INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is traditionally associated with healthcare-associated infections. The emergence of MRSA as a cause of infections in the community, in persons without traditional risk factors for healthcare-associated MRSA infection (e.g. recent hospitalization or surgery, receipt of dialysis, presence of percutaneous medical devices, or long-term residential care) or community-associated MRSA (CA-MRSA) has become a major public health concern [Reference Naimi1]. Outbreaks in athletes, school children, and prison inmates have been reported [2–4]. CA-MRSA frequently causes purulent skin or soft-tissue infections (SSTIs); however, illness can range from mild skin infections to severe conditions, including necrotizing pneumonia and invasive disease (e.g. osteomyelitis, pyomyositis, deep abscess, septic arthritis, and endocarditis) [Reference King5]. Reports of increasing numbers of cases of CA-MRSA SSTIs from healthcare providers throughout Georgia, USA during 2003–2004 and awareness of the potential severity of MRSA-related disease [Reference Klevens6] led the Georgia Department of Human Resources, Division of Public Health, to make CA-MRSA deaths and severe CA-MRSA disease reportable in October 2004. Objectives of this new passive surveillance system were to estimate the burden of severe CA-MRSA disease in Georgia, to define the population at risk, and to identify potential risk factors for severe disease and fatal outcomes.
METHODS
The passive surveillance case definitions for CA-MRSA infections were developed in consultation with infection-control practitioners (ICPs). CA-MRSA disease was defined as a clinically compatible illness associated with MRSA culture obtained before or within the first 48 h of hospitalization, in a person without history of MRSA, recent hospitalization or surgery, dialysis, percutaneous medical device use, or long-term residential care. Severe CA-MRSA disease was defined as CA-MRSA infection associated with a sterile-site isolate (invasive disease), infection requiring intensive care or operative debridement, or infection resulting in death. Only cases of CA-MRSA classified as severe were reportable to the passive surveillance system. Severe CA-MRSA cases that required hospitalization were reported to the state health department by hospital ICPs through the State Electronic Notifiable Disease Surveillance System. Georgia Division of Public Health (GDPH) epidemiologists reviewed the case reports to ensure that they met the case definition.
To determine the extent of severe CA-MRSA disease, define the population at risk, and to establish possible risk factors for severe disease and mortality (including the association of mortality with a specific clinical syndrome), data from the new passive surveillance system were analysed during 1 January 2005 to 31 December 2007. A descriptive analysis was performed by age, sex, race and reported CA-MRSA clinical syndrome for fatal and non-fatal cases. Crude risk ratios for death were computed. Multiple logistic regression was used to examine the odds of death in patients with reported pneumonia, invasive disease, and SSTI controlling for age and the presence of >1 clinical syndrome. All analyses were performed in SAS 9.1 (SAS Institute, USA). To identify possible behavioural risk factors or comorbidities associated with severe disease, chart reviews were conducted on 11 reported deaths in young and previously healthy persons aged <25 years with CA-MRSA infections reported during October 2004 to February 2007 [Reference Tuttle, Arnold and Tobin-d'Angelo7].
RESULTS
A total of 1670 cases of severe disease were reported in the survey period (Fig. 1). The median age was 34 years (range 0–90) and 59% (991/1670) were male. Of the CA-MRSA patients that reported race, 68·4% (1064/1555) were white; 28·9% (450/1555) were black; 0·64% (10/1555) were Asian; 0·3% (5/1555) were multiracial, and 1·7% (26/1555) were categorized as other. Of the patients with reported ethnicity, 5·9% (67/1131) were Hispanic. Clinical syndromes included SSTI (78·9%, 1317/1670), invasive disease (17·7%, 295/1670), and pneumonia (6·0%, 101/1670); these categories were not mutually exclusive.
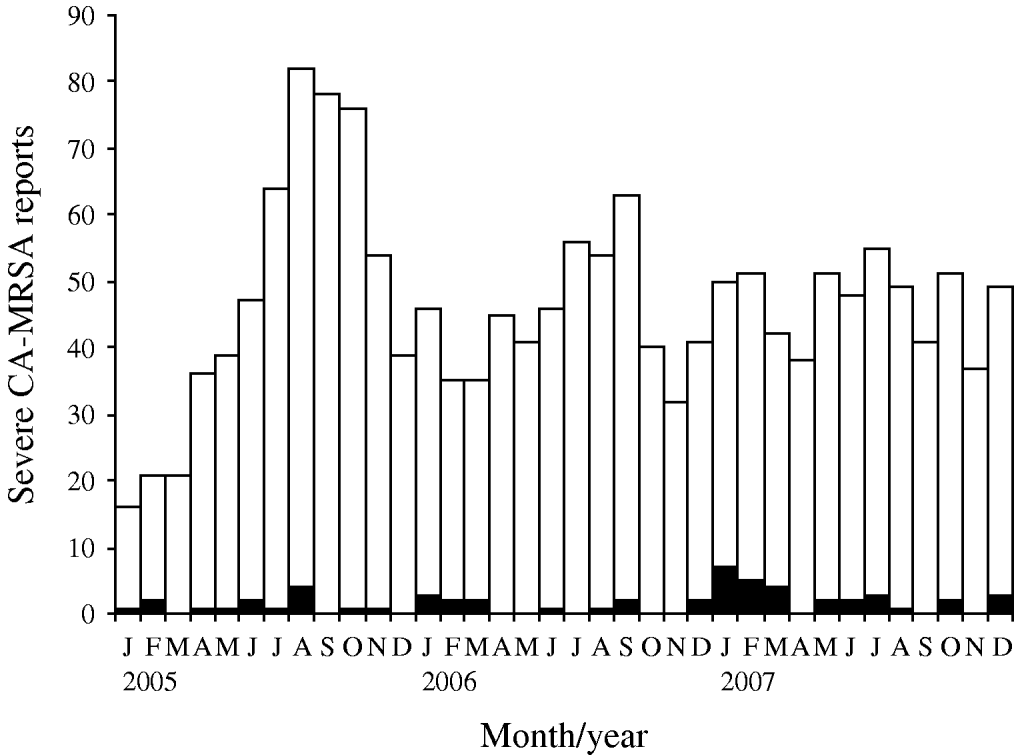
Fig. 1. Methicillin-resistant Staphylococcus aureus cases and deaths identified by the Georgia surveillance system for severe community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) disease; Georgia, 1 January 2005 to 31 December 2007. □, Non-fatal cases; ▪, fatal cases.
The case-fatality ratio was 3·4% (56/1670) during the surveillance period. The median age of patients who died was 41 years (range 0–90); 60·7% (34/56) were male, 67·9% (38/56) were white, 26·8% (15/56) black, and 1·8% (1/56) Asian; none of the fatalities with reported ethnicity were Hispanic. The median time from hospital admission until death was 4 days (range 0–28). Clinical syndromes included SSTIs (12·5%, 7/56), invasive disease (75·0%, 42/56), and pneumonia (51·8%, 29/56); these categories were not mutually exclusive.
Fatal and non-fatal cases did not differ significantly by sex or race; however, patients who died were about 15 times more likely to have been admitted with invasive CA-MRSA or MRSA pneumonia (Table 1). Invasive CA-MRSA and MRSA pneumonia remained risk factors for death when controlling for age and the presence of other clinical syndromes (Table 2). As indicated by the epidemiological curve (Fig. 1), severe CA-MRSA infections peaked during the summer months; however, the majority of deaths associated with severe CA-MRSA disease occurred during winter.
Table 1. Characteristics of patients with severe community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) disease during 1 January 2005 to 31 December 2007
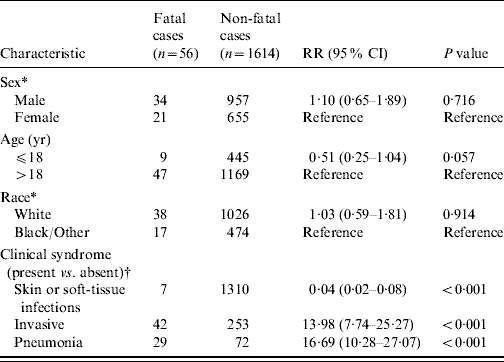
RR, Unadjusted risk ratio; CI, confidence interval.
* Missing values are excluded.
† Clinical syndromes are not mutually exclusive.
Table 2. Odds of death in patients manifesting specific severe CA-MRSA syndromes controlling for age
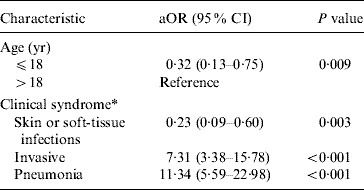
aOR, Adjusted odds ratio from logistic regression model including clinical syndrome and age; CI, confidence interval.
* Clinical syndromes are not mutually exclusive.
To establish potential behavioral risk factors or comorbidities associated with mortality, we conducted chart reviews on 11 reported deaths in young and previously healthy persons aged <25 years with CA-MRSA infections reported during October 2004 to February 2007 [Reference Tuttle, Arnold and Tobin-d'Angelo7]. Five of these patients died of sepsis after skin infections, and three had a known history of wounds having been drained at home before medical care was sought. Six patients had had primary CA-MRSA pneumonia; all six had symptoms of influenza-like illness (ILI) for 2–6 days before being admitted to the hospital. Five of these six patients were tested for influenza by rapid influenza test, three of which were positive. None of the six patients who died had a history of influenza vaccination during the previous year.
DISCUSSION
Georgia's severe CA-MRSA disease surveillance system documents severe illness and death resulting from this pathogen statewide. The actual prevalence of severe CA-MRSA disease is probably higher than reported. An analysis of sensitivity and specificity of the surveillance system was not feasible at this time, but should be attempted in the future. According to this analysis, severe CA-MRSA infections peak during the summer months; however, the majority of deaths occur during winter. This is probably attributable to higher prevalence of primary pneumonia during winter months. Sex and race are not significant risk factors for mortality; however, patients aged >18 years are at higher odds of death. In the three reported clinical syndromes (SSTI, invasive disease, pneumonia) MRSA pneumonia was associated with the highest odds of death.
The fact that the majority of patients with fatal CA-MRSA SSTIs had drained their lesions at home with no medical assistance and that all patients with CA-MRSA pneumonia had an antecedent ILI suggests important CA-MRSA risk factors for further study. The role of influenza virus co-infection in MRSA or CA-MRSA pneumonia is under investigation. However, specific recommendations, besides the recommendations for influenza vaccination of persons with underlying illnesses, regarding vaccination of colonized patients or patients with active disease have not been made. These results underscore the need for medical providers to be aware of severe CA-MRSA infections and their potentially fatal consequences and to emphasize personal hygiene and other measures for infection control. The Centers for Disease Control and Prevention (CDC), GDPH, and other public health organizations have developed messages to advise the public and the medical community about the increasing incidence of CA-MRSA SSTIs and infection prevention [Reference Gorwitz8, 9]. Because S. aureus is primarily spread by skin-to-skin contact, persons with CA-MRSA infections are advised not to share such personal items as towels, razors, or bar soap, and to practice thorough hand hygiene. Clinical guidelines for treatment of SSTIs recommend incision and drainage as the primary therapy; only in certain cases is empirical antibiotic therapy indicated [Reference Gorwitz8, 9].
In order to engage stakeholders in addressing the increase of CA-MRSA infections, GDPH and other public health partners have created a MRSA task force, with initiatives that include outreach to clinicians, patient education, and outreach to groups at increased risk (e.g. school children, athletes, trainers, and inmates of correctional facilities). Surveillance data from the severe CA-MRSA disease surveillance system was compiled and used to focus the task-force activities. The Georgia surveillance system requires resources both in public health and clinical and laboratory disease reporters. Challenges for reporting severe CA-MRSA cases include determining whether an infection is community-associated and specifying the type of clinical syndrome. The reporting definition of the described surveillance system might be altered in future to improve acceptability in reporters and to allow for more detailed risk factor assessment. One option discussed among task-force members is a laboratory-based reporting system that does not require differentiation between hospital-acquired and community-associated disease. An alternative, or possibly complementary system, might be a sentinel site surveillance system, with collection of detailed data from a smaller number of healthcare facilities. In a recent publication, the authors proposed a two-tiered model of MRSA surveillance involving both state-level coordination and participation of local reporters (e.g. hospitals, healthcare providers, clinical laboratories, and such other institutions as correctional facilities). The limitations of such a surveillance system might be the required necessary costs and resources, for which the state or local government would be responsible [Reference Simons and Alcabes10].
This is the first study to analyse the risk of death in patients with severe CA-MRSA disease associated with three different clinical syndromes – pneumonia, invasive disease, and SSTIs. GDPH plans to continue surveillance for CA-MRSA, giving special attention to CA-MRSA pneumonia cases reported during the influenza season because of the associated risk for death and the possibility of prevention through influenza vaccination.
ACKNOWLEDGEMENTS
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.





