Inflammation, oxidative stress, and metabolic and haemodynamic alterations play a central role in the pathophysiology of many disease states including diabetic nephropathy (DN)( Reference Duran-Salgado and Rubio-Guerra 1 ). DN affects up to 40 % of subjects with type 1 or type 2 diabetes mellitus (T2DM)( Reference Mahata, Argos and Verret 2 ). Patients with DN are not only at significant risk of progression to end-stage renal disease but also at risk of concomitant increase in CVD morbidity and mortality( Reference Palsson and Patel 3 ). Previous studies have reported that microalbuminuria is associated with a 2–3-fold increase in CVD risk( Reference Min, Stephens and Kumar 4 ).
Antioxidant administration including Se has been alleged to play a favourable role in the prevention of diabetic complications( Reference Bonnefont-Rousselot 5 ). DN is a good model of chronic inflammation and oxidative damage( Reference Avci, Cakir and Cevher 6 ); therefore, antioxidant supplementation, especially with Se because of its anti-inflammatory and antioxidant effects( Reference Duntas 7 ), may be useful in decreasing diabetic complications. Previous studies have demonstrated that circulating levels of Se and glutathione peroxidase (GPx) were low in diabetic patients with microalbuminuria( Reference Kornhauser, Garcia-Ramirez and Wrobel 8 , Reference Ozdemir, Ozden and Maral 9 ). In addition, data on the effects of Se supplementation on biomarkers of inflammation and oxidative stress in human and animal studies are conflicting. We have previously shown that Se supplementation for 6 weeks among women with gestational diabetes mellitus( Reference Asemi, Jamilian and Mesdaghinia 10 ) and for 6 months among patients with cervical intraepithelial neoplasia grade 1 (CIN1)( Reference Karamali, Nourgostar and Zamani 11 ) had beneficial effects on biomarkers of inflammation and oxidative stress. However, 200 µg/d Se administration among patients with arsenic-related skin lesions for 6 months did not influence protein carbonyl (PCO) concentrations( Reference Mahata, Argos and Verret 2 ).
The favourable effects of Se supplementation on biomarkers of inflammation and oxidative stress may be mediated by its impact on inhibiting the activation of NF-κB by modulating selenoprotein gene expression( Reference Kretz-Remy and Arrigo 12 ) and involvement in selenoprotein and GPx structure( Reference Ozturk, Batcioglu and Karatas 13 ). As there is evidence that intake of antioxidants such as Se has anti-inflammatory and antioxidant effects, we hypothesised that Se supplementation might help DN patients in controlling their biomarkers of inflammation and oxidative stress. The objective of this study was to evaluate the effects of Se supplementation on biomarkers of inflammation and oxidative stress among DN patients.
Methods
Trial design
This study was a prospective, randomised, double-blinded, placebo-controlled clinical trial.
Participants
For the current study, sixty DN patients aged 40–85 years and referred to Shahid Beheshti Clinic in Kashan, Iran, from March 2015 to June 2015 were included. We defined DN as diabetic renal disease with a proteinuria level of >0·3 g/24 h, with or without circulating levels of serum creatinine( Reference Gross, de Azevedo and Silveiro 14 ). Exclusion criteria were consumption of Se supplements within 3 months, patients with uncontrolled diabetes, pregnant women and those with liver or inflammatory diseases.
Ethics statement
This trial was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all patients. The present study was approved by the Ethics Committee of Kashan University of Medical Sciences (KUMS) and was registered in the Iranian website for registration of clinical trials (http://www.irct.ir: IRCT2015060622562N1).
Study design
At the beginning of the study, all subjects were matched for sex, type and dosage of hypoglycaemic medications used, duration of diabetes mellitus (DM), BMI and age. Participants were then randomly divided into two groups to receive either Se supplements (n 30: fifteen males and fifteen females) or placebo (n 30: fifteen males and fifteen females) for 12 weeks. Participants were requested not to change their regular physical activities and not to take any nutritional supplements that might influence their nutritional status during the 12-week trial. All patients completed 3-d food records and three physical activity records at study baseline, weeks 3, 6 and 9 of the intervention, and at the end of trial. Daily macronutrient and micronutrient intakes were analysed by nutritionist IV software (First Databank) modified for Iranian foods( Reference Asemi, Karamali and Esmaillzadeh 15 , Reference Bitarafan, Harirchian and Nafissi 16 ). In the present study, physical activity was described as metabolic equivalents (MET) in h/d. To determine the MET for each patient, we multiplied the times (h/d) reported for each physical activity by its related MET coefficient using standard tables( Reference Ainsworth, Haskell and Whitt 17 ). The food( Reference Lennernas 18 ) and physical activity( Reference Ainsworth, Haskell and Whitt 17 ) data were collected using validated instruments.
Intervention
In the intervention group, patients received 200 µg/d Se supplements as Se yeast( Reference Karamali, Nourgostar and Zamani 11 ) for 12 weeks. Se supplements and placebos were manufactured by Nature Made Co. and Barij Essence Co., respectively. The appearance of the placebo capsules (starch), including colour, shape, size and packaging, was identical to Se capsules. Quality check of Se supplements was performed at the laboratory of Food and Drug Administration in Tehran, Iran, by Atomic absorption spectroscopy. Following quality control, we found that the amount of Se in the prescribed capsules was at the range of 190–220 µg.
Treatment adherence
Every 4 weeks, participants were given enough supplements to last until 3 d after their next scheduled visit, and were instructed to return all unused supplements at each visit. The remaining supplements were counted and subtracted from the number provided to determine the number taken. To increase compliance, all participants received short messages on their cell phones every day reminding them to take the supplements.
Assessment of anthropometric measures
Weight and height of the participants were determined in an overnight fasting state using standard scales (Seca) at the onset of the study and after the 12-week treatment. BMI was calculated as weight in kg divided by height in metres squared.
Assessment of outcomes
Before the onset of the study and after the 12-week treatment, 10 ml (two separate tubes of 5 ml volume each) fasting blood samples were collected from each patient at the KUMS reference laboratory. Blood was collected in two separate tubes: (1) one without EDTA to separate serum, in order to determine high-sensitivity C-reactive protein (hs-CRP), transforming growth factor-β (TGF-β), matrix metalloproteinase-2 (MMP-2), advanced glycation end products (AGE) and PCO concentrations; and (2) another one containing EDTA to quantify nitric oxide (NO), total antioxidant capacity (TAC), glutathione (GSH) and malondialdehyde (MDA) levels. To separate serum, blood samples were immediately centrifuged (Hettich D-78532) at 3500 rpm for 10 min. Subsequently, samples were stored at −70°C before final analysis at the KUMS reference laboratory. In the current study, the primary outcome variables were pro-inflammatory and inflammatory markers. Serum hs-CRP concentrations were determined using an ELISA kit (LDN) with intra- and inter-assay coefficient variances (CV) of 2·6 and 4·7 %, respectively. Plasma NO concentrations were determined using the Giess method modified by Tatsch et al.( Reference Tatsch, Bochi and Pereira Rda 19 ) with intra- and inter-assay CV of<5·0 %. Serum TGF-β levels were determined using an ELISA kit (Crystal Day) with inter- and intra-assay CV of 6·7 and 8·9 %, respectively. Serum MMP-2 concentrations were quantified by using ELISA kits (Crystal Day) with inter- and intra-assay CV of 5·4 and 6·5 % for MMP-2, respectively. Secondary outcome variables were biomarkers of oxidative stress. Serum AGE were quantified by the fluorometeric method( Reference Kalousova, Skrha and Zima 20 ) with intra- and inter-assay CV of <5·0 %. Serum PCO levels were quantified using the spectrophotometric method( Reference Levine, Garland and Oliver 21 ) with inter- and intra-assay CV of <5 %. Plasma TAC concentrations were determined by the method of ferric reducing antioxidant power developed by Benzie & Strain( Reference Benzie and Strain 22 ); GSH levels were determined by the method of Beutler & Gelbart( Reference Beutler and Gelbart 23 ), and MDA concentrations were determined by the thiobarbituric acid reactive substances spectrophotometric test( Reference Janero 24 ) with intra- and inter-assay CV of <5·0 %. The assay for GPx protein levels was performed using an ELISA kit( Reference Jacobson, Narkowicz and Tong 25 ) (Bioassay Technology) with intra- and inter-assay CV of 7·4 and 9·1 %, respectively. ELISA methods appeared to significantly overestimate true plasma GPx levels. Such overestimations of GPx protein levels should be taken into account in the interpretation of our findings. Renal function was determined using the Cockroft–Gault (CG) formula in ml/min (140−age (years)×weight (kg)/72×serum creatinine×0·85 if female)( Reference Cockcroft and Gault 26 ). HbA1c levels in the whole blood were determined with the Glycomat kit (BiocodeHycel) using the method of exchange chromatography at Kashan reference laboratory. However, because of shortage of funding, we did not evaluate the effects of Se supplementation on HbA1c levels; data on HbA1c were obtained from the records of patients available in the clinic at study baseline and 12 weeks after intervention. Enzymatic kits (Pars Azmun) were used to determine serum creatinine (Jaffe method) and blood urea nitrogen (BUN) concentrations with intra- and inter-assay CV of <5·0 %.
Sample size
On the basis of a formula suggested for clinical trials, we estimated that we would need twenty-five patients in each group, considering a type 1 error (α) of 0·05 and type 2 error (β) of 0·20 (power=80 %), 1959·37 ng/ml as sd and 1600·00 ng/ml as the mean distinction (d) of hs-CRP as the key variable( Reference Razavi, Jamilian and Kashan 27 ). Assuming five dropouts in each group, the final sample size was determined to be thirty patients per group.
Randomisation
Randomisation was achieved using computer-generated random numbers. Randomisation and allocation were concealed from the researchers and participants until the final analyses were completed. Generating the randomised allocation sequence, enrolling participants and allocating them to interventions were conducted by a trained nutritionist at an internal clinic.
Statistical methods
To evaluate whether the study variables were normally distributed or not, we applied the Kolmogorov–Smirnov test. Analyses were conducted on the basis of an intention-to-treat (ITT) principle. Missing values were treated on the basis of the last-observation-carried-forward method (LOCF)( Reference Lachin 28 ). LOCF ignores whether the participant’s condition was improving or deteriorating at the time of dropout, but instead freezes outcomes at the value observed before dropout (i.e. last observation)( Reference Lachin 28 ). For non-normally distributed variables (TGF-β, MMP-2 and AGE), we applied log transformation. To detect differences in anthropometric measures as well as in macronutrient and micronutrient intakes between the two groups, we applied independent samples Student’s t test. To determine the effects of Se administration on biomarkers of inflammation and oxidative stress, we used independent samples Student’s t test. To compare within-group differences (before and after treatment), we used paired-samples t tests. ANCOVA using general linear models assessed differences between groups at the end of the study after adjustment for baseline values of biochemical parameters, age and BMI. P-values <0·05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Science, version 18 (SPSS Inc.).
Results
Among all, four participants from the Se group (withdrawn for personal reasons (n 4)) and four from the placebo group (withdrawn for personal reasons (n 4)) were excluded. In the end, fifty-two subjects (Se (n 26) and placebo (n 26)) completed the trial. However, as the analysis was based on the ITT principle, all sixty patients (thirty in each group) were included in the final analysis.
Mean age, height, weight and BMI at baseline and at the end of the trial and sex were not statistically different between the two groups (data not shown). Mean smoking, duration of DM, consumption of antidiabetic and antilipidaemic drugs, hypertension rate, and consumption of angiontensin-converting enzymes inhibitors and aldosterone receptor blockers were not statistically different between the two groups (Table 1).
Table 1 General characteristics of the study participants (Numbers and percentages; mean values and standard deviations)
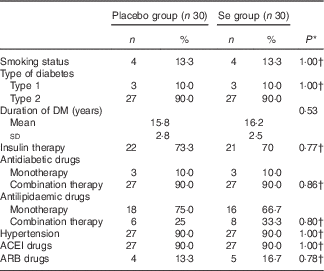
DM, diabetes mellitus; ACEI, angiontensin-converting enzymes inhibitors; ARB, aldosterone receptor blockers.
* Obtained from independent t test.
† Obtained from Pearson’s χ 2 test.
On the basis of the 3-d dietary records obtained at study baseline, at the end of the trial and every 3 weeks throughout the trial, we found no significant difference in mean dietary macronutrient and micronutrient intakes (Table 2).
Table 2 Dietary intakes of study participants throughout the study (Mean values and standard deviations)
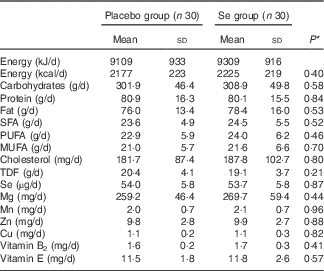
TDF, total dietary fibre.
* Obtained from independent t test.
In unadjusted analyses, after 12 weeks of intervention, compared with the placebo, Se supplementation led to a significant reduction in serum hs-CRP (−1069·2 (sd 1752·2) v. −135·3 (sd 1258·9) ng/ml, P=0·02), MMP-2 (−612·3 (sd 679·6) v. +76·0 (sd 309·1) ng/ml, P<0·001) and plasma MDA concentrations (−0·1 (sd 0·7) v. +0·4 (sd 0·9) µmol/l, P=0·01) (Table 3). In addition, a significant increase in plasma TAC levels (+174·9 (sd 203·9) v. +15·8 (sd 382·2) mmol/l, P=0·04) was observed following Se supplementation when compared with placebo. Subjects who received Se supplements experienced a borderline statistically significant decrease in serum PCO levels (P=0·06) compared with placebo. Supplementation with Se had no significant effect on NO, TGF-β, AGE, PCO, GSH, creatinine and BUN compared with placebo. The use of Se supplements also resulted in a significant rise in plasma GPx levels (+2·3 (sd 21·7) v. −27·7 (sd 35·2) U/ml, P=0·001). However, Se supplementation led to a non-significant reduction in CG (−0·9 (sd 4·5) v. −0·03 (sd 3·7) ml/min, P=0·45) and HbA1c (−0·05 (sd 0·02) v. −0·005 (sd 0·02) %, P=0·08) compared with placebo. Data on HbA1c were obtained from the records of patients available in the clinic at study baseline and 12 weeks after intervention. When the analyses were repeated without the ITT approach, similar outcomes were found (data not shown).
Table 3 Biomarkers of inflammation and oxidative stress at study baseline and after 3 months of intervention in patients with diabetic nephropathy who received either selenium supplements or placebo (Mean values and standard deviations)
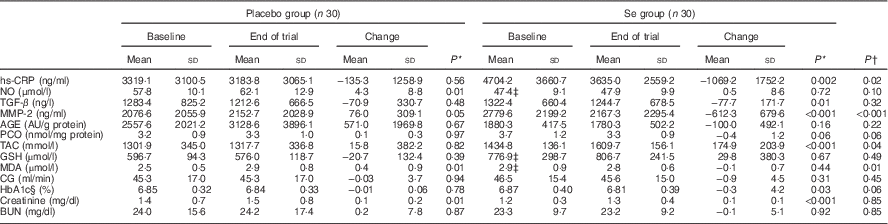
hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TGF-β, transforming growth factor β; MMP-2, matrix metalloproteinase-2; AGE, advanced glycation end products; PCO, protein carbonyl; TAC, total antioxidant capacity; GSH, total glutathione; MDA, malondialdehyde; CG, Cockcroft–Gault formula to estimate creatinine clearance; BUN, blood urea nitrogen.
* Obtained from paired samples t tests to compare baseline v. end-of-trial values within a group.
† P values represent independent samples Student’s t test to compare differences between the treatment groups.
‡ Different from corresponding placebo with independent samples Student’s t test, P<0·05.
§ Based on participants’ measured HbA1c present in their records in the clinic.
Baseline levels of plasma NO (P<0·001), GSH (P=0·003) and MDA (P=0·03) were significantly different between the two groups. Therefore, we controlled the analyses for baseline levels. When we adjusted the analyses for baseline values of biochemical variables, significant changes in plasma NO (P=0·02) and GSH (P<0·001) were observed, but the changes in serum hs-CRP (P=0·08) and plasma MDA levels (P=0·17) were not significantly different between the groups (data not shown). In addition, when we adjusted the analysis for baseline values of biochemical parameters, age and BMI, except for serum hs-CRP (P=0·14), plasma NO (P=0·04), GSH (P<0·001) and MDA levels (P=0·16), the other findings were unaltered (Table 4).
Table 4 Mean adjusted changes in metabolic variables in patients with diabetic nephropathy who received either selenium supplements or placebo (Mean values with their standard errors)
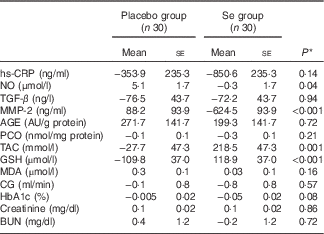
hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TGF-β, transforming growth factor β; MMP-2, matrix metalloproteinase-2; AGE, advanced glycation end products; PCO, protein carbonyl; TAC, total antioxidant capacity; GSH, total glutathione; MDA, malondialdehyde; CG, Cockcroft–Gault formula to estimate creatinine clearance; BUN, blood urea nitrogen.
* Obtained from analysis of ANCOVA adjusted for baseline values+age and baseline BMI.
Discussion
In the present randomised clinical trial, we assessed the effects of Se supplementation on biomarkers of inflammation and oxidative stress among patients with DN. We found that Se supplementation among DN patients had favourable effects on serum MMP-2, plasma NO, TAC and GSH levels, but did not affect hs-CRP, TGF-β, AGE, PCO and MDA levels. To our knowledge, the current study is the first to have assessed the effects of Se supplementation on biomarkers of inflammation and oxidative stress in patients with DN. It must be taken into account that there was a significant difference in plasma NO, GSH and MDA levels between the Se and the placebo groups at baseline. This difference might be due to several reasons. The diagnosis of DN in our study was performed on the basis of criteria of the American Diabetes Association. In other words, when proteinuria levels were >0·3 g/24 h, with or without circulating levels of serum creatinine( Reference Gross, de Azevedo and Silveiro 14 ), we considered such patients as having DN. Furthermore, we did not randomise participants on the basis of their plasma NO, GSH and MDA levels because all participants had DN. Random assignment to two groups was performed after stratification for pre-intervention BMI (<30 and ≥30 kg/m2) and age (<55 or ≥55 years), using computer-generated random numbers. Therefore, the difference in plasma NO, GSH and MDA levels between the two groups occurred randomly. In addition, when we adjusted the analyses for baseline values of these variables, significant changes in plasma NO and GSH were observed, but plasma MDA levels were not significantly different. It must be considered that, as shown in Table 4, we adjusted for several variables including baseline biochemical levels, age and BMI. We performed this analysis by ANCOVA as well as repeated-measures ANOVA. Findings from these two analyses were the same, where we found that after controlling for baseline levels, age and BMI, our intervention did not affect serum hs-CRP and plasma MDA levels. This means that elevated levels of these variables at study baseline were the main reason for their reduction, as shown in Table 4.
In the current study, no side-effects were observed following Se supplementation in DN patients throughout the study. It must be kept in mind that the mean dietary plus supplemental Se intake in our study participants was lower than the upper limits (400 µg)( Reference Monsen 29 ). However, data on the toxic effects of Se on human health are conflicting. For instance, in a study by Burk et al.( Reference Burk, Norsworthy and Hill 30 ), the results showed that intake of Se supplements from moderate (200 µg/d) to large (600 µg/d) doses for 16 weeks among volunteers aged ≥18 years was safe. However, in a Cochrane review( Reference Rees, Hartley and Day 31 ), Se was associated with a small, non-significant increase in diabetes risk, and in another study Se was associated with hair loss, dystrophic fingernail changes, gastrointestinal symptoms and memory difficulties, which are the adverse effects of Se intake( Reference Aldosary, Sutter and Schwartz 32 ). Long-term supplementation with 200 µg Se daily during the blinded phase of the nutritional prevention of cancer (NPC) trial (mean follow-up, 7·7 years) also increased the risk of T2DM( Reference Stranges, Marshall and Natarajan 33 ). In addition, the Se and vitamin E cancer prevention trial (SELECT) results clearly do not support the use of supplemental Se or vitamin E in adult life for primary prevention of cancer. The results of the SELECT study demonstrated that neither Se nor vitamin E alone or in combination decreased the incidence of prostate cancer, and that vitamin E administration significantly increased the incidence of prostate cancer among healthy men( Reference Nicastro and Dunn 34 ). In a post hoc analysis from the NPC trial in the US, Se administration alone (200 µg/d as high-Se yeast) was not significantly associated with any of the CVD end points after 7·6 years of follow-up( Reference Stranges, Marshall and Trevisan 35 ). Nonetheless, further studies are needed regarding the potential toxicity/teratogenicity of increased Se supplementation in patients with DN.
Patients with DN are susceptible to inflammation and oxidative stress( Reference Joven and Anderson 36 ). In unadjusted analyses, we found that Se administration for 12 weeks among patients with DN led to a significant reduction in serum hs-CRP and MMP-2 concentrations compared with the placebo, but did not affect plasma NO and serum TGF-β levels. When we adjusted the analyses for baseline values of biochemical variables, age and BMI, a significant change in plasma NO was observed, but the change in serum hs-CRP was not significantly different between the groups. Supporting our study, supplementation with 200 µg Se/d for 3 months did not affect CRP levels among patients with chronic kidney disease( Reference Omrani, Rahimi and Nikseresht 37 ). In addition, no significant change in hs-CRP levels was seen following the supplementation of 200 µg Se daily among haemodialysis patients for 12 weeks( Reference Salehi, Sohrabi and Ekramzadeh 38 ). Increased inflammatory markers contribute significantly to the development of chronic diseases including CVD, atherosclerosis and cancer( Reference Libby, Ridker and Maseri 39 , Reference Balkwill and Mantovani 40 ). Increased production of reactive oxygen species (ROS), free radicals and pro-inflammatory mediators such as lipid mediators and cytokines including IL-6 and TNF-α aggravates inflammation and excessive damage to host tissues( Reference Balkwill and Mantovani 40 ). The baseline characteristics of the study subjects as well as the dosage of Se supplements along with the study duration might explain the different findings.
In unadjusted analyses, our study demonstrated that taking Se supplements for 12 weeks among patients with DN was associated with a significant elevation in plasma TAC and a significant decrease in MDA levels, but supplementation did not influence serum AGE, PCO and plasma GSH concentrations. When we adjusted the analyses for baseline values of biochemical variables, age and BMI, a significant change in plasma GSH was observed, but the change in plasma MDA levels was not significantly different between the groups. Low levels of serum Se are a frequent finding in subjects with acute kidney injury or chronic kidney disease( Reference Iglesias, Selgas and Romero 41 ). Previous studies have reported that both low circulating levels of Se and renal insufficiency are associated with an increased risk of CHD mortality and all-cause mortality( Reference Eaton, Abdul Baki and Waring 42 ). In line with our study, Se supplementation reduced lipid peroxidation in the cortex and cerebellum of protein malnutrition rats along with neurobehavioural deficits( Reference Adebayo, Adenuga and Sandhir 43 ). A Few studies have evaluated the beneficial effects of Se supplementation on TAC and MDA levels. We have previously demonstrated that 200 µg/d Se supplementation for 6 months among patients with CIN1 increased plasma TAC and decreased MDA concentrations( Reference Karamali, Nourgostar and Zamani 11 ). However, a few studies did not observe such effects of Se supplementation on biomarkers of oxidative stress. For example, Se intake significantly increased MDA and hydroxyl radical levels in the lens of naphthalene-treated rats( Reference Zhu and Lu 44 ). However, human studies have yielded inconsistent results, possibly due to differences in experimental designs, in the type and dose of Se used or in clinical characteristics of the participants such as variations in baseline variables. Previous studies have shown that several biochemical components such as AGE, protein kinase C, molecular oxygen and its derivatives play a critical role in the pathogenesis of DN( Reference Cumbie and Hermayer 45 , Reference Arora and Singh 46 ). In addition, AGE promote the influx of mononuclear cells, stimulate cell proliferation( Reference Torzewski, Rist and Mortensen 47 ) and induce endothelial dysfunction( Reference Pertynska-Marczewska, Diamanti-Kandarakis and Zhang 48 ). Se intake may result in reduced oxidative stress through involvement in selenoprotein and GPx structures( Reference Ozturk, Batcioglu and Karatas 13 ) and by inhibiting production of ROS( Reference Zeng, Zhou and Huang 49 ).
The present study has some limitations. First, owing to shortage of funding, we did not evaluate serum or urine Se levels, selenoprotein P and other biomarkers of inflammation and oxidative stress, including IL-6, TNF-α, catalase and superoxide dismutase. Second, in the current study, the sample size was small. Future studies with a larger sample size are needed to confirm the validity of our findings.
All in all, the current study demonstrated that Se supplementation among DN patients had favourable effects on serum MMP-2, plasma NO, TAC and GSH levels, but did not affect hs-CRP, TGF-β, AGE, PCO and MDA levels. This suggests that Se supplementation at dosage 200 μg/d may confer advantageous therapeutic potential for patients with DN. Further research is needed in other patients and for longer periods to determine the safety of this supplemental approach. Moreover, future studies should measure the expressed levels of related variables with inflammation and oxidative stress to explore the plausible mechanism and confirm our findings.
Acknowledgements
The authors thank Arduino Arduini (CoreQuest Sagl, Manno, Switzerland) for providing helpful suggestions.
The present study was supported by a grant from the Vice-chancellor for Research, KUMS, Iran.
F. B., Z. A., M. K., A. S. and A. A. M. contributed to study conception, data collection and manuscript drafting.
The authors declare that there are no conflicts of interest.







