Introduction
Today, dragonflies (Odonata: Anisoptera) constitute about half of the roughly 6000 living species of Odonata (Dijkstra et al. Reference Dijkstra, Bechly, Bybee, Dow, Dumont and Fleck2013). Damselflies (Odonata: Zygoptera) comprise the rest, except for two species of “damsel-dragonflies” (Odonata: Anisozygoptera), a relictual Asian group that, while extant species appear to form a monophyletic group, may be paraphyletic when fossil taxa are included (Dijkstra et al. Reference Dijkstra, Bechly, Bybee, Dow, Dumont and Fleck2013).
Odonata, including Anisoptera, are predominantly tropical, with less diversity in higher latitudes. A recent study recorded 214 species in Canada and 464 in the United States of America, while Brazil had 736 species, and there appears to be a much higher number of unrecorded and undescribed species in lower latitudes (Cannings Reference Cannings2019).
Corbet (Reference Corbet1999) provided comprehensive detail on the natural history of the order, and Needham et al. (Reference Needham, Westfall and May2000), Paulson (Reference Paulson2009, Reference Paulson2011), Walker (Reference Walker1953, Reference Walker1958), Walker and Corbet (Reference Walker and Corbet1975), and Westfall and May (Reference Westfall and May2006) have written more taxonomic and distributional treatments related to North America. Much of the summary of extant dragonflies below is based on Cannings (Reference Cannings2002).
The aquatic larvae of Anisoptera are among the predominant invertebrate predators in streams, rivers, springs, lakeshores, ponds, and marshes. Adults are large, strong-flying, visually oriented hunters with large eyes, strong mandibles, and spiny legs; they feed upon a wide range of insects that are normally captured in flight.
Although their basic wing structure and arrangement of flight muscles are paleopterous, their flight performance and efficiency are remarkable. Unlike most insects, they usually beat their forewings and hind wings separately, and each wing has much independent control. This accounts for the surprising manoeuvrability of many species, which can fly upwards, sideways, and backwards as well as forwards. A large darner (Aeshnidae) can fly up to 60 km per hour.
Some dragonflies migrate to follow favourable seasonal conditions. Swarms of Pantala flavescens (Fabricius) (Odonata: Libellulidae) annually cross at least 3500 km of ocean between India and Africa as part of a possibly 18 000 km circuit of four generations, crossing the Himalayas at elevations around 6300 m (Anderson Reference Anderson2009, Hobson et al. Reference Hobson, Anderson, Soto and Wassenaar2012).
Dragonflies such as the Aeshnidae are known as flyers, spending most of their active life flying, generating additional body heat from their wing muscles. Others, like the Gomphidae and Libellulidae, are perchers, spending more time at rest, basking in the sun, and taking short flights to catch food or mate (Corbet Reference Corbet1999). Mature males often patrol breeding habitats, aggressively searching for mates and may defend a territory against conspecific males.
The giant Paleozoic insects of the Meganeuridae that are popularly thought of as dragonflies are members of the order Meganisoptera (“Protodonata”), similar-looking stem group relatives of the true odonates (Nel et al. Reference Nel, Fleck, Garrouste, Gand, Lapeyrie, Bybee and Prokop2009). Fossil Odonata are as old as the Late Triassic, and Anisoptera appear in the Middle Jurassic (Kohli et al. Reference Kohli, Ware and Bechly2016). They have a reasonably good compression fossil record, but are poorly known in amber, which has a bias for smaller insects (Bechly Reference Bechly1996; Fleck et al. Reference Fleck, Nel, De Plöeg and Masselot2000; McKellar and Engel Reference McKellar and Engel2012; Schädel and Bechly Reference Schädel and Bechly2016; Archibald et al. Reference Archibald, Rasnitsyn, Brothers and Mathewes2018). Cretaceous Anisoptera consist of modern superfamilies with some modern and numerous extinct families of varying numbers and compositions according to differing authors (Bechly et al. Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001; Rasnitsyn and Pritykina Reference Rasnitsyn, Pritykina, Rasnitsyn and Quicke2002; Grimaldi and Engel Reference Grimaldi and Engel2005). By the Cenozoic, many Mesozoic families have disappeared, leaving extant families and three others (see “Discussion” section) that are only known from the Paleocene and Eocene.
A number of Paleocene and Eocene deposits worldwide bear dragonfly fossils (Table 1), but only a single specimen has been previously reported (Archibald Reference Archibald2007; Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010: RBCM-P558, referred to there as SBA-105) in the rich Ypresian (early Eocene) localities of the Okanagan Highlands series of fossil sites of British Columbia, Canada and Washington, United States of America (Archibald et al. Reference Archibald, Greenwood, Smith, Mathewes and Basinger2011a) and there are few in museum collections of many thousands of fossil insects, all found in the last few decades. This may seem somewhat surprising, as the depositional settings of these lacustrine shales might be thought to represent ideal environments to entrap and preserve insects associated with freshwater bodies and their inflowing waters. This scarcity could, however, result from the large surface area to mass ratio of dragonflies, which increases their floating time before they sink to the substrate, thus increasing their chances of being consumed or decaying on the water surface (Martínez-Delclòs and Martinell Reference Martínez-Delclòs and Martinell1993; Wagner et al. Reference Wagner, Neinhuis and Barthlott1996). Large, unbiased collections are needed to ascertain whether the relative presence of Okanagan Highlands compression fossil Odonata meaningfully differs from that of other sites, or if differing collecting intensities and histories explain the observed abundance patterns.
Table 1. Paleocene and Eocene Anisoptera. Subfamily designations provided for Aeshnidae.
* Extinct family.
† pieces of abdomen, see Nel et al. (Reference Nel, Martínez-Delclòs, Escuillé and Brisac1994);
‡ unnamed aeshnid specimen UAPC 6190: Wighton and Wilson (Reference Wighton and Wilson1986);
§ Bechly et al. (Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001) provisionally transferred this species to Plesigomphaeschnaoides;
‖ extant genus;
# reported here;
** referred to by some authors as from Florissant (Priabonian), it is actually from the Green River Formation (Ypresian);
†† pieces of abdomen ascribed to the Libellulidae by Scudder (Reference Scudder1890);
‡‡ Fleck et al. (Reference Fleck, Nel, De Plöeg and Masselot2000);
§§ Lutz (Reference Lutz1990);
‖‖ Wappler (Reference Wappler2003);
## Nel and Fleck (Reference Nel and Fleck2014);
*** see revision of Nel and Petrulevičius (Reference Nel and Petrulevičius2010);
††† Nel and Jarzembowski (Reference Nel and Jarzembowski1999);
‡‡‡ Cordulephyidae: Nel and Fleck (Reference Nel and Fleck2014);
§§§ Schädel and Bechly (Reference Schädel and Bechly2016): they did not mention if the two undescribed Baltic amber Gomphaeschninae were likely of different genera or species;
‖‖‖ “Gomphoides occulta” of Hagen (Reference Hagen1856): inadequately described, not illustrated, specimen lost (Bechly Reference Bechly1998).
Okanagan Highlands fossil sites are exposures of a series of named and unnamed sedimentary formations that occur sporadically across about 1000 km from the Smithers region in west central British Columbia, Canada, through the southern interior of the province into north central Washington, United States of America, at the town of Republic. A general overview of the Okanagan Highlands biota and environments was provided by Archibald et al. (Reference Archibald, Greenwood, Smith, Mathewes and Basinger2011a, see map therein).
Although these forest communities existed during the Early Eocene Climatic Optimum, a time of the highest sustained global temperatures of the Cenozoic, tectonic forces had raised the region to be an upland of some considerable elevation of lakes in valleys surrounded by mountains (e.g., Ewing Reference Ewing1980; Tribe Reference Tribe2005), with mostly (both localities considered here) mesic, upper microthermal climates (microthermal: mean annual temperature ≤ 13 °C) and mild winters, with few, if any, frost days (Wolfe and Wehr Reference Wolfe and Wehr1987; Archibald and Farrell Reference Archibald and Farrell2003; Greenwood et al. Reference Greenwood, Archibald, Mathewes and Moss2005; Archibald et al. Reference Archibald, Morse, Greenwood and Mathewes2014).
The Okanagan Highlands is modelled as covered by a forest composed of a high-diversity floral assemblage in many ways analogous to that of the mixed mesophytic forests of the modern Eastern Deciduous Zone of eastern North America, but which included taxa that today inhabit East Asia, for example, Ginkgo Linnaeus (Ginkgoaceae), Metasequoia Miki (Curpessaceae), Pseudolaryx Gordon (Pinaceae) or lower latitudes, for example, palms (Arecaceae) (Wolfe and Wehr Reference Wolfe and Wehr1987; Greenwood et al. Reference Greenwood, Archibald, Mathewes and Moss2005; Moss et al. Reference Moss, Greenwood and Archibald2005; Archibald et al. Reference Archibald, Morse, Greenwood and Mathewes2014). The insect assemblage reflects this pattern, with high-diversity communities that mixed primarily boreal taxa (in the modern world) with those today restricted to low latitudes and those now found on other continents (e.g., Archibald and Makarkin Reference Archibald and Makarkin2006; Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010; Archibald and Rasnitsyn Reference Archibald and Rasnitsyn2018; and references therein). Okanagan Highlands insects of varying orders have received increasing attention in recent years (e.g., above references and Archibald et al. Reference Archibald, Mathewes and Greenwood2013, Reference Archibald, Rasnitsyn, Brothers and Mathewes2018; Makarkin and Archibald Reference Makarkin and Archibald2013; references therein, and others), but not the Odonata.
Here, we provide the first overview of Okanagan Highlands Anisoptera, describing the nine known fossils. We assign seven specimens to the Aeshnidae, six in five new species belonging to four new genera and one that we treat as Aeshnidae genus A, species A; and one to a new species in a new genus of Gomphidae. We consider the ninth, fragmentary specimen of a wing tip to be a separate species and separate genus of an undetermined family, and treat it as Anisoptera indeterminate genus A, species A. The fossil Zygoptera of the Okanagan Highlands will be considered in a future work, and there are no known fossil Anisozygoptera from this region.
Materials and methods
We examined nine fossils in lacustrine shale from sites described below. We generally follow the systematics of Dijkstra et al. (Reference Dijkstra, Bechly, Bybee, Dow, Dumont and Fleck2013) for extant Odonata, von Ellenrieder (Reference von Ellenrieder2002) for extant Aeshnidae, and authors cited below for fossil taxa as discussed.
Morphological nomenclature follows that of Riek and Kukalová-Peck (Reference Riek and Kukalová-Peck1984) as employed by Garrison et al. (Reference Garrison, von Ellenrieder and Louton2006), in common use among odonatologists. This differs slightly from the terminology often used in describing fossil Odonata, also based on that of Riek and Kukalová-Peck (Reference Riek and Kukalová-Peck1984) but as modified by subsequent authors (e.g., Bechly et al. Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001; Kohli et al. Reference Kohli, Ware and Bechly2016; Schädel and Bechly Reference Schädel and Bechly2016) in that the discoidal triangle, hypertriangle, submedian space, post-discoidal area, and IR2 of Riek and Kukalová-Peck (Reference Riek and Kukalová-Peck1984) are the triangle, supratriangle, cubito-anal space, discoidal field, and IRP2 of Garrison et al. (Reference Garrison, von Ellenrieder and Louton2006), respectively. The discoidal field is not illustrated here (see Garrison et al. Reference Garrison, von Ellenrieder and Louton2006, Fig. 6); by this we mean the area distal to the triangle to the margin, posterior to MA, and anterior of MP.
Terminology not specified by Garrison et al. (Reference Garrison, von Ellenrieder and Louton2006) (e.g., oblique vein “O”) follows Bechly et al. (Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001, see Fig. 1) or is illustrated by authors cited. The cordulegastrid gap found in Gomphaeschninae is the space without crossveins immediately basal to the nodus between RA and RP (see Fig. 2 and Bechly et al. Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001, Fig. 1, “gap”). By a vein possessing a convex curve, we mean bowed towards the anterior margin of the wing. By concave, we mean bowed towards the posterior margin; the exception is the posterior border of the pterostigma, which when bulged outwards, is considered convex.
Following Corbet (Reference Corbet1999) and widespread usage in odonatology, we refer to immature dragonflies as larvae. Contrary character states of comparative taxa are provided in brackets.
All drawings are made from both the fossil part and counterpart, both wetted with ethanol and dry. Some morphology in the drawing may, therefore, not be present or evident in the photograph of the part provided. Photographs are composites of those taken under the microscope.
Institutional collections and acronyms (if used) are as follows: Burke Museum of Natural History and Culture, Seattle, Washington, United States of America; Royal British Columbia Museum, Victoria, British Columbia, Canada (RBCM); Simon Fraser University, Burnaby, British Columbia, Canada; the Stonerose Interpretive Center, Republic, Washington, United States of America (SR and SRUI); and Thompson Rivers University, Kamloops, British Columbia, Canada.
Localities
The fossils are from sites of the Ypresian Okanagan Highlands series of localities at McAbee, near Cache Creek, in south central British Columbia, Canada, and within the town of Republic, in north central Washington, United States of America. The unnamed formation at McAbee is dated as 52.90 ± 0.83 million years old by 40Ar/39Ar analysis (Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010), and the Tom Thumb Member of the Klondike Mountain Formation exposed in Republic is estimated to be 49.4 ± 0.5 million years old by 40Ar/39Ar analysis (Wolfe et al. Reference Wolfe, Gregory-Wodzicki, Molnar and Mustoe2003). All Republic specimens were collected at exposure B4131 (Burke Museum of Natural History and Culture and Stonerose Interpretive Center locality number).
Antiquiala Archibald and Cannings, new genus
http://zoobank.org/urn:lsid:zoobank.org:act:53AF2B3C-E68F-4E2E-9A0B-90B7925382CF.
Figure 1.
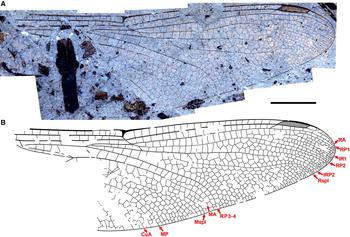
Fig. 1. Antiquiala snyderae new species, holotype forewing SR 08-10-08. A, Photograph of the part (A side); B, drawing made from both part and counterpart. Both to scale, 5 mm.
Classification. Odonata: Anisoptera: Aeshnidae: Aeshninae.
Type species. Antiquiala snyderae new species, here designated.
Diagnosis. The hind wing of Antiquiala is most easily distinguished from those of other genera of Aeshnidae (Figs. 2–4) possessing a simple IRP2 and RP2 curved basal to the pterostigma (Figs. 2–4) by combinations of the following character states:
(1) pterostigma length about four times width;
(2) supratriangle with crossveins;
(3) RP1–RP2 space becomes two cells wide about 3/4 distance from nodus to pterostigma;
(4) no cordulegastrid gap;
(5) radial planate, medial planate moderately wider in middle; radial maximum three cells wide for distance of 4–5 cells, medial maximum three wide for approximately three cells;
(6) maximum widths of RP2–IRP2, radial planate similar (RP2–IRP2 4 cells wide at maximum, radial planate 3);
(7) RP3-4–MA space about half maximum width of medial planate.
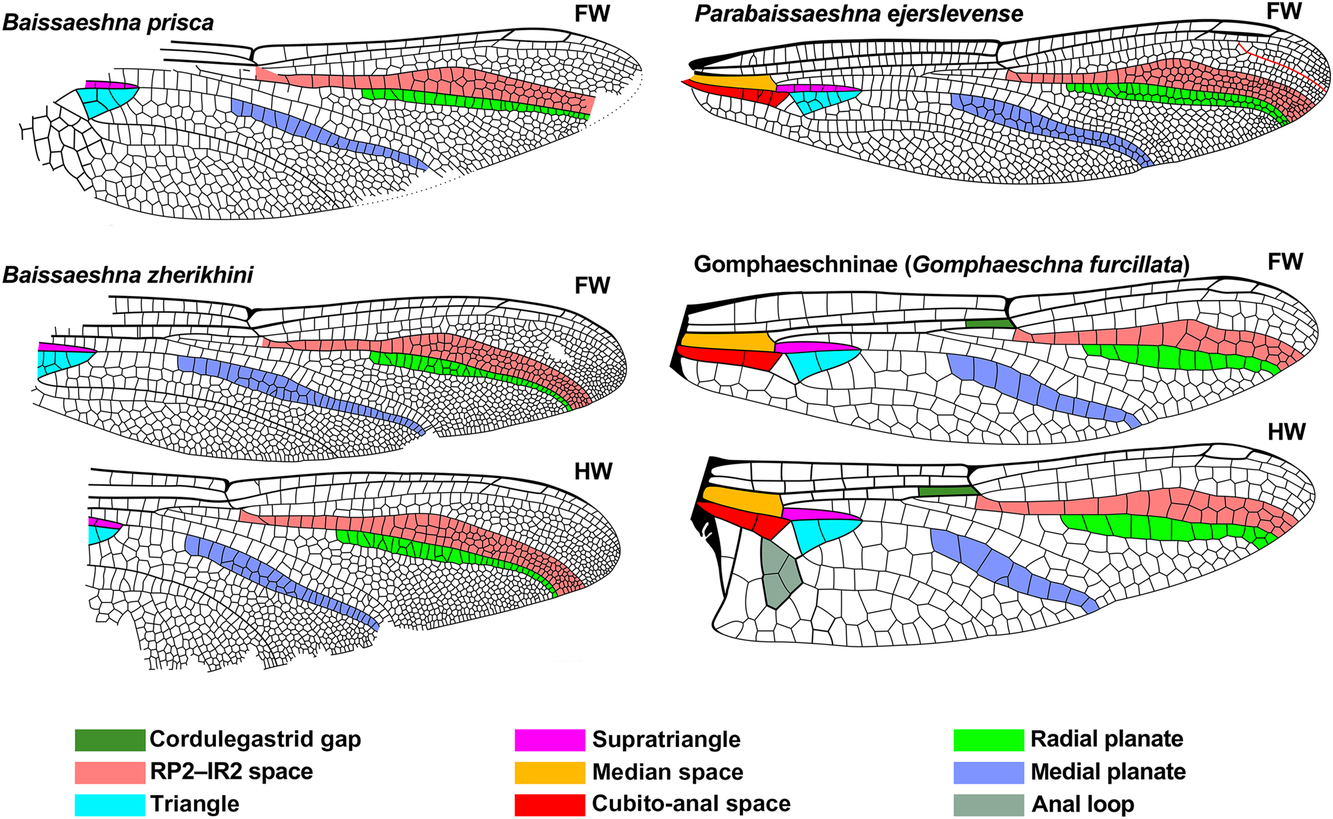
Fig. 2. Wings of comparative genera of Aeshnidae that have a simple IRP2 with wing regions discussed in the text, colour coded as indicated. Extant wings redrawn from Garrison et al. (Reference Garrison, von Ellenrieder and Louton2006), Cretaceous Baissaeshna species from Bechly et al. (Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001), and the Eocene Parabaissaeshna ejerslevense from Bechly and Rasmussen (Reference Bechly and Rasmussen2019). IR1 originating on RP1 in P. ejerslevense is rendered red, see text. Pterostigmata not coloured. To various scales (some originals without scale bars). FW, forewing; HW, hind wing.
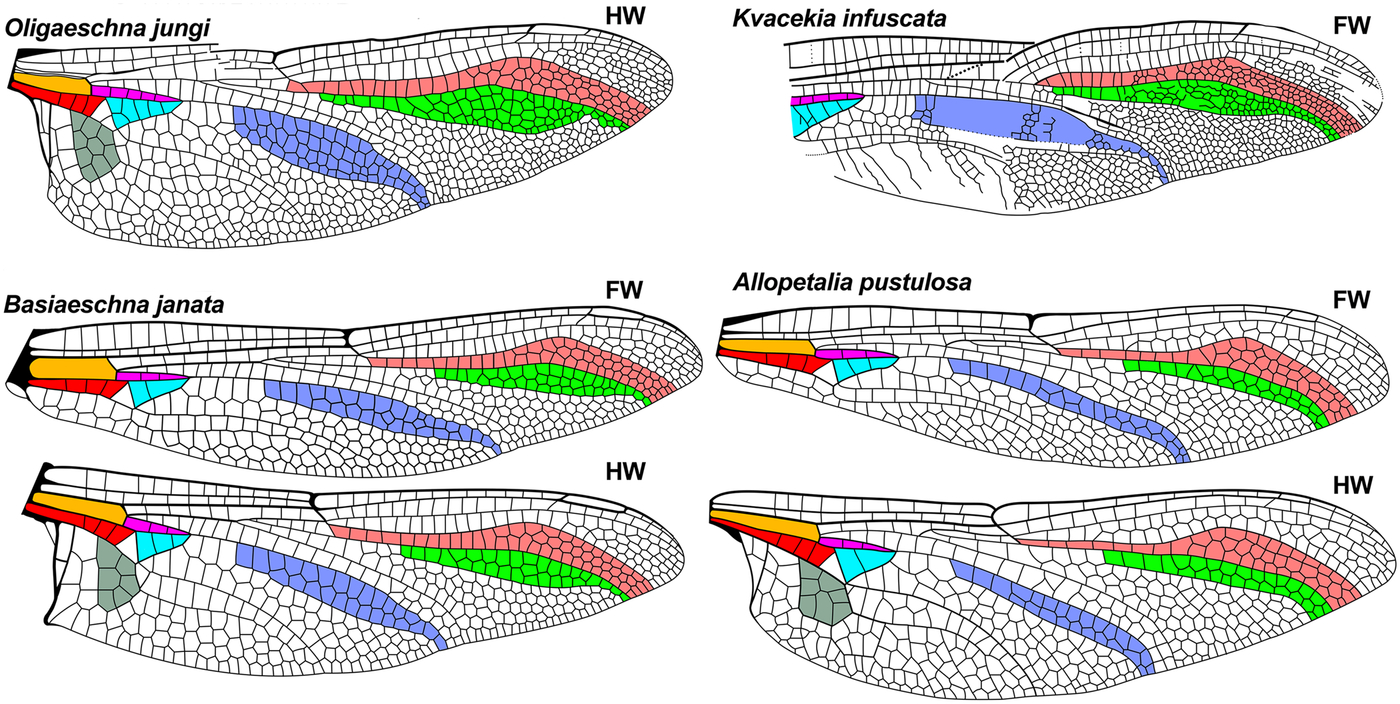
Fig. 3. Further wings of comparative genera of Aeshnidae, see caption for Fig. 2. Extant wings redrawn from Garrison et al. (Reference Garrison, von Ellenrieder and Louton2006) and the Eocene Kvacekia infuscata from Prokop and Nel (Reference Prokop and Nel2002). FW, forewing; HW, hind wing.
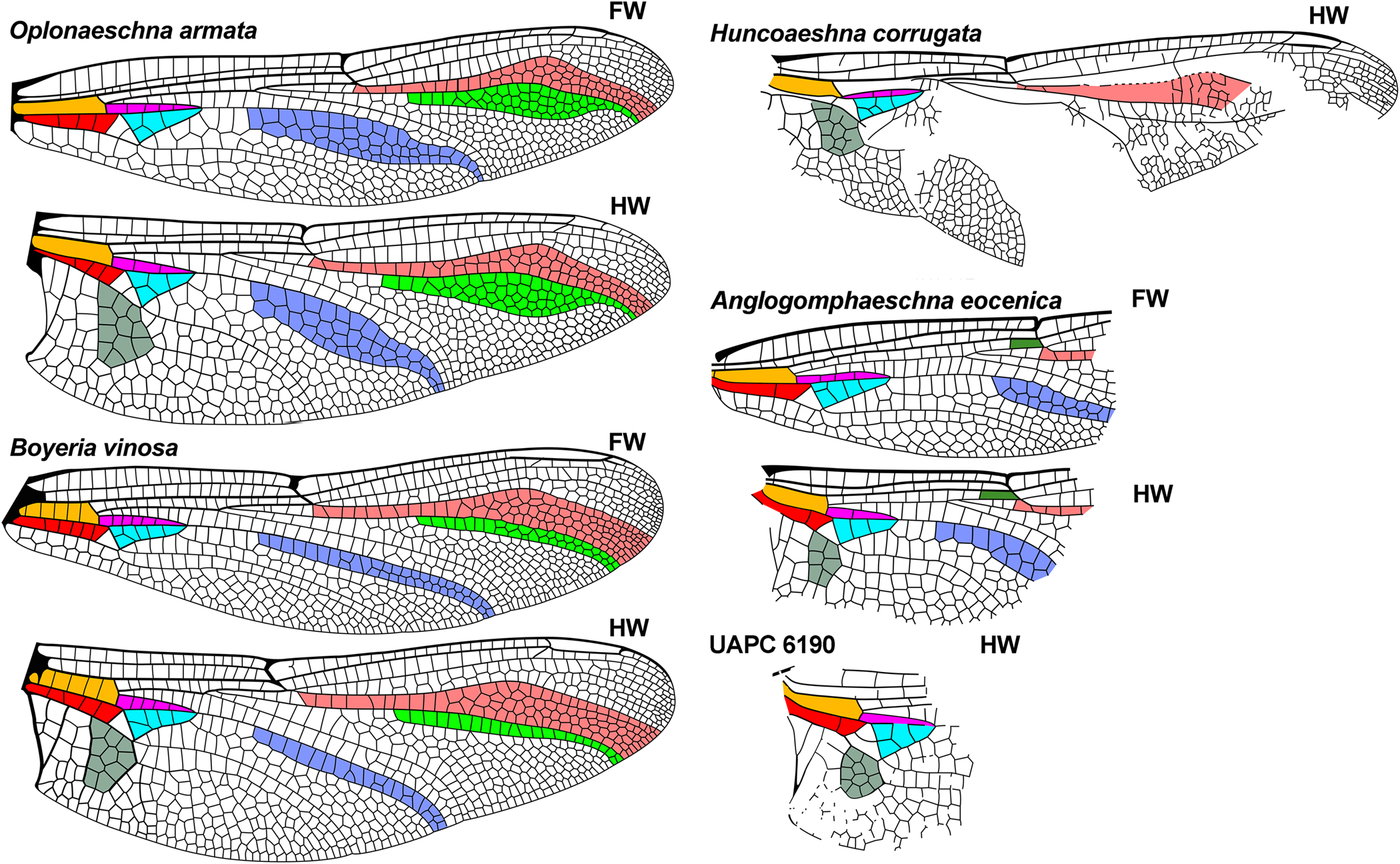
Fig. 4. Further wings of comparative genera of Aeshnidae, see caption for Fig. 2. Extant wings redrawn from Garrison et al. (Reference Garrison, von Ellenrieder and Louton2006), the Eocene Huncoaeshna corrugata from Petrulevičius et al. (Reference Petrulevičius, Nel and Voisin2010), Anglogomphaeschna eocenica from Nel and Fleck (Reference Nel and Fleck2014), and UAPC 6190 from Wighton and Wilson (1986). FW, forewing; HW, hind wing.
By these, we exclude:
Genera of Gomphaeschninae (those included discussed below) by: (2) [without], (4) [with];
Anglogomphaeschna Nel and Fleck by (most of apical half of wing not known): (3) [less than half this distance], (4) [with gap], (5) [medial maximum two cells wide];
Boyeria McLachlan by: (1) [approximately six times], (3) [13 postnodal crossveins basal to this, less than half distance from nodus to pterostigma], (5) [1–2 cells wide, not wider in middle], (6) [radial planate about a third width], (7) [similar widths];
Oplonaeschna Selys by: (1) [approximately three], (3) [one RA–RP1 crossvein basal to brace vein], (5) [both four wide, more expanded], (6) [radial planate wider], (7) [medial planate much wider];
Kvacekia Prokop and Nel by: (1) [greater than five times], (3) [less than one postnodal crossveins basad pterostigma], (5) [both apparently five wide, more expanded], (7) [medial planate much wider];
Allopetalia Selys by: (3) [less than half distance from nodus to pterostigma], (5) [two, not expanded], (6) [radial planate narrower], (7) [similar widths];
Baissaeshna Pritykina by: (3) [about half distance from nodus to pterostigma] (5) [B. prisca Pritykina: both one cell wide; B. zherikhini Bechly et al.: both one cell width, narrow in distal half, 1–2 cells, wider in basal half], both species further by: (6) [radial planate half or less width], (7) [similar maximum widths];
Oligaeschna Piton and Théobald by: (3) [distal to brace vein], (6) [radial planate wider], (7) [medial planate much wider];
Parabaissaeshna Bechly and Rasmussen by: (3) [about 1/3 distance], (5) [radial planate, medial planate maximum two cells wide], (6) [maximum radial planate width about 1/2 maximum RP2–IR2 width]. Antiquiala further distinguished from Parabaissaeshna by:
(8) region poorly preserved, but surely no more than 1–2 cells between RP, MA basal to midfork (branching of RP1-2, RP3-4), distal to triangle, [6];
(9) 22 postnodal crossveins [14];
(10) nodal vein oblique [perpendicular];
Ypshna new genus by: (3) [6], (5) [radial planate narrow, one cell wide], (6) [RP2–IRP2 space much wider than radial planate], RP3-4–MA space similar widths; and separated from Eoshna new genus as in its diagnosis, and from genus A as discussed below.
The venation of Antiquiala is closest to that of Huncoaeshna Petrulevičius et al. and Basiaeschna Selys, but may be separated from these as follows:
Huncoaeshna by: (3) [distal to brace vein], (5) [radial planate apparently two, see text], (6) [radial planate apparently about half maximum RP2–IRP2 width];
Basiaeschna by: (3) [distal to brace vein], (5) [radial planate two (one column three cells wide)]. Further from Basiaeschna by:
(11) medial planate expands to three cells wide [2];
(12) radial planate about 30 cells long [about 19–22];
(13) 22 postnodal crossveins [12–14].
Antiquiala venation denser than in Basiaeschna (e.g., 11–14) (apparently closer to Huncoaeshna in this way, as can be determined with its poor preservation); differs further from Basiaeschna by:
(14) at wing margin about 14 cells between RP1, RP2; seven in both RP1–IR1, IR1–RP2 spaces; at level of centre of pterostigma, space four cells wide between RP1, RP2; [seven cells between RP1, RP2 at wing margin, three in RP1–IR1 space, four in IR1–RP2 space; at level of centre of stigma two cells wide].
(15) RP2–IRP2 space four cells wide at maximum, just basal to pterostigma (distance of two cells), nine at wing margin [three, four at margin].
(16) MP–CuA notably widens towards margin to apparently four cells wide [slightly widens, two].
(17) 35 cells between Rspl, RP3-4 at margin as preserved, likely 40 or more total [hind wing 23];
not congeneric with UAPC 6190 (partial unnamed hind wing from the Paleocene of Alberta, Canada: Wighton and Wilson Reference Wighton and Wilson1986) by: (2) [without].
Description. As for its diagnosis, above, and its only species, below.
Etymology. Antiquiala is derived from the Latin antiquus, “ancient” and ala, “wing.” Gender, feminine.
Discussion. We assign Antiquiala to the Aeshnidae by its well-developed medial and radial planates and pterostigmal brace crossvein present and aligned with the base of the pterostigma.
It agrees with the Aeshninae sensu von Ellenrieder (Reference von Ellenrieder2002) by MA and RP3-4 not parallel (slightly but distinctly widening where one cell width becomes two, see the von Ellenrieder Reference von Ellenrieder2002, character state 13.1, Fig. 4D–F), and further by Mspl with a concave bend in the distal portion (von Ellenrieder Reference von Ellenrieder2002, character state 14.2, Fig. 4C–F); Rspl is not parallel to IRP2, with a concave bend (von Ellenrieder Reference von Ellenrieder2002, character state 16.2, Fig. 5D–G); and the absence of a cordulegastrid gap.
The wing of Antiquiala is most like that of the early Eocene Huncoaeshna and the extant Basiaeschna, also known from the late Paleocene or early Eocene (see below). Huncoaeshna has not been assigned to a subfamily due to the damaged and incomplete nature of its only known wing. Although Rspl is damaged and distally bent relative to IRP2 in Huncoaeshna, we differ from Petrulevičius et al. (Reference Petrulevičius, Nel and Voisin2010), who described its IRP2–Rspl space as three cells wide; our interpretation from their drawing is that it appears to be two, and the radial planate appears distinctly narrower than the RP2–IRP2 space, unlike in Antiquiala, where these are of similar maximum widths. The four-cell width of the RP2–IRP2 space in Huncoaeshna may be longer than in Antiquiala, but this is uncertain by preservation.
Antiquiala snyderae Archibald and Cannings, new species
http://zoobank.org/urn:lsid:zoobank.org:act:43EFA037-FE18-40D9-9DB3-49D9FF1D8D02.
Figure 1.
Type material. Holotype hind wing SR 08-10-08A, B (part, counterpart), missing the basal portion posterior to the supratriangle to the level of the nodus. Collected by Sarah Snyder, 17.vii.2007 at Republic B4131. Housed in the Stonerose Interpretive Center collection.
Diagnosis. As for the genus.
Description. Holotype hind wing SR 08-10-08A, B. Length, width not determined by preservation, see scale bar, Figure 1. Pterostigma: darkened, length about four times width; proximal, distal borders angled about 45°; anterior, posterior borders thickened, posterior border slightly convex; subtends 4.3 cells and five crossveins including well-defined brace vein aligned with proximal border. RP2 curved with convex curvature basal to pterostigma. IR1 present, well defined. IRP2 simple. Rspl with converse bend around mid-portion, not parallel to IRP2, three cells wide in mid-portion narrowing to one cell width at margin. RP3-4, MA not parallel (as defined by the von Ellenrieder Reference von Ellenrieder2002, character state 13.1). Mspl mostly rather straight with concave bend in distal portion (sensu von Ellenrieder Reference von Ellenrieder2002: Fig. 4D–F); medial planate three rows of cells in mid-region, two at the margin, mid-region about two times width of distal region; Mspl, MA not parallel. Oblique vein O one cell distal to base of RP2. Supratriangle somewhat damaged as preserved, but with at least two crossveins. Twelve antenodal crossveins preserved, some areas not preserved; 22 postnodal crossveins.
Etymology. The specific epithet is a patronymic formed from the surname of Sarah Snyder, the collector and donor of the holotype, recognising her contribution.
Idemlinea Archibald and Cannings, new genus
http://zoobank.org/urn:lsid:zoobank.org:act:7C6A197E-097F-4C91-98E3-51B158B2E2FE.
Figure 5.
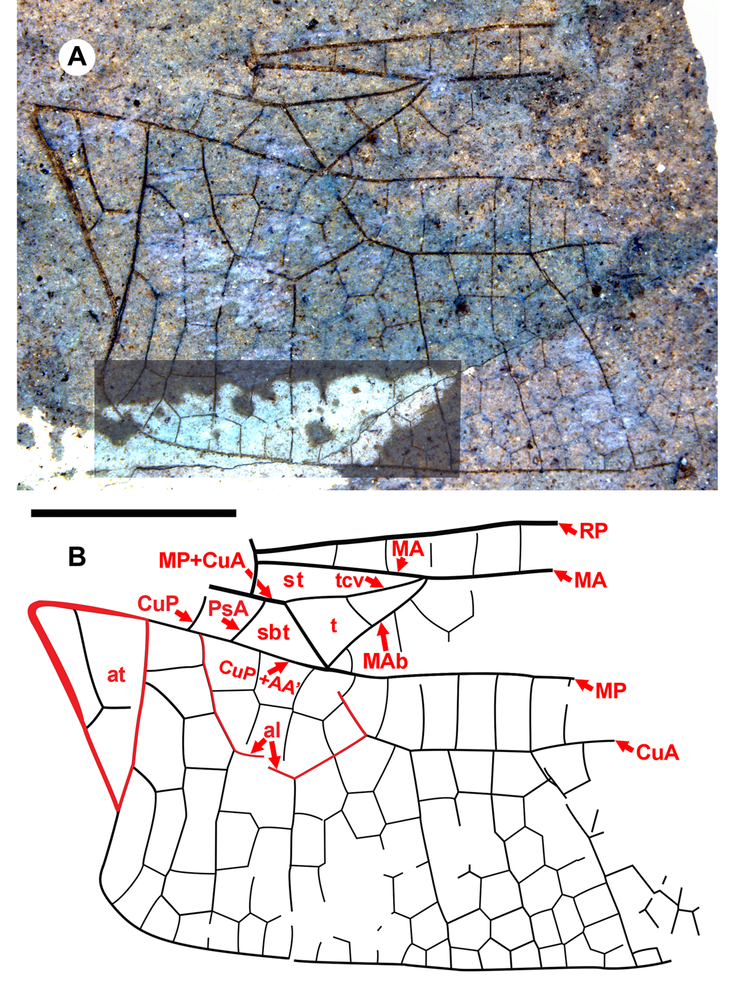
Fig. 5. Idemlinea versatilis new species, holotype hind wing SR 07-05-14. A, Composite photograph with differing exposures; B, drawing from both the part and counterpart. See text for labelling. Both to scale, 5 mm.
Classification. Odonata: Anisoptera: Aeshnidae: subfamily incertae sedis.
Type species. Idemlinea versatilis new species, here designated.
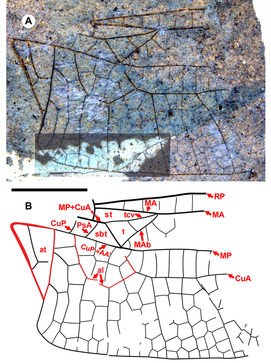
Diagnosis. Distinct from other hind wings of Aeshnidae with triangle with two cells, supratriangle free, cubito-anal space crossed by one vein, anal triangle with three cells by: supratriangle wide, maximum width 0.88 that of triangle, anterior margin (MA) rather straight, not curved; triangle rotated counterclockwise; subdiscoidal veinlet reduced to absence, so CuP + AA′, MP meet at posterior-basal corner of the triangle, aligned through meeting (for details of vein, crossvein identities in this region see Figure 12A of Riek and Kukalová-Peck Reference Riek and Kukalová-Peck1984; Fig. 1 of Bechly et al. Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001; Fig. 1 of Schädel and Bechly Reference Schädel and Bechly2016). Costal-trigonal crossvein (tcv), MA joining at anterio-basal corner of triangle angled, not in rather straight line. Subtriangle quadrilateral: PsA joins MP+CuA basal to kink in MP+CuA (basal to MP+CuA joining tcv), anterio-basal to corner of triangle; anal loop well defined with seven (damaged, but likely no more) cells.
Description. As in the diagnosis and for its only species, below.
Etymology. The genus name Idemlinea is derived from the Latin idem, meaning “the same,” and linea, “line,” referring to the major veins of the base of the hind wing (especially MP and CuA) being roughly parallel to the hind margin of the wing rather than curving posteriorly as they do in related genera. Gender, feminine.
Discussion. We place Idemlinea in the Aeshnidae by the strongly defined anal loop in this hind wing. It possesses a free supratriangle, a triangle crossed once, and only CuA crosses the cubito-anal space, a combination of character states consistent with the Gomphaeschninae as characterised by Bechly et al. (Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001, as the Gomphaeschnidae; and see Bechly Reference Bechly1996). These authors also emphasised the importance of a cordulegastrid gap in their taxon concept; however, they noted that it is not found in all members assigned to the group. Bechly et al. (Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001) included the eight Mesozoic genera Anomalaeschna Bechly et al., Sinojagoria Bechly et al., Paramorbaeschna Bechly et al., Progomphaeschnaoides Bechly et al., Plesigomphaeschnaoides Bechly et al., Gomphaeschnaoides Bechly et al., and Cretalloaeschna Jarzembowski and Nel in the taxon, and the Cenozoic Alloaeschna Wighton and Wilson (the extant genera Gomphaeschna Selys and Oligoaeschna Selys are known as fossils in the Cenozoic), grouped by wing characters (see review by Nel et al. Reference Nel, Martínez-Delclòs, Escuillé and Brisac1994). Nel and Fleck (Reference Nel and Fleck2014) assigned the Priabonian Anglogomphaeschna to it, but the genus was later transferred to the Aeshninae by Bechly and Rasmussen (Reference Bechly and Rasmussen2019), despite the presence of a cordulegastrid gap, which they note is weakly developed in this genus.
von Ellenrieder (Reference von Ellenrieder2002) considered Linaeschna Martin, Gomphaeschna, Sarasaeschna Karube and Yeh, and Oligoaeschna to constitute the extant Gomphaeschninae, which she grouped by male genitalic characters, doubting the usefulness of wing characters in defining the taxon.
By this doubt, and as the only known wing of Idemlinea is missing the portion where the presence or absence of a cordulegastrid gap may be assessed in any case, we treat the new genus as Aeshnidae subfamily incertae sedis, while suspecting it to be at least somewhat closely related to the above genera.
Idemlinea versatilis Archibald and Cannings, new species
http://zoobank.org/urn:lsid:zoobank.org:act:C5ACF4F1-7E38-47ED-8CC3-DD5B0CE0429A.
Figure 5.
Type material. Holotype SR 07-05-14A and B. The basal-most portion of a male hind wing. Collected by Karl Volkman 5.v.2007 at Republic B4131. Housed in the Stonerose Interpretive Center collection.
Diagnosis. As for the genus.
Description. Male hind wing (base of wing with an anal triangle). As in diagnosis and width RP to posterior margin 11 mm.
Etymology. The specific epithet is formed from the Latin versatilis, “revolving,” referring to the triangle that is rotated counterclockwise to the position normally seen in Aeshnidae.
Ypshna Archibald and Cannings, new genus
http://zoobank.org/urn:lsid:zoobank.org:act:5B420D6B-0FDE-45F9-B62E-0CD76BE463EF.
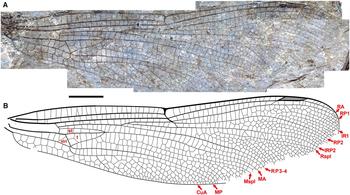
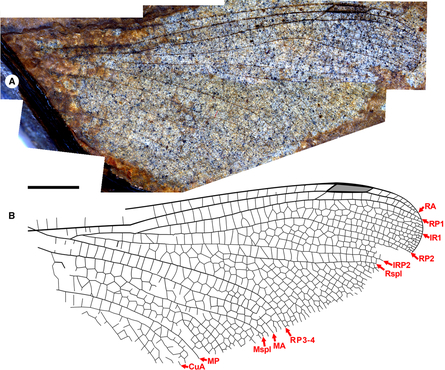
Fig. 6. Ypshna latipennata new species, holotype forewing F-1050, F-1051. A, Photograph of F-1050 (composite, with portion of F-1051 near pterostigma missing on F-1050); B, drawing made from both part and counterpart. All to scale, 5 mm.
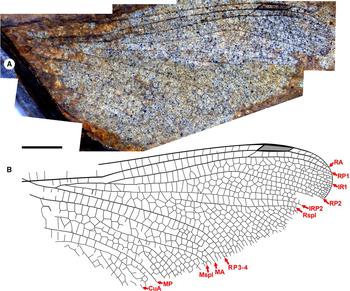
Fig. 7. Ypshna latipennata new species, paratype hind wing RBCM-P558. A, Photograph; B, drawing made from both part and counterpart. Both to scale, 5 mm.
Classification. Odonata: Anisoptera: Aeshnidae: subfamily incertae sedis.
Type species. Ypshna latipennata new species, here designated.
Included species. Ypshna latipennata new species and Ypshna brownleei new species.
Diagnosis. The forewing of Ypshna is separated from those of other aeshnid genera possessing a simple IRP2, RP2 curved basal to the pterostigma, and the median space free (listed below), by the following character states:
(1) cubito-anal space crossed by four veins (CuA + three crossveins);
(2) supratriangle with four crossveins;
(3) triangle with seven cells;
(4) triangle costal side about three times length of basal side;
(5) radial planate mostly one cell wide, maximum two, medial planate mostly two, maximum two cells wide;
(6) Rspl, IRP2 subparallel; radial planate rather straight, slightly narrows with slight downturn near margin;
(7) medial planate slightly expanded in mid-region;
(8) RP2–IRP2 space greater than two times maximum width of radial planate;
(9) no cordulegastrid gap;
(10) MA with slight concave curve, creating expansion of RP3-4–MA space (character state 13.1 of von Ellenrieder Reference von Ellenrieder2002);
(11) three cells in RP–MA space between midfork, triangle;
(12) 11 antefurcal crossveins (between RP, MA basal to midfork, distal to arculus);
(13) MP–CuA space widens near margin (see character state 12.0 of von Ellenrieder Reference von Ellenrieder2002).
Distinct from forewings of the following genera (Figs. 2–4), where known by preservation:
Genera of Gomphaeschninae by: (1) [one], (2) [none], (3) [two], (9) [present];
Anglogomphaeschna by (most of apical half of wing not known): (1) [only by CuA], (3) [less], (9) [with gap];
Oplonaeschna by: (1) [three], (4) [about 2.5], (5) [both greater], (6) [greatly expanded in middle], (7) [more expanded], (8) [similar width], (10) [concave curve greater], (13) (converging towards margin);
Oligaeschna by: (1) [variable, sometimes as in Ypshna], (3) [varies, but fewer], (4) [less than three times], (5) [both three to four], (6) [diverging widely], (7) [greatly expanded], (8) [RP2–IRP2 space similar to slightly less width as radial planate at maximum], (10) [curved more strongly].
Kvacekia by: (5) [both greater], (6) [greatly expanded in middle, greater downturn near margin], (7) [more expanded], (8) [similar widths];
Basiaeschna by: (1) [three], (2) [two], (3) [1–2], (4) [about two times], (5) [radial three], (6) [radial planate more expanded in middle], (7) [more expanded], (8) [similar widths];
Allopetalia by: (1) [three], (2) [two], (3) [three], (4) [about 2.5 times], (7) [not expanded], (10) [without curve];
Baissaeshna by: (3) [B. prisca, four; B. zherikhini, indeterminate], (4) [B. prisca, two times; B. zherikhini, indeterminate], (5) [B. prisca: radial, medial one], (6) [B. prisca, straighter; B. zherikhini, more curved];
Huncoaeshna by: (1) [one vein in space as preserved], (2) [two], (3) [five].
Parabaissaeshna by: (5) [radial planate almost entirely two cells wide], (11) [six], (12) [13]. Ypshna further distinguished from Parabaissaeshna by:
(14) 22 postnodal crossveins preserved [14 (complete)];
(15) three cells in RP–MA space between midfork, triangle [6];
(16) 11 antefurcal crossveins (between RP, MA basal to midfork, distal to arculus), [13];
(17) subtriangle free [crossed];
(18) CuA-CuP expanded at terminus on margin, five cells wide [less, by three cells];
(19) RP2 not or slightly undulate [distinctly undulate];
(20) RP1–RP2 space divides from one cell wide to two about 2/3 distance from nodus to pterostigma [about 1/3 distance];
distinct from Antiquiala, Idemlinea, Eoshna, genus A as in their diagnoses; not congeneric with UAPC 6190 by: (1) [CuA only], (2) [none], (3) [four], (4) [about 1.75 times].
Description. As in diagnosis, and IR1 originates on RP1 posterior to pterostigma.
Etymology. From Ypresian and – shna, an abbreviation of – aeshna, from Aeshna, the type genus of the family Aeshnidae. Gender, feminine.
Discussion. We assign this genus to the Aeshnidae by the well-developed medial and radial planates, and the forewing triangle width/length is within the range of the family (von Ellenrieder Reference von Ellenrieder2002: width/length between 0.23 and 0.43; here, 0.33).
Ypshna is excluded from the Aeshninae by MA and RP3-4 not parallel (see above) and from the Gomphaeschninae by character states discussed above. Following von Ellenrieder (Reference von Ellenrieder2002), who considered the extant Aeshninae and Gomphaeschninae to be the only well-supported subfamilies (and only the Aeshninae separated by wing characters), we treat Ypshna as subfamily incertae sedis.
In F-1050/1, RP2 is smoothly curved, but in RBCM-P558, it is slightly undulate (see character state 15 of von Ellenrieder Reference von Ellenrieder2002). We discount this as significant, as a forewing of Basiaeschna janata (Say) figured by Garrison et al. (Reference Garrison, von Ellenrieder and Louton2006, Fig. 224) has a similarly slightly undulate RP2, while the hind wing in that illustration does not, differing about as much as in F-1050/1.
The forewing of Ypshna is morphologically close to that of Parabaissaeshna ejerslevense Bechly and Rasmussen from the early Ypresian of the Fur Formation in Denmark (Bechly and Rasmussen Reference Bechly and Rasmussen2019) (see P. ejerslevense in Fig. 2 and Y. latipennata Fig. 6). For example, they share IR1 originating on RP1 (“pseudo-IR1” of Bechly and Rasmussen Reference Bechly and Rasmussen2019); the radial and medial planates, and RP2–IRP2 and RP3-4–MA spaces have similar shapes; the angles of the subnodus and of elements of the arculus are similar; the supratriangle of both have four crossveins; and both have a triangle with seven cells (although arranged slightly differently).
They also note that P. ejerslevense possesses an RP1–RP2 space (RA–RP1 space of Bechly and Rasmussen Reference Bechly and Rasmussen2019) that divides from one cell wide to two less than half the distance from the origin of this space to the level of the pterostigma, which they consider plesiomorphic for the Aeshnidae and uncommon in Cenozoic species. Ypshna does not share this character state; this space divides to two cells wide at about two-thirds the distance to the pterostigma (character state 20, above), that is, much closer to it. Interestingly, Eoshna thompsonensis possesses this trait (see below). Bechly and Rasmussen (Reference Bechly and Rasmussen2019) considered P. ejerslevense to be a close relative of the Cretaceous genus Baissaeshna (see Fig. 2).
If P. ejerslevense and Y. latipennata are as close phylogenetically as their forewing morphologies suggest, this would not be not surprising; a growing number of closely related insects is shared between the Okanagan Highlands and the Fur Formation, including species of the genera Ypresiomyrma Archibald et al. (Hymenoptera: Formicidae), Palaeopsychops Andersen (Neuroptera: Polystoechotidae), Protochrysa Willmann and Brooks (Neuroptera: Chrysopidae), and Cimbrophlebia Willmann (Mecoptera: Cimbrophlebiidae) (Archibald et al. Reference Archibald, Cover and Moreau2006; Archibald and Makarkin Reference Archibald and Makarkin2006; Archibald Reference Archibald2009; Makarkin and Archibald Reference Makarkin and Archibald2013). There may have been continuous forests between the Fur Formation and the Okanagan Highlands in the Ypresian across land bridges in climatically mild high latitudes, facilitating large-scale Holarctic dispersal of mammals and plants as well as insects (e.g., Woodburne and Swisher Reference Woodburne, Swisher, Berggren, Kent, Aubry and Hardenbol1995; Tiffney and Manchester Reference Tiffney and Manchester2001; Archibald et al. Reference Archibald, Johnson, Mathewes and Greenwood2011b).
Ypshna latipennata Archibald and Cannings, new species
http://zoobank.org/urn:lsid:zoobank.org:act:7EAB42EF-7C03-4BFF-8411-791BA202461B.
Catalogue. Odonata: Aeshnidae: Archibald Reference Archibald2007, 81 (RBCM-P558, referred to there as SBA-105); Odonata: Aeshnidae: Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010, supplement 12 (RBCM-P558, referred to there as SBA-105).
Type material. Holotype forewing F-1050, F-1051 (part, counterpart) (Fig. 6). An almost complete forewing, unknown collector, unknown date. Housed in the Thompson Rivers University collection. Paratype RBCM-P558 (part, counterpart), also labelled SBA-105A, B (Fig. 7). Distal half of a hind wing. Collected by SBA at McAbee, 20.vi.2000. Housed in the Royal British Columbia Museum collection.
Diagnosis. Forewing most easily separated from that of Ypshna brownleei new species by broader shape: ratio of length from base of pterostigma to arculus (distal-posterior point): maximum width (at/near distal-most point of CuA meeting margin) is 1: 0.36 [Y. brownleei, 1: 0.29].
Description. Holotype forewing F-1050, F-1051, part, counterpart (Fig. 6). An almost complete forewing. Length 47 mm, width 11 mm, nodus to apex 25 mm. Twenty-five antenodal crossveins. Pterostigma darkened, length about 5.5 times width; proximal, distal borders angled about 40°; anterior, posterior borders thickened, posterior border slightly convex; subtends five cells and six crossveins including well-defined brace vein aligned with proximal border. No cordulegastrid gap. RP1–RP2 space basal to pterostigma narrowing to two cell widths, then one; RP2 evenly curved with convex curvature basal to pterostigma. IR1 present, well defined. Median space free. IRP2 simple, Rspl, IRP2 mostly parallel except narrowing at margin, radial planate maximum two cells wide. Cubito-anal space with five cells (CuP plus three crossveins); supratriangle longer than anterior side of triangle, with four crossveins; triangle elongate, with seven cells. Discoidal field immediately distal to triangle two cells wide, three from about third of distance to level of nodus. Anal area with two cell rows. RP3-4, MA parallel. Mspl straight; one row of cells in medial planate (Mspl-MA space) from base to near level of base of Rspl, two rows distal to margin; Mspl, MA subparallel, with mid-region about two times width of distal region; MP–CuA space widens near wing margin (von Ellenrieder Reference von Ellenrieder2002, character state 12.0). Oblique vein O immediately distal to base of RP2.
Paratype hind wing RBCM-P558. Length approximately 35 mm from branching of RP1-2, RP3-4 to apex, width approximately 16 mm as preserved likely close to true width. Pterostigma darkened, length about 4.5 times width, apparently narrowing slightly distally; proximal, distal borders angled about 45°; bordering veins slightly thickened, posterior border hardly convex; subtends five cells and five crossveins, including well-defined brace vein aligned with proximal border. No cordulegastrid gap. Median space free. Slightly undulate RP2. IRP2 simple. IR1, RP2, IRP2, Rspl, radial planate, RP3-4, MA, medial planate, Mspl, distal portion of MP as in F-1050/1, except that radial planate only one cell width throughout (distal-most portion near margin not preserved). MP–CuA space widens near wing margin.
Etymology. The specific epithet latipennata is formed from the Latin latus (broad) and pennatus (winged), referring to the broader wing that distinguishes it from its congener.
Discussion. The hind wing RBCM-P558 agrees with the forewing very closely, with the exception of the mildly undulate RP2, as discussed above.
Ypshna brownleei Archibald and Cannings, new species
http://zoobank.org/urn:lsid:zoobank.org:act:CC98EDC0-E412-4E37-BC25-8F1B53E5A4C7.
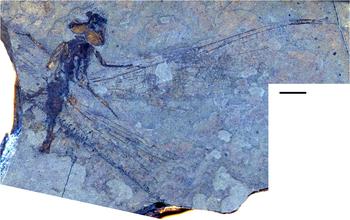
Fig. 8. Ypshna brownleei new species, holotype SR 01-06-13. Photograph. Image reversed left/right so that wing apices are to the right for ease of comparison. Scale bar is 5 mm.
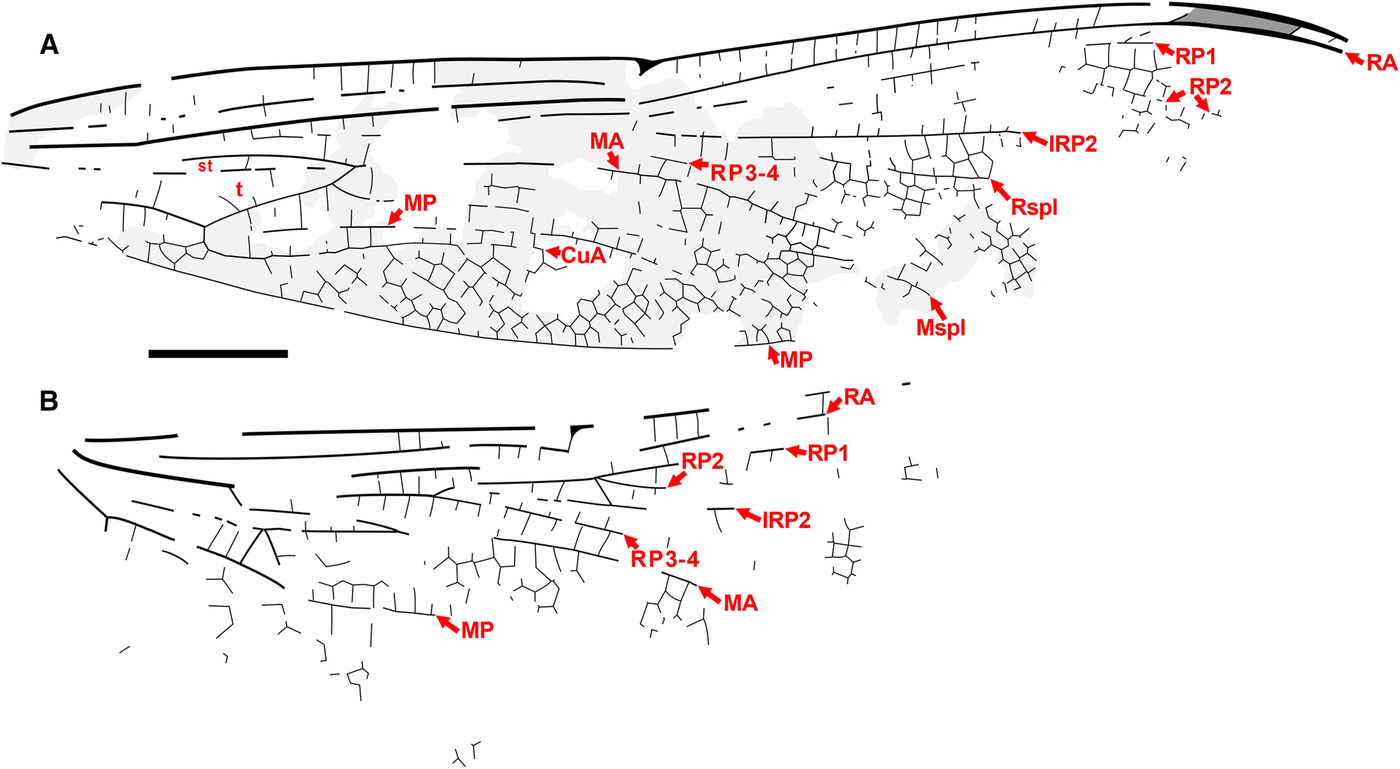
Fig. 9. Ypshna brownleei new species, holotype SR 01-06-13. A, Forewing; B, hind wing. Image reversed left/right so that wing apices are to the right for ease of comparison. Regions of light infuscation indicated on forewing might be present more widely (or not); unclear by preservation. Both to scale, 5 mm.
Diagnosis. Forewing of Ypshna brownleei most easily separated from that of Y. latipennata by narrower shape as described in its diagnosis.
Type material. Holotype SR 01-06-13. Much of a body with legs, missing the distal half of the abdomen, preserved mostly in dorsal aspect, head in dorsal aspect, with most of the right forewing, much of the basal portion of the right hind wing, and small fragments of the basal portions of the left wings, which are partly disarticulated from the thorax. Collected by Don Brownlee 15.x.2001 at Republic B4131. Housed in the Stonerose Interpretive Center collection.
Description. Male. Body with rather delicate build. Head. Dorsum of eyes, vertex preserved; eye seam short, probably entire; head anterior to eyes flattened, pushed forward in preservation. Thorax. Badly damaged, distorted, apparently mostly left side visible. Abdomen. Indicates male, segments 1–2 thickened; segment 2 in dorsolateral aspect with suggestions of secondary genitalia; segment 3 (dorsal view) constricted as in males of many taxa, including many aeshnids. Forewing. Length approximately 50 mm, width approximately 10 mm, nodus to apex approximately 25 mm. Light infuscation preserved on portions, unclear by preservation if more widespread. Pterostigma slightly darkened, length about 5.5 times width; proximal, distal borders angled about 45°; bordering veins slightly thickened, posterior border hardly convex; area posterior damaged, associated cells, crossveins not preserved; brace vein aligned with proximal border. Median space free. Supratriangle with at least one crossvein (region poorly preserved), triangle base not preserved, but appears similar shape (length/width) as that of Y. latipennata (base present in hind wing specimen); likely with at least four cells indicated by partial crossveins present, those detected agree with Y. latipennata. At least one vein crosses cubito-anal space (region poorly preserved; probably at least two, judging from relationships of veins in hind wing). Possible section of RP2 preserved, if so, then bend is before pterostigma, RP2–IRP2 space relatively narrow. Presence/absence of cordulegastrid gap not established. Small section of Rspl preserved, see discussion; radial planate widening before pterostigmal region. MA gently curved. Sections of Mspl preserved, medial planate appears similar in shape as that of Y. latipennata. Hind wing. Length, width not known by preservation (see Fig. 9 for scale bar). Not known by preservation if light infuscation preserved on portions of forewing present on hind wing. Median space free. Supratriangle with three crossveins preserved (could be more: region poorly preserved); cubito-anal space crossed by at least two veins; triangle with at least five cells evident by zigzag of portions of preserved crossveins. Presence/absence of cordulegastrid gap not established. Oblique vein O present at branching of RP1, RP2. Rspl, Mspl regions not preserved.
Etymology. The specific epithet is formed from the surname of Don Brownlee, collector of the type specimen, in recognition of his contribution.
Eoshna Archibald and Cannings, new genus
http://zoobank.org/urn:lsid:zoobank.org:act:CB2B336B-3FD9-4B4E-AD46-B27EE2C68EAF.
Figure 10.

Fig. 10. Eoshna thompsonensis new species, holotype hind wing F-1487. A, Photograph; B, drawing. Both to scale, 5 mm.
Classification. Odonata: Anisoptera: Aeshnidae: subfamily incertae sedis.
Type species. Eoshna thompsonensis new species, here designated.
Diagnosis. The hind wing of Eoshna possesses the following character states:
(1) cubito-anal space basal to PsA crossed by six veins (CuP + five crossveins, one faintly preserved);
(2) supratriangle with at least three crossveins;
(3) triangle with at least 10 cells, but in part poorly preserved;
(4) triangle costal side about 2.5 times length of basal side;
(5) anal loop well defined with at least 22 cells indicated;
(6) radial planate maximum three cells wide; medial planate maximum four;
(7) radial planate slightly widened in middle, rather straight with slight downturn, narrowing near margin;
(8) medial planate expanded in basal half, more than radial;
(9) RP2–IRP2 space greater than two times maximum width of radial planate, maximum 5–6 cells wide;
(10) no cordulegastrid gap;
(11) MA with slight concave curve (apparently: this portion damaged), with slight expansion of RP3-4–MA space (von Ellenrieder Reference von Ellenrieder2002, character state 13.1).
These exclude all other aeshnid genera with a simple IRP2, RP2 curved basal to the pterostigma, and the median space free, and Boyeria, in which the median space is crossed, as the apparent free median space in Eoshna might be an artefact (Figs. 2–4):
Gomphaeschninae by: (1) [one], (2) [none], (3) [two], (10) [with];
Anglogomphaeschna by (most of apical half of wing not known): (1) [only crossed by CuA], (3) [less], (6) [medial planate two (preserved portion)], (10) [with gap];
Oplonaeschna by: (1) [three], (3) [four], (5) [11], (6) [radial, four], (8) [both greatly expanded], (9) [similar], (11) [greater curve];
Oligaeschna by: (3) [variable, less than 10], (7) [greatly widened], (8) [medial planate maximum width similar to radial], (9) [radial planate greater width], (11) [greater curve].
Kvacekia by: (3) [apparently seven, in any case, much fewer, but poorly preserved], (6) [both five], (7) [more wide], (8) [wide for greater length], (9) [similar];
Basiaeschna by: (1) [three], (2) [two], (3) [forewing, one; hind wing, three], (4), [about two times], (5) [six], (6) [medial, two], (8) [similar widths], (9) [similar widths];
Allopetalia by: (1) [three], (2) [two], (3) [seven], (5) [seven], (6) [both, two], (7) [not narrowing], (8) [not expanded, similar to radial], (11) [not];
Boyeria by: (1) [five], (4) [about three times], (5) [10], (6) [radial planate one, medial planate, occasionally two], (8) [medial planate not so expanded], (11), [without], further by:
(12) male anal angle of hind wing rounded (see von Ellenrieder Reference von Ellenrieder2002, character state 18.1), [angulate];
Baissaeshna by: (3) [B. prisca: four; B. zherikhini: unknown by preservation], (4) [B. prisca, two times; B. zherikhini: unknown], (6) [radial: B. prisca, forewing one, B. zherikini, two; medial: B. prisca, forewing one; B. zherikini, 2], (7) [B. prisca, straight, not widened, one cell wide; B. zherikhini, similar but maximum two cells wide];
Huncoaeshna by: (1) [one], (2) [two], (3) [five], (4) [three times], (5) [apparently 11];
Parabaissaeshna by: (1) [three (one forked)], (3) [seven], (4) [three times], (6) [both two]. Parabaissaeshna further distinguished from Eoshna by:
(13) at least 24 postnodal crossveins, surely a few more, [14];
(14) damaged, apparently two cells in RP–MA space between midfork, triangle, [six];
(15) subtriangle free, [crossed];
(16) RP1–RP2 space divides from one cell wide to two less than half distance from nodus to pterostigma, [about 1/3 distance];
Ypshna by: (1) [four], (3) [seven], (4) [about three times], (6) [both two], (7) [not bowed out], (8) [medial, more expanded];
not congeneric with UAPC 6190 by: (1) [CuA only], (2) [none], (3) [four], (4) [about 1.75 times], (5) [10].
Description. As in diagnosis.
Etymology. From Eocene and – aeshna, from Aeshna, the type genus of the family Aeshnidae. Gender, feminine.
Discussion. The anal triangle is in part poorly preserved, but there is at least one crossvein, and so there are two or more cells.
We assign this genus to the Aeshnidae by the well-developed medial and radial planates, pterostigmal brace crossvein present and aligned with the base of the pterostigma, and the well-defined anal loop. As in Ypshna, MA and RP3-4 are parallel, excluding it from the Aeshninae, and as with that genus, we treat it here as subfamily incertae sedis. Also, as in Ypshna latipennata specimen RBCM-P558, RP2 is somewhat undulate, but we hesitate to interpret this as distinctive enough to be informative.
Bechly and Rasmussen (Reference Bechly and Rasmussen2019) note that the RP1–RP2 space dividing from one cell wide to two less than half the distance from the origin of this space to the level of the pterostigma (character state 16) is plesiomorphic (see discussion of Ypshna, above) only found in the Cretaceous Baissaeshna (especially B. zherikhini), the Ypresian Parabaissaeshna, the Priabonian Anglogomphaeschna, and the extant Allopetalia and Boyeria.
Eoshna thompsonensis Archibald and Cannings, new species
http://zoobank.org/urn:lsid:zoobank.org:act:1293AECA-02AD-4BDB-BCC3-52D63D398209.
Figure 10.
Material. F-1487, part only. The hind wing of a male, mostly complete. McAbee, British Columbia, Canada; collected by unknown person, unknown date. Housed in the Thompson Rivers University collection.
Diagnosis. As for the genus.
Description. Holotype male hind wing F-1487. As in genus diagnosis, and: 23 postnodal crossveins preserved (nodus to pterostigma in C–RA space). Length 54 mm, width 16 mm, nodus to apex 33 mm. Pterostigma mostly not clearly preserved, but base at about 2/3 of nodus to apex distance; proximal border angled about 45°, aligned with brace vein; subtends at least four cells, four crossveins (estimated: distal border not preserved, possibly more). Length of pterostigma unknown by preservation. IR1 present, well defined. RP2 with convex curvature basal to pterostigma; oblique vein O one crossvein distal to branching of RP1, RP2. Median area free (but: possibly this is a preservational artefact). Rspl, IRP2 subparallel, radial planate three cells wide in middle, narrows to one cell wide at margin. Discoidal field with three rows of cells distal to triangle. RP3-4, MA parallel. Mspl concave in middle, then convex; Medial planate four cells wide in middle narrowing to one at margin; MP–CuA space appears widens for single cell wide to apparently three at margin (distal-most portion not preserved) as in von Ellenrieder (Reference von Ellenrieder2002) character state 12.0.
Etymology. The specific epithet thompsonensis is formed from the name of the nearby Thompson River.
Discussion. With a hind wing length of 54 mm, Eoshna thompsonensis was a large species, almost as large as an average-sized Epiaeschna heros, the largest Canadian dragonfly today (male hind wing is 53–58 mm long). All Okanagan Highland aeshnids appear to be mid-sized to large compared with modern North American Aeshnidae. The male hind wings of modern Basiaeschna janata, a small aeshnid, are about 32–40 mm long, and that of the Paleocene or Eocene Basiaeschna alaskaensis Garrouste and Nel from the Chickaloon Formation of Alaska, United States of America is estimated to have been about 40 mm long (Garrouste and Nel Reference Garrouste and Nel2019); those of Aeshna interrupta, a mid-sized aeshnid, are 43–47 mm long (wing measurements are from Walker Reference Walker1958). The fragmentary wing fossil SR 07-16-14 described below as Anisoptera genus A, species A probably was about the same size as E. thompsonensis.
Aeshnidae genus A, species A
Figure 11.

Fig. 11. Aeshnidae genus A, species A, SRUI 09-97-61, a hind wing. A, Photograph; B, drawing. Both to scale, 5 mm.
Material. SRUI 09-97-61, part only. A male hind wing, mostly complete, but with portions obscured throughout. Collected at Republic B4131 by Gregg Wilson, 21.iv.2008. Housed in the Stonerose Interpretive Center collection.
Description. Male hind wing. Length 47 mm (almost complete, missing tip likely approximately 1 mm), nodus to apex 24 mm (as preserved), width 15 mm. Pterostigma only slightly darkened, length about 4.5 times width; anterior, posterior borders hardly thickened, posterior border not convex; cells posterior to pterostigma as well as brace vein region not preserved. Presence/absence of cordulegastrid gap not established. IR1 present. RP2–IRP2 space width relative to radial planate not known; RP2 simple, curved basal to pterostigma, not known by preservation if evenly curved or undulate. Oblique crossvein O present, distal to and near branching of RP1, RP2. Supratriangle with at least three crossveins. Region where Rspl might occur not well preserved. MA gently curved, distal portion poorly preserved, but apparently bent towards the posterior margin near it; portions of Mspl preserved, therefore medial planate present, similar width as RP3-4–MA space in their mid-sections, apparently about half width of RP3-4–MA space near margin. Anal loop well defined with likely 12 cells. Anal triangle with at least, but probably three cells. Regions of triangle, subtriangle, median space, cubito-anal space poorly or not preserved.
Discussion. SRUI 09-97-61 bears the following hind wing character states useful in assessing its relationship with genera compared below:
(1) cubito-anal space crossed by more than two veins (unclear how many more);
(2) supratriangle with at least three crossveins;
(3) triangle with at least three cells (unclear how many, poorly preserved);
(4) triangle costal side length likely about 1.5 times basal side (poor preservation: indicated), distal side (MAb) with strong kink at crossvein;
(5) medial planate maximum of two cells wide, moderately expanded in middle, narrowing to one cell wide near margin (radial planate not known);
(6) MA rather straight, gently curving, possibly without small curve, see the von Ellenrieder (Reference von Ellenrieder2002) character state 13.1;
(7) anal loop with likely 12 cells;
(8) damaged, but apparently two cells in RP–MA space between midfork, triangle;
(9) RP1–RP2 space divides from one cell wide to two about 3/4 distance from origin to level of pterostigma (damaged, apparently).
We compare SRUI 09-97-61 with the following aeshnid genera (Figs. 2–4), where IRP2 is simple as above, but we add Boyeria, as it is unknown if the median space possesses crossveins.
Excluded from genera of Gomphaeschninae by: (1) [one], (2) [zero], (3) [two];
Anglogomphaeschna by: (1) [only crossed by CuA], (7) [five], (9) [less than half distance];
Oplonaeschna by: (5) [four, greatly bowed out], (6) [with distinct curve];
Oligaeschna by: (4) [greater than or equal to two times], (5) [3–4 cells wide, greatly expanded], (6) [with distinct curve].
Kvacekia by: (5) [five cells wide, more bowed out];
Basiaeschna by: (2) [two], (7) [six];
Allopetalia by: (2) [two], (7) [seven];
Baissaeshna by: (6) [more curved];
Huncoaeshna by: (4) [greater than three times, distal side smoothly curved];
Boyeria by: (4) [about three times, distal side without similar sharp angle], (5) [not bowed out];
Parabaissaeshna by: (6) [more curved], (8) [six], (9) [about 1/3 distance].
Eoshna by: (4) [about 2.5 times], (5) [four, more expanded];
Ypshna by: (4) [three times], (5) [two cells wide in distal half], (6) [more curved];
not congeneric with UAPC 6190 by: (1) [CuA only], (2) [none], (7) [10].
We assign this wing to the Aeshnidae by its well-developed medial planate and anal loop. Although this wing may be separated from those of similar genera as above, we hesitate to name a new genus and species based on this fossil because of its incomplete preservation. We await the discovery of a more complete wing where enough of the median and cubito-anal spaces, triangle, subtriangle, supratriangle, and radial planate are preserved so that they can be clearly characterised and strongly define the genus and species of the specimen.
Auroradraco Archibald and Cannings, new genus
http://zoobank.org/urn:lsid:zoobank.org:act:36186C3F-1EB4-45FB-976D-2C7C71715A40.
Figure 12.

Fig. 12. Auroradraco eos new species, holotype forewing F-238, F-239. A, combination of photograph of F-239, with the basal posterior portion F-238 (regions damaged in F-239); B, drawing from both the part and counterpart. Abbreviations: c-a is the cubito-anal space, see text for other labeling. Both to scale, 5 mm.
Classification. Odonata: Anisoptera: Gomphidae: subfamily incertae sedis.
Type species. Auroradraco eos new species, here designated.
Diagnosis. Forewing distinct by combination of: well-defined trigonal sector (“ts,” Fig. 12) subtending eight cells; crossvein Ax0 present in costal (C) space, no crossveins in subcostal (ScP) space as preserved; nodus at about mid-length of wing; arculus at right angle to main veins; triangle not elongate (about as high as wide); triangle, supratriangle, subtriangle without crossveins; cubito-anal space only crossed by CuP; posteriodistal side of triangle (MAb, “b” in Fig. 12) rather straight, not at all sigmoid; two crossveins (asterisks in Fig. 12) in RP space, that is, between arculus, branching of RP1-2, RP3-4; CuA pectinate, with five branches (i.e., branched four times).
Description. As in diagnosis, and: triangle with anterior side parallel to main veins, distal corner not rotated posteriorly.
Etymology. The genus name is formed from the Latin words aurora, dawn and Draco, dragon. Gender, masculine.
Discussion. Most authors diagnose the Gomphidae using non-wing character states; however, Schädel and Bechly (Reference Schädel and Bechly2016 and references therein) state that the following combination of wing character states are only found within the family (but these are not present in all members; most lack one or more): a distinct PsA (proximal side of the subtriangle), the anterior side of the supratriangle curved, a rather straight arculus in the forewing, and a slightly angled to strongly sigmoidal posteriodistal side of the triangle (MAb) (Schädel and Bechly Reference Schädel and Bechly2016 and references therein). The new genus conforms to all of these, except that MAb is rather straight, which we consider to be distinctive of this genus (more so than in Burmalindenia Schädel and Bechly, see below). While a few subfamilies of the Gomphidae are well supported by recent molecular analyses, much of its higher systematics remains in flux (Dijkstra et al. Reference Dijkstra, Bechly, Bybee, Dow, Dumont and Fleck2013 ; Carle et al. Reference Carle, Kjer and May2015; Ware et al. Reference Ware, Pilgrim, May, Donnelly and Tennessen2016) and we hesitate, therefore, to assign Auroradraco to a subfamily.
The distinct trigonal sector in the forewing of the new genus is uncommon (Carle Reference Carle1986; Garrison et al. Reference Garrison, von Ellenrieder and Louton2006; Schädel and Bechly Reference Schädel and Bechly2016). Those genera where it is present mostly differ from Auroradraco by having an elongate triangle with a strongly sigmoid distal side (MAb of Bechly et al. Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001; ddv of Schädel and Bechly Reference Schädel and Bechly2016; “b” in Fig. 12), or by having at least one crossvein in at least one triangle. The mid-Cretaceous Burmalindenia from Burmese amber has a trigonal sector, an Ax0 crossing the C space, and MAb not strongly sigmoid (Schädel and Bechly Reference Schädel and Bechly2016). However, its triangle is elongate and it and the supratriangle have crossveins. Further, although its MAb is not strongly sigmoid, it is notably more kinked than in the new genus. The presence of an Ax0 crossvein is rare within the Gomphidae.
The forewing of the monotypic Australian genus Odontogomphus Watson shares many character states with Auroradraco: an Ax0 crossvein, a trigonal sector; the triangle, subtriangle, and supratriangle lacking crossveins; and the cubito-anal space crossed only by CuP. Odontogomphus differs, however, in that there are five crossveins in the RP space, CuA has two branches, the arculus is distinctly angled towards the base anteriorly, and the nodus is distal to the mid-wing (Watson Reference Watson1991).
Auroradraco eos Archibald and Cannings, new species
http://zoobank.org/urn:lsid:zoobank.org:act:184E4FDE-3846-47BE-952A-E4E17C8DCBD3.
Figure 12.
Type material. Holotype F-238, F-239 (part and counterpart), an almost complete forewing, but with a somewhat damaged basal posterior portion. Collected at McAbee by unknown person, unknown date. Housed in the Thompson Rivers University collection.
Diagnosis. Forewing, as for the genus.
Description. Forewing as in genus and: Length 36 mm, width 9 mm, apex to nodus 17 mm. Pterostigma darkened, rather short, slightly shorter than supratriangle, length about four times width, borders thickened, posterior margin convex, subtending 3.5 cells and four crossveins including strong brace vein aligned with proximal border. IR1 originates on RP1 posterior to pterostigma. RP2 simple, parallel with IRP2, evenly convex from about region of base of pterostigma. Oblique vein O present as second crossvein in RP2–IRP2 space, at level of first crossvein distal to branching of RP1, RP2. Twelve antenodal crossveins (costal space), 10 postnodal crossveins. IRP2 simple (two vague “branches,” posterior more distinct, originating at fifth crossvein distal to O; not equivalent to strong, distinct branch characteristic of Lindeniinae). RP3-4 close to, subparallel with MA. MA gently curved in middle, MA, RP3-4 mostly separated by one cell width, two cells closer to margin, three at margin, mostly rather straight. CuA space apparently four cells wide, but region damaged. CuA, MP slightly divergent at margin (separated by at least four cells, possibly five, but surely no more).
Etymology. The specific epithet is formed from Eos, dawn (Greek), from which the word Eocene is derived.
Discussion. Although the fossil Okanagan Highlands Aeshnidae are mid-sized or large for the family in North America today (see above), the forewing of A. eos is of average length compared to those of extant gomphids.
Family indeterminate, genus A, species A
Figure 13.
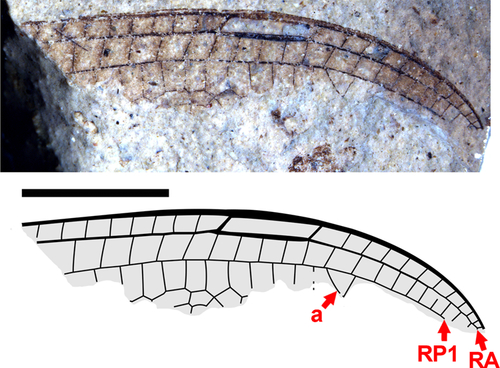
Fig. 13. Anisoptera indeterminate genus A, species A from Republic, SR 07-16-14. A, Photograph; B, drawing. Vein labelled “a” may be the origin of IR1 or an adventitiously angled crossvein, see text. Both to scale, 5 mm.
Classification. Odonata: Anisoptera: family incertae sedis.
Material. SR 07-16-14, an anterior-apical fragment of a wing, including the pterostigma. Collected at Republic B4131, 28.v.2005, by Gregg Wilson. Housed in the Stonerose Interpretive Center collection.
Description. Membrane darkly infuscate; pterostigma pale, rather short, length about 3.5 times width, borders thickened, posterior margin slightly convex, subtending five crossveins (four cells); venation rather dense, 11 crossveins distal to pterostigma in costal-RA space; no brace vein; angled vein (“a” in Fig. 13: base of IR1 or perhaps an adventitiously angled crossvein) in RP1 space originating on RP1, one row of cells in RA–RP1 space distal to pterostigma; RP1–RP2 space more than one cell wide, far basal to pterostigma as preserved (greater than three RA–RP1 cells basal).
Discussion. Although this is a small wing fragment, it is of interest in its difference from all other Okanagan Highlands dragonfly fossils. Infuscation is only seen elsewhere on the holotype forewing of Y. brownleei, but it is distinctly darker here, and the pterostigma is pale. Further, the short pterostigma and lack of a pterostigmal brace vein are unique in this assemblage.
These character states separate this wing from all dragonfly genera described here except for Idemlinea, where only the base of the wing is known. It may be distinguished from Idemlinea, however, by its denser venation (apparently more so than any other Okanagan Highlands species) and larger size. Although the fragment is small, we estimate the complete wing to have been about the same length as that of Eoshna, that is, approximately 53–54 mm, and likely as wide (16 mm), whereas the Idemlinea wing is hardly more than 12 mm wide (estimated complete). Such a difference cannot readily be explained by sexual dimorphism or intraspecific variation.
The family placement of this specimen is problematic because the small fragment preserved presents little comparative data. The relatively dense venation (the cells posterior to the costa and RA veins are virtually square), the short pterostigma with four square cells posterior to it, the lack of a pterostigmal brace vein, and the exact alignment of the crossveins at the ends of the pterostigma with the crossveins posterior to it in the RA–RP1 space are distinctive. These characteristics eliminate the extinct families known from the early Paleogene (Paleocene and Eocene) (Urolibellulidae, Bolcacorduliidae, and Palaeomacromiidae; details in Discussion below) as well as, for the most part, the extant Corduliidae, Macromiidae, Cordulegastridae (all three usually with fewer crossveins posterior to the pterostigma and none aligned with both its ends), Neopetaliidae (brace vein present), and Petaluridae (pterostigma extremely long and narrow). Most genera of Aeshnidae and Gomphidae have a brace vein associated with the pterostigma, but a number do not and, in many genera, the crossveins at the ends of the pterostigma are aligned with those immediately posterior to it in the RA–RP1 space. Although the evidence is inconclusive, it is likely that the fragment SR 07-16-14 comes from a gomphid or aeshnid lacking a brace vein and having an unusual arrangement of crossveins around a short pterostigma.
Discussion
Worldwide, the largest family of dragonflies today is the Libellulidae, with approximately 1037 described species, followed by the Gomphidae, with 980 described species (Dijkstra et al. Reference Dijkstra, Bechly, Bybee, Dow, Dumont and Fleck2013). When undescribed species are included, however, the Gomphidae may equal or exceed the Libellulidae in species (Carle et al. Reference Carle, Kjer and May2015). The Aeshnidae is smaller (456), with the remaining families steeply diminishing in numbers (Dijkstra et al. Reference Dijkstra, Bechly, Bybee, Dow, Dumont and Fleck2013).
In the early Paleogene, however, the dragonfly assemblage is quite different (Table 1). The Libellulidae have not yet appeared; their oldest reliable record is late Rupelian, about 29.2 million years old, some 20 million years younger than the youngest Okanagan Highlands dragonflies (see discussion by Kohli et al. Reference Kohli, Ware and Bechly2016). The Libellulidae are widely considered to be the most derived of Anisoptera families (Ware et al. Reference Ware, May and Kjer2007; Fleck et al. Reference Fleck, Ullrich, Brenk, Wallnisch, Orland, Bleidissel and Misof2008; Dijkstra et al. Reference Dijkstra, Bechly, Bybee, Dow, Dumont and Fleck2013). The majority of known dragonflies in the early Paleogene belong to the Aeshnidae: 31–33 species belong to the family of 56 dragonfly species based on wings, described and undescribed, named and unnamed, including those described here (Table 1).
According to Kohli et al. (Reference Kohli, Ware and Bechly2016), the oldest crown group Aeshnidae is the Early Cretaceous gomphaeschnine Gomphaeschna inferna Pritykina from the Zara Formation of Russia. For those who treat Gomphaeschninae as a separate family and recognise Allopetalia and the Early Cretaceous Baissaeshna to constitute a distinct Allopetaliidae (Bechly et al. Reference Bechly, Nel, Martinez-Delclòs, Jarzembowski, Coram and Martill2001; Huang et al. Reference Huang, Cai and Nel2017; Garrouste and Nel Reference Garrouste and Nel2019), however, its oldest fossil occurrence would be Basiaeschna alaskaensis from the late Paleocene or earliest Eocene, shortly before Okanagan Highlands times, that is, the latter half of the early Eocene. This occurrence of the extant genus Basiaeschna, a rather derived member of the family according to the cladistic analysis of von Ellenrieder (Reference von Ellenrieder2002), quickly followed by a notable expansion of known genera within only a few million years suggests either a revealed diversity of a lineage previously hidden by taphonomic factors in the Cretaceous (see above), or a previously small lineage rapidly diversifying in the Ypresian.
For those who include gomphaeschnines and Baissaeshna as aeshnids (e.g., Bechly and Rasmussen Reference Bechly and Rasmussen2019), the view of Aeshnidae evolution would be similar, except that part of the Cretaceous record is known.
The next largest family in the early Paleogene is the Corduliidae, with five or six species, and the remainder have between one and four species each. Three extinct families are present in the Eocene: Urolibellulidae (one species from the Green River Formation of Colorado, United States of America: Zeiri et al. Reference Zeiri, Nel and Garrouste2015), Bolcacorduliidae (one species from Monte Bolca, Italy: Gentilini Reference Gentilini2002), and Palaeomacromiidae (one species from Monte Bolca, Italy: Gentilini Reference Gentilini2002). Palaeomacromiidae is the only extinct dragonfly family known from the Paleocene (three species from Argentina: Petrulevičius et al. Reference Petrulevičius, Nel and Muzón1999; Petrulevičius and Nel Reference Petrulevičius and Nel2002, Reference Petrulevičius and Nel2007).
Consistent with this, the Aeshnidae dominate dragonflies in the Okanagan Highlands, with the six species described here (five named) in five genera (four named). (Specimen SR 07-16-14 family indeterminate genus A, species A likely belongs to either the Aeshnidae or Gomphidae.) The sole species of Okanagan Highlands Gomphidae, Auroradraco eos, is the only currently recognised occurrence of the family within the roughly 66-million-year interval from mid-Cretaceous Burmese amber (earliest Cenomanian: Schädel and Bechly Reference Schädel and Bechly2016) to the Oligocene of France (Rupelian: Nel and Paicheler Reference Nel and Paicheler1994). The oldest known gomphid is from the Late Jurassic Solnhofen limestones of Bavaria, Germany (Kohli et al. Reference Kohli, Ware and Bechly2016). Nel and Paicheler (Reference Nel and Paicheler1994) suggested that gomphids were more numerous and had a greater diversity from the Late Jurassic to the mid-Cretaceous than at any subsequent time. It is not surprising that they are unknown in the Cretaceous younger than Burmese amber, as it is poor in insect-bearing shale sites and occurrences in amber are rare, but their absence in the increasingly well-known fossil record of the Cenozoic before the Rupelian seems puzzling. If their modern presence in the region is limited by colder stream temperatures than where they are more diverse, this could have been true as well in the early Eocene, when the Okanagan Highlands was a cooler upland of depositional lake basins set among mountains of some elevation. It would not, however, explain their absence in coeval hot lowland localities such as those of the Green River Formation in Wyoming, Colorado, and Utah, United States of America. A few fossils were assigned to the Gomphidae in late Eocene (Priabonian) Baltic amber in the mid-nineteenth century (reviewed and revised by Nel and Paicheler Reference Nel and Paicheler1994). This was, however, when family concepts differed, they were inadequately described by modern standards, were never illustrated, and the types have been lost, apparently long ago: none are now considered to belong to the family (Nel and Paicheler Reference Nel and Paicheler1994; Bechly Reference Bechly1996; Bechly and Wichard Reference Bechly and Wichard2008; Wichard et al. Reference Wichard, Gröhn and Seredszus2009; Weitschat and Wichard Reference Weitschat, Wichard and Penny2010). They have a modest post-Eocene fossil record (Nel and Paicheler Reference Nel and Paicheler1994).
Acknowledgements
We thank Collections Manager Nancy Van Wagoner at Thompson Rivers University for loans of specimens in their collections; Director Katherine Meade and Collections Manager Travis Wellman at the Stonerose Interpretive Center for loans of specimens and facilitating research at Republic; Victoria Arbour, Curator of Paleontology and Erica Wheeler, Head of Collections Care and Conservation at the Royal British Columbia Museum, for access to collections and facilitating accession of SBA-105 into the Royal British Columbia Museum collection; collectors who donated specimens to these collections as noted in the text; Günter Bechly (Biologic Institute, Redmond, Washington) for discussion of Parabaissaeshna ejerslevense; André Nel (Muséum National d’Histoire Naturelle, Paris, France) for providing copies of his works; Rosser Garrison (California Department of Food and Agriculture, Sacramento, California, United States of America, retired) and Dennis Paulson (Slater Museum of Natural History, University of Puget Sound, Tacoma, Washington, retired) for morphological advice; Klaas-Douwe Dijkstra and Rory Dow (Naturalis Biodiversity Center, Leiden, The Netherlands), for images of and information on Old World gomphids; Alexandr Rasnitsyn (A.A. Borissiak Paleontological Institute, Russian Academy of Sciences, Moscow, Russia) for supplying Russian literature; Brian Farrell and Emily Blank (Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, United States of America) for providing photographs of the holotype of Oligaeschna separata; and Vladimir Makarkin (Russian Academy of Sciences, Vladivostok, Russia) for advice on Latin. S.B.A. fieldwork was funded by a Putnam Expeditionary Grant. S.B.A. thanks Rolf Mathewes (Simon Fraser University) for funding and facilitating work at Simon Fraser University, and R.A.C. thanks Joan Kerik for her support.