Much progress has been made in the screening, diagnosis and management of important pregnancy disorders, but little progress has been made in the prevention of such disorders. Pre-eclampsia remains the second most common cause of maternal death worldwide( Reference Say, Chou and Gemmill 1 ). Prematurity, mostly due to spontaneous preterm birth, is the leading cause of death among infants worldwide( Reference You, Hug and Ejdemyr 2 ). This is followed by growth restriction in term infants( Reference Morisaki, Esplin and Varner 3 ). Furthermore, infants born preterm or with low birth weight have an increased risk of CVD and metabolic diseases later in life, the leading cause of death in adulthood( Reference Abitbol and Rodriguez 4 ). The incidence of gestational diabetes mellitus (GDM) is increasing rapidly worldwide( Reference Lawrence, Contreras and Chen 5 ) and is associated with lifelong risks of metabolic disease in both mother and baby( Reference Whincup, Kaye and Owen 6 , Reference Gunderson, Chiang and Pletcher 7 ).
An association between low vitamin D and adverse pregnancy outcomes was first identified in the early 2000s( Reference Sabour, Hossein-Nezhad and Maghbooli 8 ); consequently, vitamin D has been postulated as a possible intervention strategy to reduce pregnancy complications. A recent meta-analysis found that low vitamin D, defined variably by the authors of included studies, was associated with an increased likelihood of pre-eclampsia (OR 1·79; 95 % CI 1·25, 2·58), small-for-gestational age (SGA) babies (OR 1·85; 95 % CI 1·52, 2·26) and GDM (OR 1·49; 95 % CI 1·18, 1·89)( Reference Aghajafari, Nagulesapillai and Ronksley 9 ). Preterm birth was not included as an outcome. However, most included studies did not correct for important confounders, and those that did were small. In addition, vitamin D deficiency was variably defined between 35 and 80 nmol/l or analysed in late pregnancy( Reference Aghajafari, Nagulesapillai and Ronksley 9 ).
Late pregnancy may not be the optimum time to measure vitamin D, as it is believed that the origins of most late pregnancy disorders are in early pregnancy. Moreover, the expressions of both CYP25B1 (the vitamin D activating enzyme) and vitamin D nuclear receptor( Reference Zehnder, Evans and Kilby 10 ) are highest during the first trimester. In the current study, factors that may alter the development of pregnancy conditions were investigated at the beginning of the second trimester – a time when critical vascular development of the placenta occurs( Reference Pijnenborg, Bland and Robertson 11 ).
The aim of this study was to investigate the relationship between early pregnancy 25-hydroxyvitamin D concentration and subsequent development of pre-eclampsia, spontaneous preterm birth, SGA and GDM in a large, well-phenotyped, prospective cohort.
Methods
Participants
Samples were collected from Auckland participants recruited to the Screening for Pregnancy Endpoints study (SCOPE) between 2005 and 2008. SCOPE is an international, prospective, pregnancy study of nulliparous women, which aims to identify early pregnancy predictors of late pregnancy complications( Reference Chappell, Seed and Myers 12 ). The study was approved by the Auckland University Ethics Committee (AKX/02/00/364). The trial was registered with the Australia New Zealand clinical trial registry (ACTRN 12607000551493).
Non-fasting serum samples were collected at 15 weeks of gestation and stored at −80°C. At the time of sample collection, participants were asked whether they took multivitamins, and the brand used was recorded. Data on socio-economic status( Reference Davis, McLeod and Ransom 13 ) were recorded, and participants were asked whether they smoked. Participants were also asked to rate their physical activity (over the previous 3 months) as vigorous, moderate, recreational walking or no physical activity, and the frequency at which they engaged in physical activity was recorded. Data were entered into an Internet-accessed, password-protected, centralised database with complete audit train (MedSciNetAB).
Pre-eclampsia was diagnosed according to the International Society for the Study of Hypertension in Pregnancy criteria( Reference Brown, Lindheimer and de Swiet 14 ) as gestational hypertension (systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg on at least two occasions) with proteinuria or any multisystem complication of pre-eclampsia( Reference North, McCowan and Dekker 15 ). SGA was defined as weight below the 10th customised birth weight centile( Reference McCowan and Stewart 16 ), and spontaneous preterm birth was defined as delivery before 37 weeks of gestation. Women were screened for GDM between 24 and 28 weeks of gestation with a non-fasting 50-g polycose challenge in community laboratories, according to the Auckland District Health Board Guidelines. Those with a positive polycose test (defined as 1 h post-challenge glucose of ≥7·8 mmol/l) were further tested with a fasting 75-g oral glucose tolerance test (OGTT). A total of forty-four women considered to be at high risk of GDM underwent an OGTT without a polycose test. GDM was diagnosed if fasting glucose was ≥5·5 nmol/l or if the 2-h post-glucose challenge showed values ≥9·0 mmol/l.
Sample analysis
Serum samples were prepared using PhreeTM phospholipid removal cartridges (Phenomenex) after addition of the internal standard deuterated 25-hydroxyvitamin D3 (Isosciences). Separation of 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 was achieved with a KinetexTM pentaflurophenyl 100×3-mm 2·6-μm HPLC column (Phenomenex). 25-Hydroxyvitamin D3, c3-epi 25-hydroxyvitamin D3 and 25-hydroxvitamin D2 were analysed by liquid chromatography tandem MS (Finnigan TSQ Quantum Ultra™ triple quadrupole mass spectrometer; Thermo Electron Corporation). Total 25-hydroxyvitamin D was calculated by the sum of these three compounds. The intra- and inter-assay CV were 7·3 and 4·9 % for 25-hydroxyvitamin D3 and 8·2 and 5·8 % for 25-hydroxyvitamin D2, respectively. Quality control was ensured through participation in the Vitamin D External Quality Assessment Scheme DEQAS programme operating out of Charing Cross Hospital, London( Reference Carter, Berry and Gunter 17 ), and the use of commercially available control materials of known vitamin D concentration. Vitamin D binding protein (VDBP) was analysed using an ELISA from R&D systems.
Statistical analysis
All statistical analyses were performed in SPSS (IBM) version 21. Normality was tested by assessing the histogram and tests for skewness and kyphosis. Continuous variables are reported as mean values and standard deviations. χ 2 Tests were performed for categorical variables and the t test for continuous variables. Fisher’s exact test was performed when χ 2 was not appropriate. The dates of sample collection were categorised into seasons as follows: summer, December to February; autumn, March to May; winter, June to August; and spring, September to November. Logistic regression analyses were performed to determine whether 25-hydroxyvitamin D concentration was associated with the development of GDM, pre-eclampsia, SGA and spontaneous preterm birth. Only participants screened for GDM were included in the analysis for GDM. Multiple logistic regression with backwards selection using the likelihood ratio criteria was used to incorporate known risk factors for adverse pregnancy outcomes, including age, BMI, ethnicity, smoking status and socio-economic status. The analysis was performed with 25-hydroxyvitamin D as a continuous variable and vitamin D level as a categorical variable. Vitamin D categorical variables were created with cut-offs at 25, 50 and 75 nmol/l. These cut-offs were based on the Institute of Medicine definition of deficiency (<50 nmol/l)( Reference Ross, Taylor and Yaktine 18 ) and the Endocrine Society clinical practice guidelines definition of insufficiency (<75 nmol/l) and severe deficiency (<25 nmol/l)( Reference Holick, Binkley and HA 19 ). Hosmer–Lemeshow goodness of fit was performed to test the adequacy of the model (a value of 1 indicates perfect correspondence between the model-predicted and observed risks).
Results
A total of 2065 women were recruited to the SCOPE study in Auckland. Of those women, 355 did not have 15-week serum or plasma samples for analysis. In total, 1710 women were included in the final analysis. Of those, 166 participants were not screened for GDM, and 1544 women were included in the final analysis for that outcome. Among them, thirty-two (2·1 %) women were diagnosed with GDM, seventy-three (4·2 %) women were diagnosed with pre-eclampsia, seventy (4·1 %) women spontaneously delivered preterm infants and 170 (9·9 %) women delivered SGA infants (Fig. 1). The majority of women were of New Zealand (NZ) European ethnicity (83·8 %). The remaining ethnic groups were Asian (n 91, 5·3 %), Maori (n 57, 3·3 %), Indian (n 65, 3·8 %), Pacific Islanders (n 34, 2·0 %) and African (n 30, 1·8 %) (combined as ‘other ethnicities’ in Table 1).
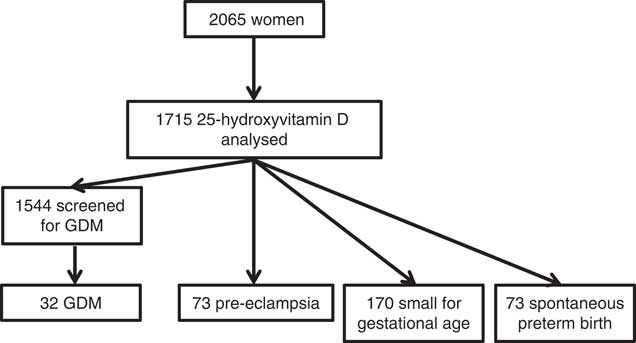
Fig. 1 Cohort flow diagram of Auckland SCOPE participants from recruitment at 15 weeks’ gestation.
Table 1 Maternal characteristics, 25-hydroxyvitamin D concentration at 15 weeks of gestation and pregnancy outcomes (Numbers and percentages; mean values and standard deviations)
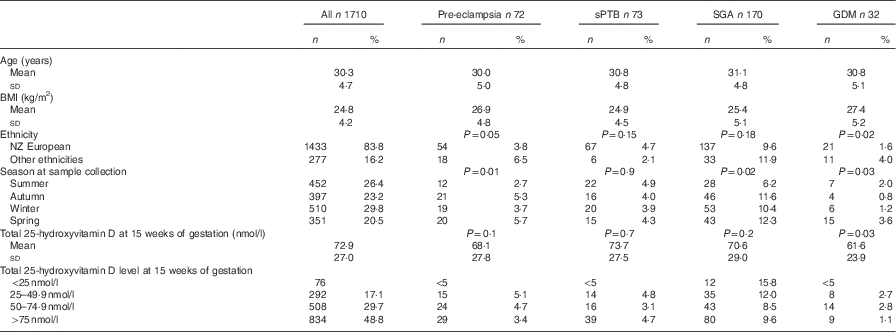
sPTB, spontaneous preterm birth; SGA, small for gestational age; GDM, gestational diabetes mellitus.
Overall, 55 % of women took multivitamin supplements containing vitamin D; one brand contained vitamin D2 and was taken by twenty women. The dose of vitamin D supplement ranged from 5 to 20 μg/d. Women who took multivitamin supplements containing vitamin D had significantly higher 25-hydroxyvitamin D concentrations than those who did not (93·6 (sd 29·6) v. 75·8 (sd 32·6) nmol/l; P<0·001). The proportion of women who took multivitamin supplements containing vitamin D was lower among those who developed GDM, although this was not statistically significant (38 v. 55 %; P=0·4).
Concentrations of 25-hydroxyvitamin D were approximately normally distributed (skewness −0·03, se 0·05; kurtosis −0·3, se 0·1). Just over half of the sample (53 %) had 25-hydroxyvitmamin D concentrations <75 nmol/l, 22·7 % had concentrations <50 nmol/l and 4·4 % had concentrations <25 nmol/l. There was a significant difference in the mean BMI between women who developed GDM and pre-eclampsia and women who did not (P=0·001) (Table 1). The prevalence of GDM and pre-eclampsia varied significantly depending on ethnicity (P=0·02 and 0·05, respectively), with Indian women having the highest prevalence of GDM and NZ European women the lowest. Pacific Island women had the highest prevalence of pre-eclampsia. The mean total serum 25-hydroxyvitamin D concentration was lower in those who developed GDM than in those who did not (61·6 (sd 23·9) nmol/l as compared with 72·9 (sd 27·0) nmol/l; P=0·03) but not lower in women who developed pre-eclampsia (68·1 (sd 27·8) nmol/l) or delivered preterm (73·7 (sd 27·5) nmol/l) or SGA infants (70·6 (sd 29·0) nmol/l) (Table 1). Of women who developed GDM, 72 % had a concentration <75 nmol/l and 22 % had a concentration <50 nmol/l. Women of European decent were the largest ethnic group; there was no significant difference in mean total 25-hydroxyvitamin D in those who developed GDM and in those who did not (70·6 v. 75·9 nmol/l; P=0·35).
There was an increased likelihood of developing GDM if total 25-hydroxyvitamin D was <75 nmol/l (OR 2·3; 95 % CI 1·1, 5·1). However, after adjustment for BMI and ethnicity, this difference was not significant (adjusted OR 1·8; 95 % CI 0·8, 4·2). There was no increased likelihood of developing any of the adverse pregnancy outcomes at concentrations <50 and <25 nmol/l (Table 2).
Table 2 Pregnancy outcome associations with vitamin D as a continuous variable and at cut-offs <50 nmol/l (compared with ≥50 nmol/l) and <75 nmol/l (compared with ≥75 nmol/l), unadjusted and adjusted for BMI and ethnicity (Odds ratio (OR) and 95 % confidence intervals)
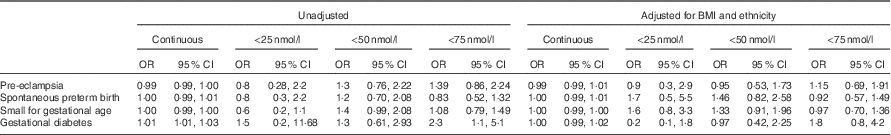
On logistic regression, 25-hydroxyvitamin D concentration as a continuous variable was associated with an increased likelihood of developing GDM with a 1 % increase in risk for every 1 nmol/l reduction in 25-hydroxyvitamin D (OR 1·01; 95 % CI 1·01, 1·03; P=0·03) (Table 2). Following inclusion of BMI and ethnicity in the model, the relationship between 25-hydroxyvitamin D concentration and GDM was no longer significant. Using the International Association of Diabetes in Pregnancy criteria for the diagnosis of GDM increased the number of GDM cases (n 48) but did not increase the significance of the relationship with 25-hydroxyvitamin D.
In a logistic regression model including vitamin D and season, vitamin D was not a significant contributor. After adjustment for BMI and ethnicity, season remained a significant risk factor for GDM (adjusted OR 4·2; 95 % CI 1·3, 13·0; P=0·04). Season was also a significant predictor of developing pre-eclampsia (adjusted OR 2·2; 95 % CI 1·0, 4·5; P=0·04) and delivering SGA infants (adjusted OR 2·1; 95 % CI 1·3, 3·5; P=0·003). After adjustment for BMI and ethnicity, exercise (P=0·1), age (P=0·7), socio-economic status (P=0·8) and smoking status (P=0·2) were not statistically associated with GDM and are not included in the model shown in Table 2.
VDBP was analysed, and free 25-hydroxyvitamin D was calculated for a subgroup of participants (32 GDM with 4 BMI- and ethnicity-matched controls). VDBP concentration was significantly correlated with 25-hydroxyvitamin D concentration (P=0·03). No correlation between calculated free 25-hydroxyvitamin D and GDM was found.
Discussion
In this large, well-phenotyped cohort, we found that 25-hydroxyvitmain D concentration was not lower at 15 weeks of gestation in women who later developed pre-eclampsia, spontaneous preterm birth or who had SGA infants. Concentrations were lower in women who developed GDM but did not predict GDM when adjusted for BMI and ethnicity.
This cohort differs from most other pregnancy cohorts in that it is relatively vitamin D replete. Although 23 % of Auckland SCOPE participants were vitamin D deficient (25-hydroxyvitamin D<50 nmol/l), half to more than 90 % of pregnant women have been found to be deficient in previous studies( Reference Flood-Nichols, Tinnemore and Huang 20 – Reference Park, Yoon and Ryu 22 ). The SCOPE Auckland cohort may be more vitamin D replete in comparison with the obstetric Auckland population and the wider New Zealand population. Auckland is in the northern region of New Zealand where it is estimated that 24 min of hand and face exposure to the sun daily is adequate. This is not the case for much of New Zealand’s geographical distribution( Reference Nowson, McGrath and Ebeling 23 ). NZ European women had the highest 25-hydroxyvitamin D concentrations by ethnicity, and although they represented 83·8 % of Auckland Scope, at the time of sample collection, they represented 49·0 % of women delivering at Auckland City Hospital( 24 ), and according to the most recent National Women’s report they represented 44 % of women delivering at Auckland city hospital( 25 ).
In the SCOPE cohort, there are limited proportions of women with vitamin D concentrations <25 nmol/l (4·4 %). We believe that this has not significantly altered the association of other outcomes, given that previous studies investigating the relationship between 25-hydroxyvitmain D concentration and pregnancy outcomes have reported both positive and negative associations in populations with high and low prevalence of vitamin D deficiency (summarised in Table 3).
Table 3 Comparison of observational studies of vitamin D status and pregnancy outcomes with the Screening for Pregnancy Endpoints study (SCOPE) cohort (Mean values and standard deviations, interquartile range (IQR), 95th percentile and range)
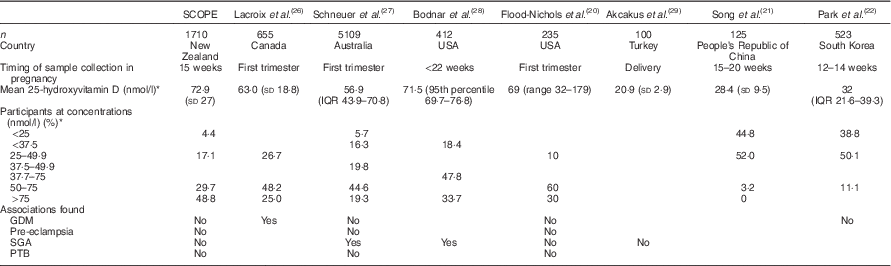
GDM, gestational diabetes mellitus; SGA, small for gestational age; PTB, preterm birth.
* ng/ml has been converted to nmol/l for the purpose of comparison.
In some vitamin D-replete populations of pregnant women, significant associations with GDM( Reference Lacroix, Battista and Doyon 26 ) and SGA( Reference Schneuer, Roberts and Guilbert 27 ) have been found. In a similarly replete population, investigators found no association between vitamin D status and pregnancy outcomes including pre-eclampsia, SGA, preterm birth and GDM( Reference Flood-Nichols, Tinnemore and Huang 20 ). However, in vitamin D-deficient populations, no association between 25-hydroxyvitamin D concentration and SGA( Reference Hoffmeyr, Lawrie and Atallah 30 ) and GDM( Reference Park, Yoon and Ryu 22 ) was found (Table 3).
The main limitation to our study was the comparatively low prevalence of late pregnancy disorders, particularly GDM; this was due to the purpose for which SCOPE was established – that is, to investigate predictors of late pregnancy disorders in early pregnancy in low-risk, nulliparous women( Reference Chappell, Seed and Myers 12 ). We did not have data on sunlight exposure – a factor that contributes most to 25-hydroxyvitamin D concentration. Given the lack of an association between vitamin D and pregnancy outcomes in this study, we do not believe these data would have altered the outcome.
Our findings are supported by a recent meta-analysis on trials of vitamin D supplementation in pregnancy and pregnancy outcomes( Reference Pérez-López, Pasupuleti and Mezones-Holguin 31 ). There was no difference in the incidence of pre-eclampsia, preterm birth, low birth weight and GDM with vitamin D supplementation in pregnancy. However, a recently updated Cochrane systematic review on vitamin D supplementation in pregnancy found a reduced incidence of pre-eclampsia, preterm birth and low birth weight (<2500 g)( Reference De-Regil, Palacios and Lombardo 32 ). These results were sensitive to the inclusion, or not, of trials using low-dose vitamin D supplementation. This highlights the need for pregnancy outcomes to be evaluated in ongoing, randomised trials of vitamin D supplementation in pregnancy.
There may be a number of reasons for the inconsistency in the literature. The aetiology of pre-eclampsia, preterm birth, SGA and GDM are not fully understood and is likely to be multi-factorial. Vitamin D deficiency maybe an important predictor in some populations and not in others. For example, because vitamin D increases intestinal absorption of Ca, in a population where dietary calcium is particularly low, vitamin D status maybe an important predictor for developing pre-eclampsia but not when dietary Ca is adequate( Reference Hoffmeyr, Lawrie and Atallah 30 ). Genetic polymorphisms of the vitamin D nuclear receptor and vitamin D binding protein have been associated with a wide variety of conditions( Reference Gnagnarella, Pasquali and Serrano 33 , Reference Koplin, Suaini and Vuillermin 34 ), and may explain why a study in the USA found that vitamin D deficiency (<37·5 nmol/l) was a risk factor for SGA in white women (adjusted OR 7·5; 95 % CI 1·8, 31·9) but not in black women (adjusted OR 1·5; 95 % CI 0·6, 3·5)( Reference Bodnar, Catov and Zmuda 28 ). This study found a non-linear association between 25-hydroxyvitamin D concentration and SGA, with an increased risk of SGA with concentrations >75 nmol/l (OR 2·2; 95 % CI 1·2, 3·8).
Vitamin D may be a surrogate marker for other causative factors that vary between populations, particularly those with seasonal variation. We found a significant variation in GDM, pre-eclampsia and SGA with season that was not explained by 25-hydroxyvitamin D concentration. Factors that vary with season may be population specific, especially dietary patterns. A study on pregnant women in New Zealand found significant variations in macronutrient and micronutrient intakes according to season, including lower dietary Ca consumption in winter and summer( Reference Watson and McDonald 35 ). A recent study has demonstrated seasonal variation in gene expression in human lymphocytes and adipocytes( Reference Dopico, Evangelou and Ferreira 36 ). These cell types play important roles in insulin sensitivity and inflammation. Although it is not yet known how cell function is affected, these variations in gene expression may be relevant to GDM and pre-eclampsia risk. Others have found a significant variation in the prevalence of pre-eclampsia with season( Reference TePoel, Saftlas and Wallis 37 ). In our data, the association of season and GDM remains significant after adjustment for BMI and ethnicity, consistent with greatest risk if early pregnancy occurs during the winter and early spring, when 25-hydroxyvitamin D concentrations are lowest.
In this vitamin D-replete pregnancy cohort, 25-hydroxyvitamin D concentration did not predict pregnancy outcomes including pre-eclampsia, SGA, spontaneous preterm birth and GDM when adjustments were made for confounders. Season was identified as a significant predictor for GDM. This does not exclude a potential contribution of vitamin D to GDM risk, but also raises the possibility of other pertinent seasonal factors.
Acknowledgements
The authors acknowledge Rennae Taylor (manager), Auckland SCOPE biobank, Department of Obstetrics and Gynaecology, The University of Auckland, and Associate Professor Timothy Kenealy, School of Medicine, The University of Auckland.
Funding was provided by Gravida via Uniservices (grant no. 33015.001), a Centre of Research Excellence of the NZ Government Tertiary Education Commission.
P. N. B. and L. C. K. conceived the study, provided supervision and reviewed the manuscript. V. T. B. analysed samples and the data and drafted the manuscript. E. B. T. assisted with sample analysis and reviewed the manuscript. D. M. assisted with sample analysis. M. B. J. assisted with data analysis and reviewed the manuscript. L. M. E. M. provided the samples and reviewed the manuscript.
The authors have no conflicts of interest to declare.







