Hospital employees, and healthcare workers (HCWs) in particular, are at increased risk for exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Reference Grant, Malik, Elkholy and Van Kerkhove1 They may present with pauci- or asymptomatic coronavirus disease 2019 (COVID-19). Reference Jung, Park and Kim2 Detecting acute infections (using RT-PCR) is thus subject to many limitations; infections may be unrecognized or not medically attended, and the true infection rate may be largely underestimated. For these reasons, serological studies assessing the presence of specific IgG against SARS-CoV-2 in this population are needed to reflect adequately the infection rate over time, as well as to estimate the risk from occupational exposures.
Several IgG-based serologic point-prevalence studies have suggested higher SARS-CoV-2 seropositivity among HCWs up to 31.6%, Reference Garcia-Basteiro, Moncunill and Tortajada3–Reference Grant, Wilmore and McCann6 compared to 5.0%–10.8% in the community. Reference Pollán, Pérez-Gómez and Pastor-Barriuso7,Reference Stringhini, Wisniak and Piumatti8 This higher prevalence may be driven by community acquisition, close contact with other HCWs, or transmission from infected patients. Few large-scale longitudinal cohort studies of hospital employees have been conducted across multiple professional categories assessing nosocomial and community risk factors for SARS-CoV-2 seroconversion while accounting for nosocomial COVID-19 outbreaks and time-varying exposures. Early in the first pandemic wave in Europe, we conducted a hospital-wide study to determine trends and independent risk factors for SARS-CoV-2 seroconversion among hospital employees with different occupational exposures during a 3-month exposure period.
Methods
Study design and participants
We conducted a longitudinal seroprevalence cohort study from March 30, 2020, to June 12, 2020, on a convenience sample of all volunteering employees working at Geneva University Hospitals (HUG), regardless of their prior symptoms or documentation of SARS-CoV-2 infection. Starting from date of enrollment, 2 follow-up visits were scheduled every 3 weeks from the baseline visit. Recruitment and follow-up are further described in Appendix 1 (online). Employees unable to provide consent or for whom serum samples were not collected were excluded. We stratified hospital sectors with high exposure to COVID patients (adult intensive care units [ICUs], anesthesiology, COVID testing center, COVID-19 cohorting wards and emergency ward, intermediate exposure (non–COVID-19 medical wards, non–COVID-19 geriatric and rehabilitation wards, surgery, pediatrics, gynecology, obstetrics, radio-oncology, and hemato-oncology wards), and low exposure (office workers in administrative sector) (Supplementary Fig. 1 online). The primary outcome was the proportion of seroconverted employees, defined as the cumulative proportion of employees seropositive for SARS-CoV-2 using a 2-step diagnostic strategy based on a combination of 2 serologic assays (described in the following section). In the presence of an emerging virus, the first detection of SARS-CoV-2–positive employees in the 20 days prior to the beginning of the study, and an immunologically naive population, we assumed that employees positive at baseline had recently seroconverted (as further discussed in Appendix 2 online). Secondary outcomes included the proportion of asymptomatic seroconverted employees and prevalence ratios of risk factors for SARS-CoV-2 seroconversion. This study was performed in accordance with the STROBE statement for cohort studies (Appendix 1 online). Reference Altman and Egger9

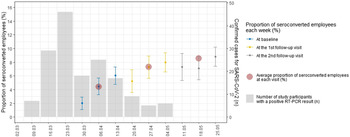
Fig. 1. Evolution of the proportion of SARS-CoV-2 seroconverted employees during follow-up visits, with counts of positive SARS-CoV-2 PCRs among all study participants.
Study setting and local COVID-19 epidemiology
HUG is the largest tertiary-care center in Switzerland, with >2,000 beds and 11,945 employees. 10,11 After the first COVID-19 patient was hospitalized in Geneva on February 27, 2020, the daily number of hospitalized COVID-19 patients peaked on March 31, 2020. Based on our screening program recommended for HUG employees (Appendix 3 online), 697 of 3,790 tested employees (18.4%) had PCR-confirmed SARS-CoV-2 infection by July 1, 2020. Multiple interventions were implemented at the community and hospital levels to control the spread of SARS-CoV-2 (Appendix 4 and Supplementary Figs. 1 and 2 online).
Sampling and data collection
As described in Appendix 1 (online), study nurses visited wards and office areas to recruit volunteering employees. The recruitment period occurred from March 30, 2020, to April 17, 2020, with 2 follow-up periods from April 17 to May 11 and from May 11 to June 12. Each participant was asked to attend every 3 weeks. To ensure a high follow-up rate, participants received e-mails and telephone calls as reminders. At each visit, a serum sample was drawn, and health-related data were collected using a self-completed questionnaire (Supplementary Table 1 and Appendix online) under the supervision of medical students. In addition to demographic data, hospital sector, and profession, data from the 20 days prior to each visit were collected and included symptom-related data, nosocomial exposures (aerosol-generating procedures, close contact of <1 m with a COVID-19 patient), community exposures (close contact with SARS-CoV-2–positive persons within or outside the household, number of household members, use of public versus private transportation), preventive measures (use of surgical mask versus N95 respirators, knowledge of SARS-CoV-2–related institutional infection prevention and control [IPC] recommendations, 12 and self-reported adherence to IPC recommendations). Categorization and definitions of these variables are detailed in Appendix 5 (online). We assumed, according to our hospital setting, that all office workers worked in the administrative sector. Other professional activities or undetermined sectors mainly included employees with no available information or with mixed assignments (eg, working in the whole institution). Time-varying exposures included hospital sectors; aerosol-generating procedures; and nosocomial contact with positive employees, patients, or community contacts. Data were entered into a REDCap database. Whenever available, results from RT-PCR tests performed during routine screening were matched to participants in the electronic database (Appendix 3 online).
Table 1. Characteristics of SARS-CoV-2 Seroconverted and Nonseroconverted Employees

a Unless otherwise indicated.
b Same population considered interchangeably.
c,d,e Categories were merged in the regression model.
Serological testing of anti-SARS-CoV-2 IgG antibodies
In serological investigations, we applied a 2-tiered diagnostic strategy (Appendix 6 online) using a first S1 protein-based IgG enzyme-linked immunosorbent assay (ELISA; EUROIMMUN AG, Lübeck, Germany, no. EI 2606-9601 G). Following the 2-tiered strategy, all samples with undetermined and positive results were retested with an ElectroChemiLuminescence ImmunoAssay (ECLIA) Elecsys Anti-SARS-CoV-2 Ig (Roche Diagnostics, Germany) according to the manufacturer’s instructions. First-step equivocal and positive results were considered positive only if confirmed by the ECLIA test; all others were considered negative.
Sample size calculation
As of April 23, 2020, 3.82% of employees (205 of 5,366) working in hospital sectors with low, intermediate, and high exposure were positive by PCR for SARS-CoV-2 at rates of 3.5% in the low-exposure group, 4.5% in the intermediate-exposure group, and 15.5% in the high-exposure group. The comparisons of interest concerned hospital sectors with low versus high exposure and intermediate- versus high-risk areas. The maximum sample size of the 2 comparisons was retained. With 80% power and a 2.5% false-positive rate accounting for Bonferroni correction, 2-sided power calculations (using the “pwr” package) yielded a sample size of 131 participants by group, or 393 in total. Anticipating a conservative 30% dropout rate, 188 participants were required in each group, or 564 in total.
Statistical analysis
Main analyses. The overall proportion of seroconverted employees during the whole study period in the 3 categories of COVID-19 exposure (high, intermediate, and low) were compared using χ2 tests with Bonferroni correction. We performed univariate and multivariate mixed-effect Poisson regression to estimate prevalence ratios of risk factors for SARS-CoV-2 seroconversion over all participants and visits. Reference Zou13–Reference Sinclair and Bracken15 Random effects on individuals and on weeks of follow-up were included to account for the multilevel nature of the data. These consisted of repeated measurements within subject and multiple subjects within weeks of follow-up, thus accounting for time-varying infection risks. Candidates for the multivariate model were selected using stepwise regression, Lasso penalized regression, Bayesian model averaging (BMA) methods, and clinical relevance (Appendix 7 online). To account further for the time-varying exposure relative to the first wave of the pandemic, the full cohort was categorized into subcohorts based on their enrollment week (S1, S2, or S3) (Supplementary Figs. 3 and 4 online) and were entered as fixed effects in the multivariate model. To allow model convergence, certain hospital sectors and professional subcategories were merged. Any possible collinearity and confounding effects were examined.
Missing data. Using the cumulative nature of the main outcome, missing serological results were inferred (Appendix 8 online). Remaining missing serological data were handled using mixed-effects models under the assumption of missing at random (Appendix 8 online).
Secondary analyses. An additional analysis examined self-reported symptoms in the previous 20 days among seroconverted employees. The amplitude of a potential selection bias was assessed by comparing (1) PCR-based SARS-CoV-2 prevalence between study participants and nonparticipants and (2) basic demographic information between study participants and the overall population of HUG employees (Appendix 9 online). All analyses were conducted using R studio version 4.0 software (R Foundation for Statistical Computing, Vienna, Austria) including the “lme4” package.
Ethical considerations
This study was approved by the Ethics Committee of the Canton of Geneva (no. 2020-01330). Each study participant provided written informed consent.
Results
Among 11,945 hospital employees, we recruited 3,436 employees (29%), of whom 3,421 were included in the final analysis, with an overall follow-up rate of 92% (Supplementary Figs. 3 and 4 online). At the baseline visit, 3,402 employees were tested. At the first follow-up visit, 3,119 employees were tested. At the second follow-up visit, 3,154 employees were tested. After using inference for missing events, 3,419 cases were included in the baseline group; 3,275 cases were included in the first follow-up group; and 3,173 cases were included in the second follow-up group. In total, 15 recruited persons were not sampled: 8 with incomplete consent, 3 withdrawals, and 4 without information (Supplementary Fig. 5 online). Most participants worked in geriatric and rehabilitation (G&R) wards, followed by COVID-19 cohorting wards and pediatric, gynecologic, and obstetric wards (Table 1). Regarding profession, nurses comprised the largest group of participants, followed by physicians and nursing assistants. We observed no impact of data inference for missing events (Appendix 8 online).
Proportion of seroconverted employees with SARS-CoV-2 IgG
Confirmatory tests were performed on 983 samples with equivocal screening tests and 871 samples with positive screening tests. Among first-step equivocal and positive samples, respectively, 76 (7.7%) and 546 (62.7%) were confirmed positive by the second test. IgG seropositivity among hospital employees increased from 4.4% (95% CI, 3.7%–5.1%) at recruitment to 7.3% (95% CI, 6.4%–8.2%) at the first follow-up visit and to 8.5% (95% CI, 7.6%–9.5%) at the second follow-up visit (Fig. 1). Results from the first-step test are detailed in Supplementary Table 2 (online). Over all time points, the cumulative proportion of seroconverted employees was 7.9% (271 of 3,421; 95% CI, 7.0–8.8) (Supplementary Tables 3 and 4 online). Among the 3 subcohorts, the baseline proportion of seroconverted employees varied from 2.0% (19 of 949) the first week to 6.0% (83 of 1,373) the third week of recruitment (Supplementary Fig. 6 online).
Over all time points, sectors with high exposure had significantly more seroconverted employees than sectors with low exposure (10.0% vs 4.9%; P = .005) and intermediate exposure (6.8%; P = .005). At the last visit, large differences emerged among subsectors; the highest proportions were observed in COVID-19 wards (32.3%; 95% CI, 24.5–40.2) and in non–COVID G&R wards (12.3%; 95% CI, 9.5–15.2). At the final visit, we observed the lowest rates of seroconversion among office workers (4.9%; 95% CI, 2.7–7.2), in ICU and anesthesiology workers (3.9%; 95% CI, 1.2–6.6), in hemato-oncology workers (3.3%; 95% CI, 0.7–5.8), in workers in the COVID-19 testing center (2.1%; 95% CI, 0.0–6.2), and in surgery workers (1.8%; 95% CI, 0.0–3.8) (Fig. 2 and Supplementary Table 5A online). Slightly increased proportions of seroconverted employees were observed when considering only employees working in the same hospital sector during the entire study period who were present at all 3 visits (n = 2,380) (Supplementary Fig. 7 online). At the last visit, we observed a higher proportion of seroconverted employees in COVID-19 cohorting wards compared to noncohorting wards (15.1% [95% CI, 12.0–18.2] vs 10.0% [95% CI, 7.8–12.1]; P = .008). We observed a higher proportion of seroconverted employees in wards with a confirmed COVID-19 outbreak than in wards without outbreaks (28.1% [95% CI, 21.3–35.0] vs 6.9% [95% CI, 6.0–7.8]; P < .001) (Supplementary Figs. 8 and 9 online).

Fig. 2. Evolution of the proportion of SARS-CoV-2 seroconverted employees among follow-up visits, stratified by professional activities and subsectors. Despite the cumulative nature of this outcome, certain observations might decrease among sectors and follow-up visits because of missing results, or seroconverted employees changing ward affiliations between visits.
Over all time points and among all professional categories compared to office workers, only nursing assistants had a statistically significant higher risk of seroconversion (11.7% vs 4.9%; P = .006). At the last follow-up visit, the highest proportions of seroconversion occurred among nursing assistants (11.7%; 95% CI, 8.9%–14.5%), nurses (8.1%; 95% CI, 6.5%–9.5%), physicians (8.9%; 95% CI, 6.4%–11.3%), and allied health professionals (8.1%; 95% CI, 4.2%–12.0%) (Fig. 2 and Supplementary Table 5B online). Among 271 seroconverted employees, 227 (84%) had reported symptoms prior to seroconversion. At baseline, 151 were positive, including 126 (83%) who had reported symptoms in the previous 20 days.
Risk factors for SARS-CoV-2 seroconversion among employees
Risk factors retrieved by univariate analysis are shown in Supplementary Table 6 (online). By multivariate analysis, significant predictors for seroconversion (Table 2) included use of public transportation and close community contact with a known COVID-19 case. Compared to sectors with intermediate exposure to COVID-19 patients, working in undetermined sectors (eg, mixed assignments or undefined assignments), G&R COVID-19 wards, and non–COVID-19 G&R wards were also associated with increased risk of seroconversion. We observed other independent associations for wards reporting a COVID-19 outbreak and among participants with self-reported higher adherence to SARS-CoV-2–related IPC recommendations. Compared to physicians, other professional activities (eg, midwives, hospital cleaners, and technicians) had a lower risk of seroconversion. Reported use of respirators (compared to surgical masks) also lowered the risk of seroconversion. Exposure to aerosol-generating procedures was not associated with an increased risk of seroconversion (Table 2).
Table 2. Adjusted Prevalence Ratios of Risk Factors for SARS-CoV-2 Seroconversion From the Multivariate Mixed-Effect Poisson Regression Model Among All Visits of Participating Hospital Employees

Note. IPC, infection prevention and control.
a Office workers and administrative sectors are used interchangeably. Because of collinearity, the variable administrative sector has been dropped from this model.
Assessment of selection bias
The study population shared basic demographic characteristics with the overall population of hospital employees (Appendix 9 online), though nursing assistants and nurses were slightly overrepresented in our study population: 17% versus 8% of nursing assistants and 38% vs 31% of nurses. Furthermore, study participants who underwent PCR-based testing had a positivity rate of 14.7%, whereas the proportion of positive PCR results among tested nonparticipants was 19.9% (535 of 2,690).
Discussion
The following principal findings summarize the results of this hospital-wide cohort study: (1) The proportion of seroconversion was relatively low among employees of this large Swiss University Hospital during the early phase of the pandemic. (2) The G&R wards had significantly higher seroconversion rates compared to other sectors traditionally considered to be high risk. (3) Only a small proportion of seroconversions were asymptomatic. (4) Exposure to community risk factors increased the risk of seroconversion. And the use of respirators compared to surgical masks seemed to be protective against seroconversion. (5)
The proportion of seroconverted employees observed in our study is similar to the 9.3% in a Spanish cross-sectional survey among 578 HCWs and to another Italian study that reported a seroprevalence of 7.4% among 202 HCWs, both in early April 2020. Reference Garcia-Basteiro, Moncunill and Tortajada3,Reference Sotgiu, Barassi and Miozzo4 In New York, seroprevalence among 40,329 employees from April through June in a hospital network was observed to be 13.7%. Reference Moscola, Sembajwe and Jarrett5 A higher seroprevalence of 31.6% was observed among 2,004 British hospital employees in May–June 2020. Reference Grant, Wilmore and McCann6 Differences across these studies can be explained by heterogeneity across populations, study design, sampling schemes, and serological tests used. Reference Arora, Joseph and Wyk16 The baseline estimate of asymptomatically infected employees observed in our study (17%) was similar to those in other reports (15.5%–20.2%). Reference Mizumoto, Kagaya and Zarebski17
Employees working in what are conventionally considered to be high-risk sectors (e.g. ICU) had similar proportions of seroconversion compared to office workers (<5%). Potential explanations include case-mix differences and possibly a higher adherence to IPC measures in these sectors. In addition, risk perceptions may not always align with true high-risk situations, especially in intubated patients with protected airway circuits. By contrast, prevalence ratios were particularly high for employees working in wards reporting nosocomial COVID-19 outbreaks or COVID-19 cohort wards in geriatrics. The high risk in nondedicated G&R wards might be explained by the mixing of COVID-19–dedicated and nondedicated wards in the same institution, which probably facilitated cross infections as well as the occurrence of several large-scale nosocomial clusters involving 185 HCWs, with spillover effects. Alternative determinants include limited staff resources, high staff turnover, insufficient infection prevention training, and frailty of patients requiring close nursing care. Reference Mendes, Serratrice and Herrmann18,Reference Pagani, Thomas and Huttner19
We observed that use of public transportation and community contact with persons with COVID-19 were predictors of seroconversion. Public transportation was also a significant exposure among Brazilian hospital employees. Reference Costa, Giavina-Bianchi and Buss20 Notably, wearing masks in public transportation in Geneva only became mandatory after July 1, 2020. Community acquisition by HCWs was already demonstrated in other reports that observed either epidemiological links with community contacts, Reference Safdar, Moreno and Braun21 genetic relatedness between hospital and community clusters, Reference Sikkema, Pas and Nieuwenhuijse22 or high genetic diversity suggestive of community transmission. Reference Olmos, Campaña and Monreal23
We observed several interesting associations concerning infection control measures, toward increased risk of seroconversion for reported use of surgical masks compared to N95 respirators and employees with higher self-reported adherence to IPC recommendations. However, this latter association may have been artificially increased by social desirability bias and thus does not represent true clinical adherence, as has been previously reported for other IPC measures such as hand hygiene. Reference Kelcikova, Mazuchova, Bielena and Filova24 Finally, the reported use of respirators (FFP2/N95 masks) showed a protective effect with lower seropositivity. This observation might reflect either true protection or residual confounding (eg, higher general adherence to IPC recommendations), which needs to be elucidated in well-designed clinical trials.
Our study has several strengths, including the large sample size and high follow-up rate, which allowed a robust estimate of the seroconversion dynamics among different professional categories and across hospital sectors. Furthermore, the analysis accounted for various confounders, including community and time-varying exposures, and we used a 2-tiered strategy to correct for testing biases.
This study also had some limitations. First, using a convenience sample with 3,421 participants among 11,945 employees and recruiting only certain hospital sectors at specific working hours might have introduced a selection bias. Lower PCR-based prevalence among participants suggests that PCR-positive employees had fewer incentives or time to participate, which might have slightly underestimated the true seroconversion rate. Misclassification bias is always possible with serological testing, due to frequent cross reactivity among coronaviruses. However, adequate performance was observed by the chosen assays that were thoroughly validated by our laboratory. Recall bias might have been present regarding past exposure history of HCWs. We decreased this risk by only collecting exposure histories for the 20 days prior to each visit. Residual confounding might persist because not all possible confounders were included in the questionnaire regarding additional community or nosocomial exposures (eg, cafeteria visits). Office workers might also have had occasional contacts with patients in the context of their administrative duties. However, we hypothesized that these contacts were nonsignificant. Indeed, compared to other countries, few office workers work in clinical areas at HUG. Finally, the 3 periods occurred in different epidemiological contexts with multiple time-varying confounders (eg, nonpharmaceutical interventions implemented in community). Comparisons between visits must be made with caution, though adjustments for correlation within individuals and within weeks were made in the statistical models.
In conclusion, likelihood of SARS-CoV-2 seroconversion among employees was influenced by both community and healthcare-related exposures. Increased risk of seroconversion was particularly observed in units reporting a nosocomial outbreak, and in G&R wards, highlighting additional efforts needed to protect HCWs working in elderly care wards. The role of routine respirator use, as opposed to surgical face masks, requires further validation.
Acknowledgments
The authors acknowledge the invaluable support from Dre Laure Vieux, Prof Idris Guessous, Dre Silvia Stringhini, PhD Andrew Azman, Dr Lauriane Lenggenhager, Rachel Brung, Didier Robert-Tissot, Marie-Sophie Desaunay, Anne Thierstein, Catherine Vasseur, Jean-Luc Loretz, Giancarla Vendetti, Elodie De Jesus, Valérie Dard, Garance Gutknecht, Laetitia Didierlaurent, Camille Tissier, Marianne Rousseau, Murielle Ares, Marie Peyron, Audrey Grosmaire, Anita Froissard, Julien Fonck, Fabienne Favre, Carla Leon, Coralien Di Santo, Chloé Deforel, Nikita Grieder, Sami Uslu, Johann Pignat, Aurélie Piletta, Van-Dai Nguyen, Hagar El Bentiri, Chayma Bettaieb, Yaëlle Aeschimann, Akcasoy Fatma, Guza Lorela, and all employees from the virological laboratory who greatly achieved a gigantic amount of work in very little time.
Financial support
This study was supported by the Geneva University Hospitals and Faculty of Medicine, an unrestricted grant from Fondation Pictet (Geneva, Switzerland), and the Swiss National Science Foundation (grant no. 31CA30_196197). R.M. was partially supported by the Swiss National Science Foundation (grant no. 407240_177454). The funders were not involved in the design, conduct, analysis, interpretation, or reporting of the study. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Conflicts of interest
The authors report no potential conflicts of interest related to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2021.117