Pulmonary regurgitation and pulmonary stenosis exist as important morbidity causes after the surgery for CHDs such as Rastelli, Ross, and total repair of tetralogy of Fallot. Percutaneous pulmonary valve implantation has been performed as a less invasive alternative to surgery for the last 20 years.Reference Bonhoeffer, Boudjemline and Saliba1
Since then, the successfully treated cases increase and favourable outcomes have been established which are equal to or better than the surgical outcomes.Reference Holzer and Hijazi2–Reference Asoh, Walsh and Hickey5 The patients with percutaneous pulmonary valve implantation have lower morbidity and need very seldom re-operations compared to the ones with the surgery.Reference Nordmeyer, Coats and Bonhoeffer6,Reference Lurz, Gaudin, Taylor and Bonhoeffer7 The significant cost benefits have been also shown in percutaneous pulmonary valve implantation procedures over the surgery.Reference Haas, Carere and Kretschmar8 The complications of percutaneous pulmonary valve implantation are stent fracture, conduit rupture, arrhythmias, coronary compression, and endocarditis.Reference Haas, Carere and Kretschmar8 Although Harmony (Medtronic) self-expandible valve has FDA approval in the USA, Pulsta (Taewoong medical) and Venus P (Venustech) valve are still pending certification. There are so far two CE-marked and FDA-approved valve options for commercial use such as the Melody transcatheter pulmonary valve (Medtronic, Minneapolis, Minn, USA) and the Sapien Edwards XT (Edwards Lifesciences, Irvine, Ca, USA).
Although Melody valve has been established as a good alternative in percutaneous pulmonary valve implantation in 2006 and excellent results have been achieved according to 11 years follow-up, there are limitations such as stent fracture and high rate of infective endocarditis, and this system is not appropriate for the right ventricular outflow tracts larger than 24 mm.9–Reference Eicken, Ewert and Hager11 Melody has larger landing zone size which makes it vulnerable for the fracture, so pre-stenting is necessary.Reference Cools, Brown and Budts12 Endocarditis was also detected in 11% of the cases 6 years post-procedure and 16% of patients 8 years post-procedure series.Reference Eicken, Ewert and Hager11,Reference Biernacka, Rużyłło and Demkow13 The Edwards Sapien which received a CE label for the pulmonic position in 2010 was approved as an alternative for dysfunctional conduits and has an advantage over Melody regarding less infective endocarditis risk and fracture rate.Reference Nordmeyer, Ewert and Gewillig14–Reference Sinha, Aboulhosn and Asnes17 The further development of Sapien, the Sapien S3, is also used in an off-label setting.Reference Morgan, Sadeghi and Salem18
Percutaneous pulmonary valve implantation was proven as a safe and less invasive method with better outcomes but needs still improvements to reduce complications such as pulmonary insufficiency and conduit dysfunctions. Considering the limited options in a such promising area, alternative valves are needed to be evaluated. Herein, we present a case series that is treated by off-label use of a new transcatheter valve system Meryl Myval ® (Meril Life Sciences Pvt. Ltd., Vapi, Gujarat, India) which is designed for transcatheter aortic valve implantation procedures.
Matherials and methods
Product
Myval ® is characterised by a nickel–-cobalt alloy frame composed of a single element – hexagon arranged in a hybrid honeycomb fashion.Reference Sinha, Aboulhosn and Asnes19 This unique structure of hybrid honeycomb allows 53% of the frame to have large open cells toward the aortic end, which preserves the coronary flow, and 47% of the frame to have closed cells with higher annular radial force toward the ventricular end (Fig 1).Reference Sengottovelu, Kumar and Seth20
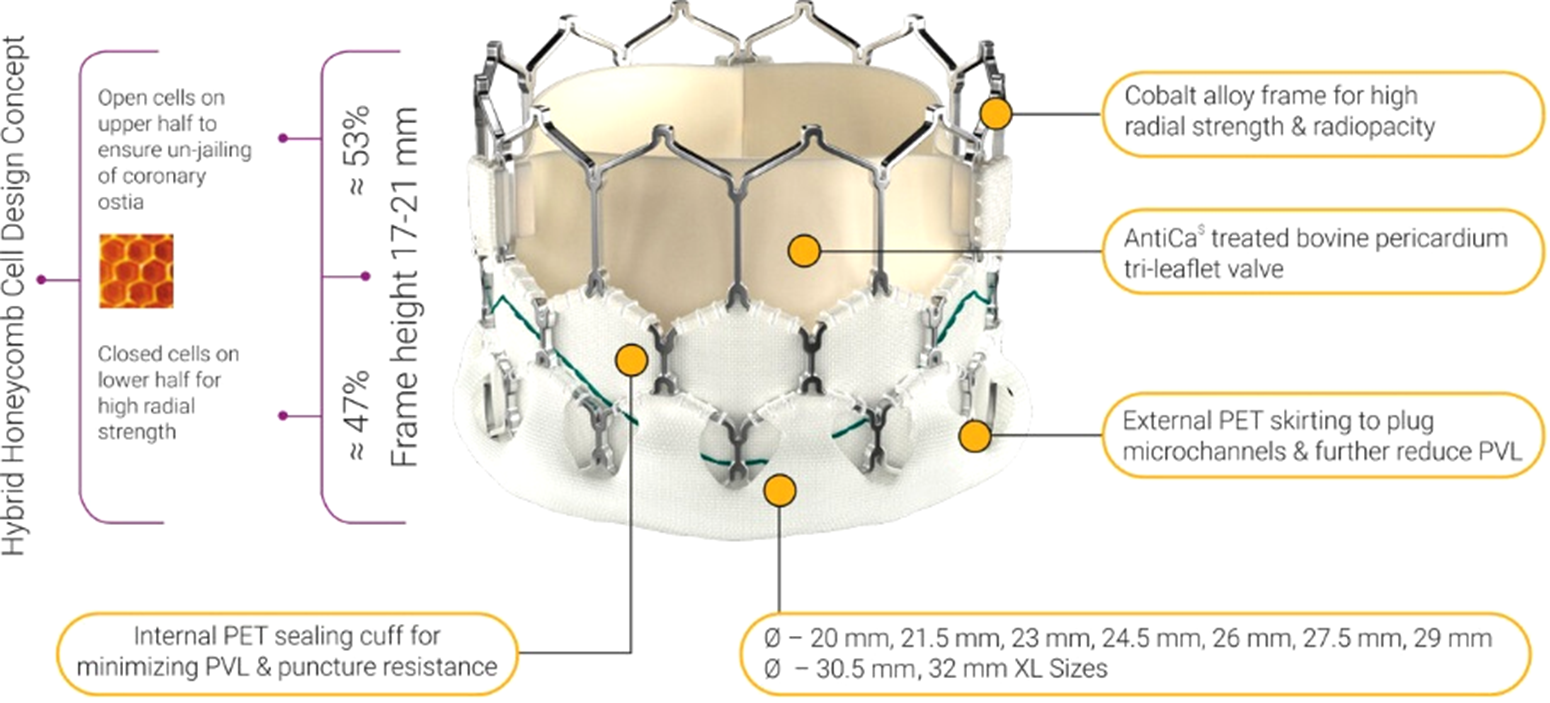
Figure 1. Myval ® product details.
Myval ® system cobalt alloy frame, sewn bovine pericardium leaflets. All size from 20 to 32 mm sizes in 1.5-mm increaments are available in the markets. The length of the valve is changing from 17 to 21 mm. Half of the valve is a closed cell and an outline polyethylene terephthalate skirt exists in to avoid paravalvular leakage.Reference Sinha, Aboulhosn and Asnes19–Reference Seth24
The Navigator delivery system (Fig 2) has two stoppers to prevent embolisation during advancement of the valve. Python 14 F expendable sheath is quite similar to the Edwards e-sheath and allows to introduce Myval ® into the vessel. Valve is crimped on to the balloon directly instead of the delivery pusher catheter (Fig 3). Therefore, there is no need for repositioning of the valve in the vessel.
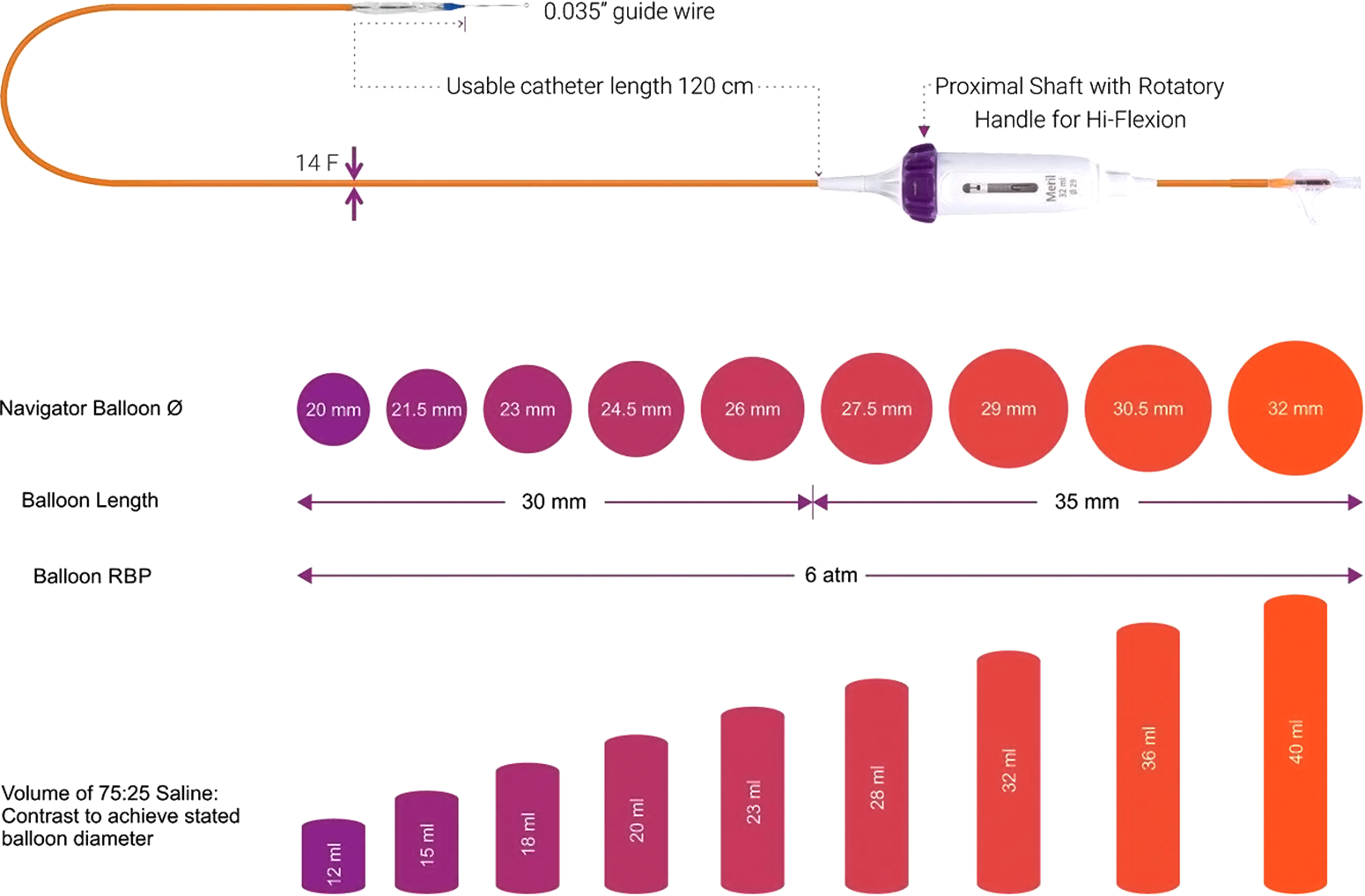
Figure 2. Details of the delivery system and the available sizes of the Myval ®.
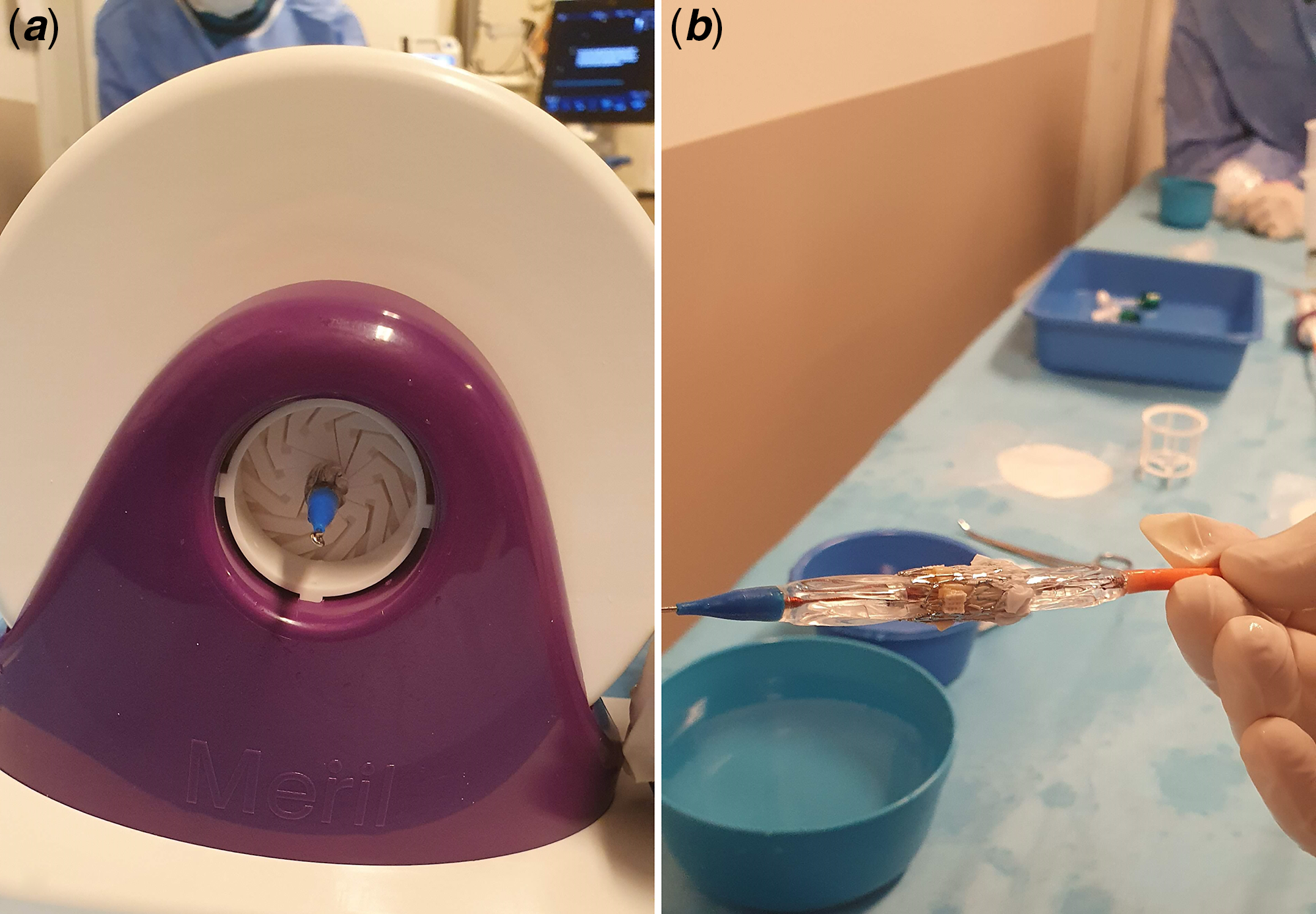
Figure 3. ( a ) Crimping of the valve by crimping device. ( b ) Valve on the balloon after crimping.
Patients
We present our experiences with nine cases between 8 and 34 years of age using Myval for the pulmonary position between June and November, 2020. The demographic, clinical characteristics, and pre-procedural MRI findings are shown in Table 1, and procedural details of the patients are summarised in Table 2. Tetralogy of Fallot, transposition of great arteries with ventricular septal defect, pulmonary atresia, and pulmonary stenosis are the diagnosis of our patients. Three of the patients had conduit dysfunction. In six patients, the valve was implanted into the native right ventricular outflow tract. MRI was performed for all patients before the procedure to reveal the volume and function of right ventricle and severity of the pulmonary regurgitation. MRI findings of the second patient were not consistent with the indications, but the procedure was performed because of the severe symptoms.
Table 1. Demografic and clinical characteristics of the patients

IVS+PA = intact ventricular septum with pulmonary atresia; PS = pulmonary stenosis; RVEDV = right ventricular end diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end systolic volume; TGA = transposition of the great arteries; TOF = tetralogy of Fallot; VSD = ventricular septal defect.
Table 2. Technical details of the prestenting and valve implantation procedure

CP = Cheatham Platinum Stent.
Procedure
Cephazolin, 50 mg/kg, was given intravenously for prophylaxis before and 6 hours after procedure. Introducers were placed into the femoral artery and vein. Intravenous heparin 50 units per kilogram was given, and additional dose was applied according to ACT levels during procedure. Right heart catheterisation was performed, and right ventricle and pulmonary artery pressure were measured. Right ventricle angiogram performed in right anterior oblique 40 and cranial 20 and lateral 90-degree angle. The narrowest part and the length of the right ventricle outflow tract or conduit were measured (Fig 4a and b). A lunderquist wire was placed into the right pulmonary artery as deep as possible. Balloon occlusion testing for coronary compression interrogation was executed for all patients. Stent implantation was done in all cases before the valve implantation step (Fig 4c and d). The details of the procedure were shown in Table 3. Two or three millimeters larger than the stent diameter size of Meryl valve selected for implantation for native right ventricular outflow tracts. The valve was crimped by the company specialist outside of the patient as before definedReference Gupta21–Reference Seth24. The valve was advanced to the inside of the stent and the balloon inflated slowly during at least 10 heartbeats (Figs 4e and 5d). A control angiogram was performed after the procedure (Figs 4e and 5e). Valve functions were also evaluated after implantation by transthoracic echocardiography. Patients received acetylsalicylic acid, 5 mg/kg for antiaggregation for 1 year.
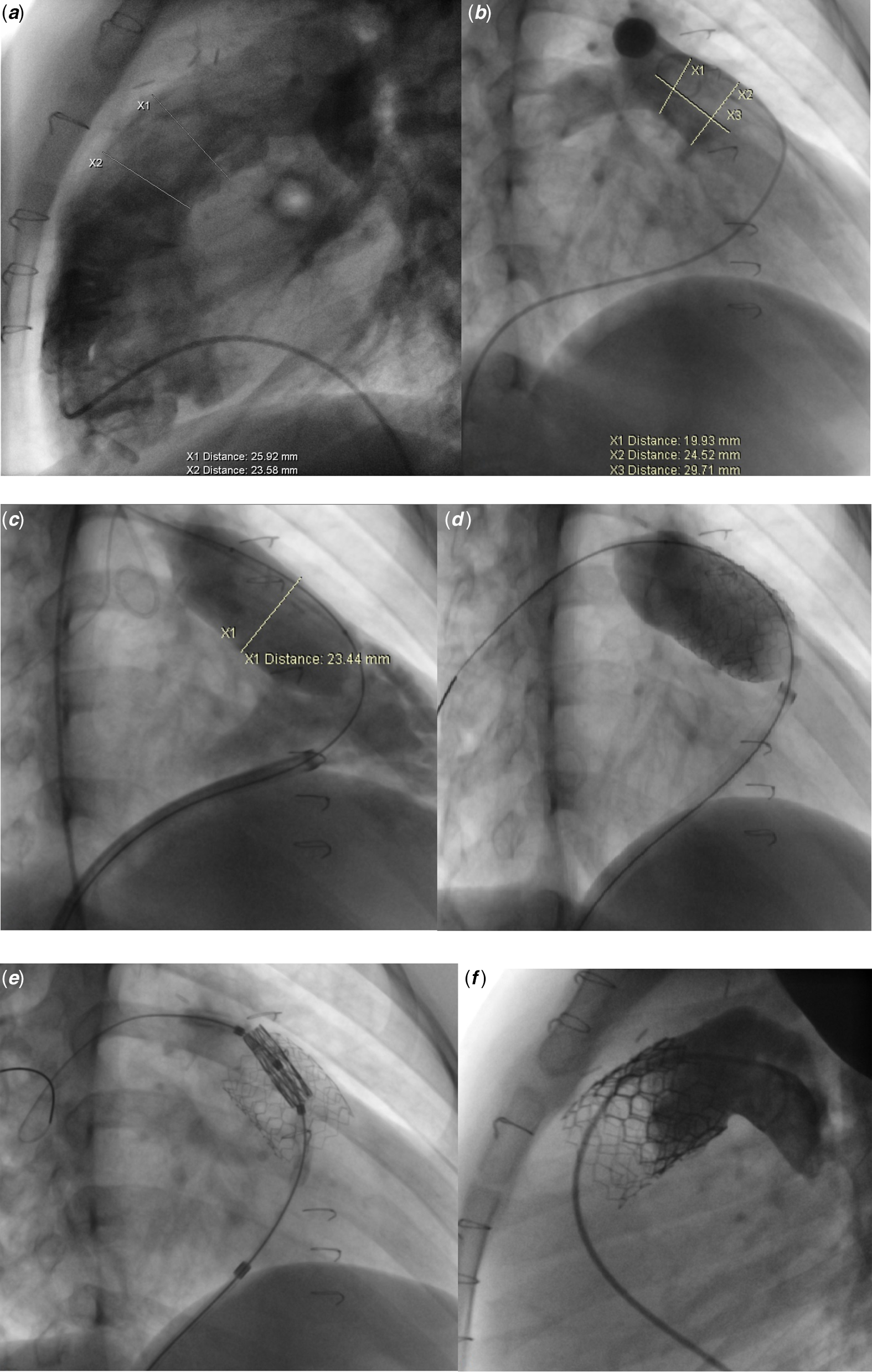
Figure 4. Myval ® implantation procedure steps into the native right ventricular outflow tract. ( a and b ) Measurements of the right ventricular outflow tract and pulmonary arteries in lateral ( a ) and RAO40-cranial 20 position ( b ). ( c and d ) Balloon occlusion test of the right ventricular outflow tract ( c ) and stent implantation ( d ). ( e and f ) Valve implantation ( e ) and control angiogram after implantation showing no regurgitation.
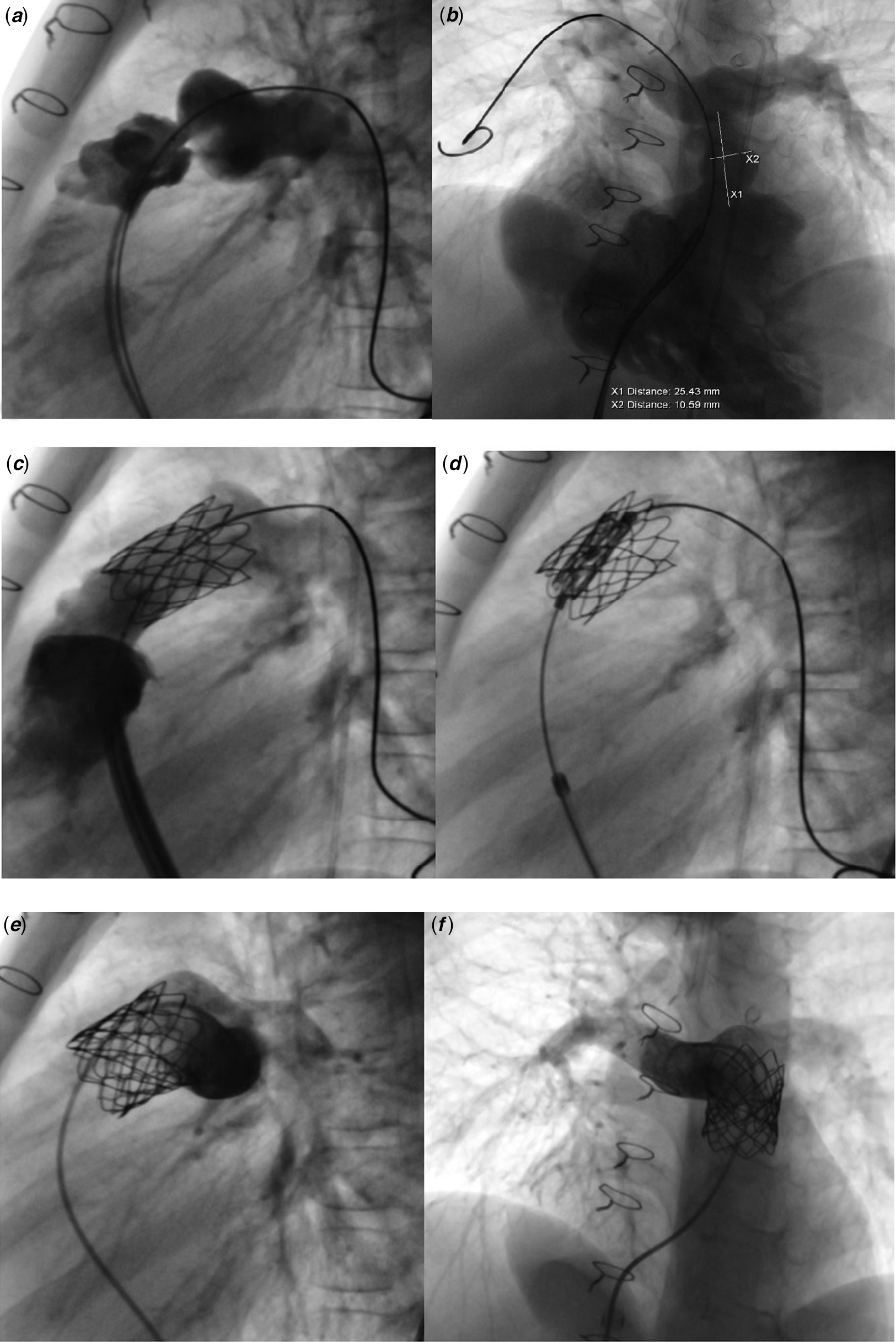
Figure 5. Myval ® implantation procedure steps into the conduit. ( a and b ) Lateral and left oblique-cranial angiogram of right ventricle and conduit. ( c and d ) Implanted stent into the conduit for landing zone ( c ) and valve implantation ( d ). ( e and f ) Lateral and cranial oblique control angiograms of the valve shows no regurgitation throughout valve.
Table 3. Procedural and hospitalisation characteristics of the patients

Results
In all patients, the stent implantation was preceded the valve implantation and the valve implantation was performed successfully. In two patients with native right ventricular outflow tract, the stent and valve were implanted in the same session. Two-month interval between the stent and valve implantation was required for endothelization in other cases. The valve size was 23 mm for the patients with the conduit, 29 mm for the two cases, and 26 mm for the four cases with native right ventricular outflow tract. There is no valvular leakage immediately after the procedure by TTE and angiography. The patients were examined by TTE, ECG, and chest X-ray before the discharge and in the first 3 and 6 months. No dysrhythmia was detected throughout the procedure and during follow-up. The valve functions are also competent after the sixth month. Total follow-up period of the shortest is 6 months and the longest is 14 months (mean 9.8 months). Chest X-ray was investigated in terms of stent or valve frame fracture which did not exist. The right ventricle pressures were higher than two-thirds of the systemic in patients with conduit before the valve implantation and decreased to normal at the end of the procedure as gradient between right ventricle and pulmonary artery less than 15 mm Hg in all patients.
Discussion
Myval ®System received CE mark approval in 2019 for the aortic position; however, it was not yet approved for the pulmonic position. The first case with Myval ® in pulmonary position was published in May 2020 who was a 26-year-old female patient with a history of multiple cardiac surgeries. After the procedure, the patient was asymptomatic and no pulmonary regurgitation or stenosis was detected.Reference Sharma25 Although the article is a successful example, the data are not sufficient. This is the first series of the Myval ® usage in the native right ventricular outflow tract. Additionally, this is the first report regarding using of the Myval ® in patients with large native right ventricular outflow tract.
Myval ® has a similar appearance to the Edwards S3 ® valve, yet it has some differences.
An ideal delivery system for percutaneous pulmonary valve implantation can be advanced from a smaller size of the introducer sheath, easily drivable throughout right ventricular outflow tract and the valve should be retrievable. Considering these properties, Myval ® has advantages over the previously valves. As 29-mm Myval ® requires 14-F sheath, 29-mm Edwards S3 valve 16-F e-sheath is necessary. Melody valve delivery system is 22 F for 24-mm size valves, incomparably larger than Myval ® e-sheath.
Edwards S3 valve is not recommended for retrieval; however, retrieving the Myval ® is quite a straightforward procedure and can be repetitive.
Myval ® delivery system is quite flexible and softer comparing to especially Edwards valve Commander ® delivery system. This property of the system gives an important advantage to advance the valve throughout curved right ventricular outflow tract which is very tricky in some cases with Edwards Commander ® delivery system (Movie S1). The stabilisation of the stent is not safe in most of the cases with native right ventricular outflow tract, so valve implantation was postponed for 2 months in order to achieve endothelization. Because of the flexible and soft property of the Myval ® delivery system, it does not push the stent forcibly and avoids migration. Therefore, in many cases, valve implantation is being possible at the same session as stent implantation.
The radial strength of the valve is quite satisfying and allows using the valve in severely stenotic conduits. Although, we have implanted a stent to create a landing zone and there is no stent fracture or valve dysfunction observed during the follow-up of the patients.
Another difference is that it is crimped on the balloon catheter system, which may be an advantage because the SAPIEN valves are mounted on the balloon in the inferior vena cava.
A 32-mm size valve is an extra advantage for large right ventricular outflow tracts. With this valve size, many of the native right ventricular outflow tract patients can be treated safely comparing the 29-mm Edwards valve.
Additionally, Myval ® reveals a “dog bone” configuration during inflation which is an advantage regarding positioning of the valve, especially in valve in valve procedures (Movie S2).
Implanted valves are functional during the follow-up period and paravalvular leakage is not detected. Because of the external polyethylene terephthalate skirt, paravalvular insufficiency risk is lower.
In conclusion, this study demonstrates our first experiences with Myval ® in the pulmonic position for both conduit and native right ventricular outflow tract. Regarding this experience, Myval ® is a clinically feasible, safe, and effective system for use in the pulmonic position. Further follow-up requires for the assessment of the valve efficacy in long-term period.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951121004650
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Confict of interest
None.
Ethical standards
Informed consent form was obtained from all the patients or the parents. Ethical committee approval was achieved from the Koc University Faculty of Medicine.











