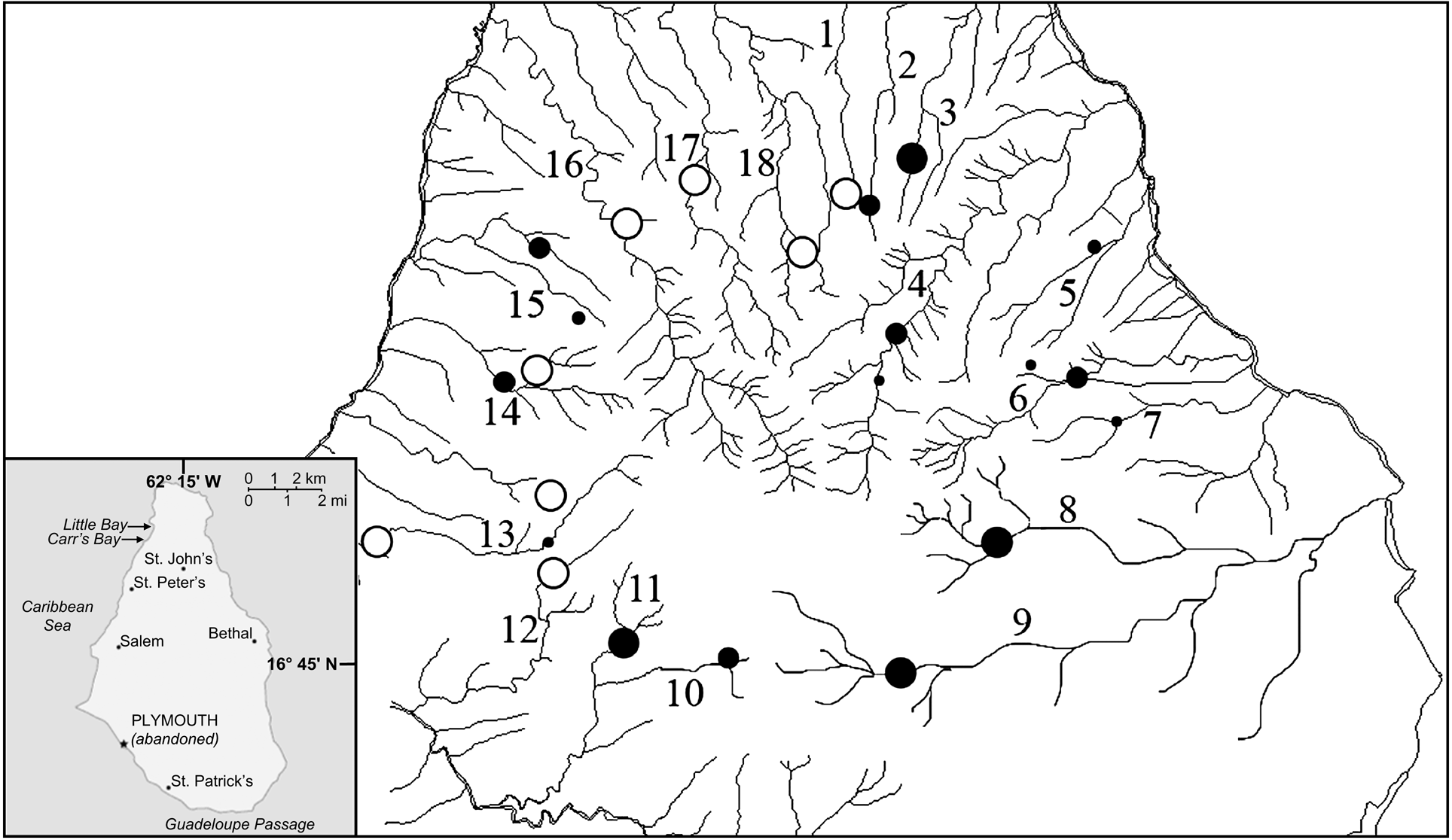
Fig. 1 The Centre Hills area of Montserrat (see inset for location on the island), indicating the 19 sampling sites: 1, Oushie Spring; 2, Cat Ghaut; 3, Sweet Water Ghaut; 4, Bottomless Ghaut; 5, Cedar Ghaut; 6, Pelican Ghaut; 7, Mournful Ghaut; 8, Lee River; 9, Farm River; 10, Daly River; 11, Sappit River; 12, Spring River; 13, Nantes River; 14, Cassava Ghaut; 15, Bunkum River; 16, Soldier Ghaut; 17, Caines River; 18, Collins River; 19, Garibaldi Hills. Black circles, sites where skin swabs and serum samples were taken from mountain chickens, skin swabs from B. marinus and toe clippings from E. johnstonei (size of the circle denotes relative sample sizes of mountain chickens). Open circles, sites where only skin swabs from B. marinus and toe clippings from E. johnstonei were taken.
Ranaviruses cross-react with a polyclonal antibody raised against epizootic haematopoietic necrosis virus (EHNV; Hyatt et al., Reference Hyatt, Gould, Zupanovic, Cunningham, Hengstberger, Whittington, Kattenbelt and Coupar2000). We used the competitive ELISA described by Zupanovich et al. (Reference Zupanovic, Lopez, Hyatt, Shiell and Robinson1998a) to detect antibodies against ranaviruses. Minor modifications were made to the protocol, including the use of 1% ovalbumin as the blocking agent, and the co-incubation of the test sera with a monoclonal antibody (1:2) generated against EHNV (7A7(131)). All sera were diluted at 1:10 and 1:50, and analysed in duplicate. We included the following controls within each assay following Hyatt et al. (Reference Hyatt, Eaton, Hengstberger and Russel1991) and Zupanovich et al. (Reference Zupanovic, Lopez, Hyatt, Shiell and Robinson1998a,Reference Zupanovic, Musso, Lopez, Louriero, Hyatt, Hengstberger and Robinsonb): (1) laboratory raised antibodies (rabbit polyclonals against Bohle iridovirus (BIV) and EHNV, (2) known positive sera from experimentally infected B. marinus, and (3) sera from uninfected B. marinus. Results are expressed as percent inhibition (%). Rabbit antisera against EHNV was included within the assay as a positive control; it returned percent inhibitions of approximately 90%, indicating all components of the assay were viable and performing optimally. The serum derived from experimentally infected B. marinus was included to give an estimate of what percent inhibition should be expected from infected amphibians. Of a total of 57 L. fallax serum samples screened for ranavirus and assuming a cut-off for sero-positive animals at 60% (at 1:10) and 40% (1:50), values equivalent to that of the positive controls, we detected no seropositive animals.
DNA extraction and real-time Polymerase Chain Reaction (PCR) amplification was performed following Boyle et al. (Reference Boyle, Boyle, Olsen, Morgan and Hyatt2004). Real-time PCR was conducted using an Applied Biosystems Prism 7700 Sequence Detection System. Primers were sourced from MWG Biotech AG (Ebersberg, Germany) and the Taqman MGB2 probe from Applied Biosystems (Foster City, USA). Negative controls and four standards (100, 10, 1 and 0.1 zoospore-equivalents) were included on each plate and all samples, and standards and controls, were replicated at least once. We estimated maximum prevalence of infection for B. dendrobatidis using the relationship described by DiGiacomo & Koepsell (Reference DiGiacomo and Koepsell1986): n = log(1-C)/log(1-P), here n = sample size, C is the desired probability of detecting at least one infected individual, and P is the prevalence of disease in the population. In all cases we set C at 95% and assumed random sampling. Of a total of 319 wild L. fallax on Montserrat screened for B. dendrobatidis all tested negative for the presence of the fungus. Swabs from B. marinus and toe clips from E. johnstonei also tested negative for B. dendrobatidis.
Recent concern over global amphibian declines has prompted analyses of threats to species in the West Indies and Latin America (Lips et al., Reference Lips, Burrowes, Mendelson and Parra-Olea2005). In Puerto Rico species such as Eleutherodactylus karlschmidti and the Puerto Rican live bearing frog Eleutherodactylus jasperi may have already become extinct, and two stream-associated species on Hispaniola (Eleutherodactylus semipalmatus and Hyla vasta) appear to have declined in recent years, probably due to the alteration of riparian habitats through deforestation (Blair Hedges, Reference Blair Hedges1993).
The absence of unusual morbidity or mortality suggests that the amphibians of the Centre Hills are not experiencing declines due to infectious disease. Additionally, at least until 2005, there was no evidence of infectious disease or of infection with B. dendrobatidis or ranavirus in the amphibians of the Centre Hills, although this is with the caveat that endemic infections may occur without disease-related die offs (Garner et al., Reference Garner, Walker, Bosch, Hyatt, Cunningham and Fisher2005).
At least 70% of the amphibians in the Caribbean are threatened with extinction (IUCN et al., 2005). This is mostly due to extensive habitat loss as well as the incidence of disease. The mountain chicken could be devastated if cutaneous chytridiomycosis spreads from Dominica, where the disease is causing high mortalities. The most likely route of any introduction is via the inadvertent arrival of amphibians in the regular shipments (1-2 per week) of fresh vegetables and fruits from Dominica. A risk analysis of the potential for the introduction of B. dendrobatidis to Montserrat has already been completed, biosecurity recommendations have been presented to the Montserrat government, and precautions are being contemplated (Horton, Reference Horton2005). A major biodiversity assessment effort led by Durrell, the Royal Society for the Protection of Birds (RSPB) and the Montserrat Government has been recently completed and is aiding the management and declaration of the Centre Hills as a National Park. Protection of the area will encourage conservation of the mountain chicken and other species but an action plan for the species is urgently required. Durrell, with the RSPB and the Montserrat Forestry Department, are currently seeking funds to undertake this task.
Acknowledgements
We are grateful to Claude Gerald and the Department of Agriculture in Montserrat for permission to work in Montserrat, and especially to Lloyd ‘Lloydie’ Martin, James ‘Scriber’ Daly, John ‘Gambie’ Martin, Lloyd ‘Big Lloyd’, and Philemon ‘Pie’ Murrain for support in the field. We thank Sarah MacIntyre for her help in the field and Matthew Perkins for technical support.
Biographical sketches
Durrell has been working on the mountain chicken in Montserrat since 1997, initially in association with Fauna & Fauna International, and jointly with the Montserrat Forestry Department. The authors were involved in a variety of ways, reflecting their interests, in this research on the mountain chicken. Since 2003 the project has been led by John Fa. Gerardo Garcia undertook the fieldwork reported here, assisted by Agnieszka Ogrodowczyk and Calvin Fenton. Skin swab samples for chytrid detection were sent to Andrew Cunningham at the Institute of Zoology, London, and processed by Daniel Horton and Trenton Garner of the Institute. Blood samples were examined for ranaviruses by Alex Hyatt and Sandra Hengstberger. Veterinary procedures were supervised in Durrell by Javier Lopez.



