Linezolid is an oxazolidinone antibiotic active against Gram-positive bacteria, approved in Europe in 2001 [Reference Brauers1]. Linezolid-resistance in enterococci was observed soon after its introduction and most reports describe resistance to this agent in vancomycin-resistant enterococci (VRE) recovered from patients who received long courses of therapy [Reference Meka and Gold2–Reference Pai5]. Here we describe the emergence of linezolid-resistant enterococci (LRE) in a university hospital in which linezolid had been in use since 2003. During 2004 and at the start 2005 the hospital was affected by an extensive outbreak of VRE [Reference Borgmann6]. Although the VRE outbreak could be contained LRE emerged during and after the outbreak. For further analysis LRE isolated from clinical specimens from June 2004 to June 2006 were characterized and data including linezolid therapy and hospital location were ascertained. Additionally, the extent of linezolid usage over the last 2 years was documented.
Bacteria were identified to the species level by the Streptrapid (bioMérieux, Nürtingen, Germany) or Vitek 2 (bioMérieux) systems. Antibiotic susceptibility testing was performed according to Clinical and Laboratory Standards Institute (CLSI) criteria either by the agar diffusion method or broth microdilution testing in Vitek 2 and confirmed by E-test analysis (AB Biodisk, Solna, Sweden). The genetic relatedness of LRE SmaI-digested (Roche Biochemicals, Mannheim, Germany) chromosomal DNA from isolates was analysed by pulsed-field gel electrophoresis (PFGE). The restriction fragments were separated in a contour-clamped homogenous electric field apparatus (CHEF-DRII; Bio-Rad, Munich, Germany) in 0·5× Tris-borate-EDTA buffer at 14°C and 200 V with ramped pulsed times of 1–20 s for 24 h. Gels were interpreted according to the criteria of Tenover et al. [Reference Tenover7].
The Table summarizes the data for all LRE detected in the period from June 2004 to June 2006 in chronological order. E-test analyses revealed minimal inhibitory concentrations (MICs) with values ranging from 4 to >256 μg/ml. This is in agreement with an observed gene-dosage effect showing an association between the number of loci containing the mutation and the magnitude of the MIC [Reference Marshall8]. Most LRE isolated during the first period until the second term of 2005 (VRE outbreak situation) were vancomycin-resistant carrying the vanA gene and examination of the patients' data revealed prior exposure to linezolid in most cases. The first isolate is an exception to this trend. This vancomycin-susceptible E. faecium isolate without prior drug exposure was most probably imported from Italy [Reference Schulte9]. In the second period (end of 2005 up to 2006) the situation changed: LRE were isolated more frequently from patients who had never received this antibiotic and resistance was also observed in vancomycin-susceptible enterococci. Investigation of linezolid usage at our university hospital revealed that the striking increase in resistance in the second quarter of 2006 was paralleled by an increase in consumption of the antibiotic (Fig.). Usage of linezolid became more common because of good clinical outcomes in the treatment of VRE infections. To elucidate genetic relatedness and possible nososcomial spread of LRE, PFGE of 17 isolates was performed. As expected the DNA profiles of the strains isolated in the first period (patients B–G; Table) were identical to VRE outbreak strains (profiles 2 and 3) with the exception of the first isolate which showed no clonal affinity to the other strains. The DNA profiles of the isolates in the second period from patients I–Q were more variable. Amongst VRE isolates the former epidemic outbreak strain (patient J) could be found as well as non-epidemic strains (patients L, M). Interestingly, patient J harboured a vancomycin-susceptible and -resistant clone of the outbreak strain presumably indicating loss of the vanA gene during colonization. The vancomycin-susceptible strain of patient K isolated from a blood culture showed no similarities to any other strain. This patient had not received linezolid and had no local and temporal contact with the other patients. Vancomycin-susceptible strains isolated from patients N, O, and Q had identical DNA profiles indicating nosocomial spread of this strain. While patients O and Q received the drug prior to isolation of the strains and shared the same ward, patient N did not. No association between patient N and the others was evident. Although the first isolate was detected in patient N without exposure to linezolid, it can be assumed that the LRE was generated in one of the patients under linezolid pressure with subsequent spread to the other two patients. At that time linezolid was not routinely tested in vancomycin-susceptible enterococci and thus LRE may have been overlooked in previous specimens.

Fig. Number of linezolid-resistant enterococci (LRE, ■) isolated at the university hospital in Southwest Germany from June 2004 to June 2006 [by quarter (qr)] compared with the consumption of linezolid (–◆–) (DDD/1000 PD=defined daily doses per 1000 patient days) in the same period.
Table. Characteristics of linezolid-resistant enterococcal strains in chronological order
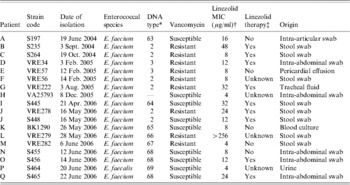
* DNA profile pattern type defined by PFGE, pattern types 2 and 3 representing linezolid-resistant clones of a vancomycin-resistant Enterococcus faecium (VRE) outbreak, types 63–69 representing linezolid-resistant clones of non-epidemic VRE strains or vancomycin-susceptible strains.
† Susceptibility breakpoint for linezolid ⩽2 μg/ml, resistance breakpoint ⩾8 μg/ml.
‡ Linezolid therapy before isolation of resistant enterococcal isolate.
Linezolid resistance in clinical isolates is associated with G2576U mutation in domain V of the 23S rRNA [Reference Marshall8]. Only a few reports on the occurrence of LRE in patients without prior exposure to linezolid are available. In all cases nosocomial transmission of resistant strains was regarded as the most probable route of acquisition or this was not able be ruled out [Reference Jones10–Reference Bonora12]. We also isolated LRE from patients without previous therapy. In our opinion nosocomial spread of these isolates via the hands of health-care workers or via contaminated objects is most likely, although spontaneous mutation cannot be excluded. Identical PFGE patterns in the cases of patients N, O and Q support the hypothesis of nosocomial spread. Currently reports on the spread of LRE always deal with vancomycin-resistant strains [Reference Herrero, Issa and Patel13, Reference Dobbs14]. We report the first case of nosocomial spread of vancomycin-susceptible but linezolid-resistant E. faecium.
In conclusion, increasing consumption of linezolid was associated with rising enterococcal resistance which extended to vancomycin-susceptible strains. This suggests that linezolid should be used with caution and susceptibility testing of isolates should be performed prior to its use to detect decreasing linezolid susceptibility over time. Isolation precautions and screening of contacts should be considered to avoid further spread of resistant isolates.
DECLARATION OF INTEREST
None.




