Major depressive disorder (MDD) is the leading cause of disability for individuals 15-44 years of age. Current treatments for MDD are effective, but only 60% of patients respond to the first treatment. Reference Gartlehner, Thaler, Hill and Hansen1,Reference Hansen, Gaynes, Thieda, Gartlehner, Deveaugh-Geiss and Krebs2 Non-response in patients constitutes a large proportion of the ongoing costs and disease burden of MDD. The total annual cost of depressive illness in Europe was estimated to be e92 billion in 2010, Reference Olesen, Gustavsson, Svensson, Wittchen and Jonsson3 making the effective identification and treatment of depression an economic imperative as well as a major healthcare priority. Although clinical assessment is the cornerstone of patient management in depression, there are currently no agreed-upon pre-treatment clinical predictors for treatment outcomes. Reference Kemp, Gordon, Rush and Williams4 The shortcomings of the standard clinical measures in use have led to a recent focus on the development of novel mechanism-based biomarkers that reflect disruptions to the underlying brain circuitry. Reference Insel, Cuthbert and Garvey5 Despite recent advances, there are currently no useful biomarkers for predicting response to antidepressant medications. Reference Rush, Wisniewski, Warden, Luther and Davis6
The functions of frontal-limbic circuits, specifically the amygdala-hippocampal complex and the anterior cingulate region, are central to the conceptual model of MDD and the action of antidepressants. Reference Mayberg, Brannan, Mahurin, Jerabek, Brickman and Tekell7-Reference Costafreda, Chu, Ashburner and Fu9 The key structures within these circuits are the subgenual anterior cingulate cortex (ACC) (Brodmann area 25) and the amygdala. The subgenual ACC has strong axonal connections to the medial prefrontal cortex, orbitofrontal cortex, the medial temporal lobe and to the anterior and posterior components of the ACC. Reference Gutman, Holtzheimer, Behrens, Johansen-Berg and Mayberg10,Reference Freedman, Insel and Smith11 These connections involve centres that influence learning, memory, motivation and reward behaviour - all of which are abnormal in MDD. Reference Drevets, Price and Furey12 White matter alterations related to these connections within the reward circuitry have been found altered with depression and risk for developing depression. Altered cingulate bundle white matter has been found in participants with a high familial risk for depression and was also associated with symptoms of subclinical anhedonia in these individuals. Reference Keedwell, Chapman, Christiansen, Richardson, Evans and Jones13 White matter related to the medial forebrain bundle, which is a key structure of the reward system and connects the ventral tegmental area with the nucleus accumbens, dorsolateral and orbitofrontal prefrontal regions, was found to characterise melancholic subtype of depression and was also associated with both depression severity and anhedonia. Reference Bracht, Horn, Strik, Federspiel, Schnell and Hofle14,Reference Blood, Iosifescu, Makris, Perlis, Kennedy and Dougherty15 Deep brain stimulation of this bundle was also found to improve symptoms in treatment-resistant depression, suggesting that white matter connectivity of the key limbic regions may be associated with treatment outcome. Reference Schlaepfer, Bewernick, Kayser, Madler and Coenen16 Convergent data from electroencephalogram (EEG) theta activity, Reference Pizzagalli, Pascual-Marqui, Nitschke, Oakes, Larson and Abercrombie17 pre-treatment baseline functional magnetic resonance imaging (MRI), Reference Siegle, Thompson, Collier, Berman, Feldmiller and Thase18 structural grey matter density measured using MRI Reference Costafreda, Chu, Ashburner and Fu9 and positron emission tomography (PET) data Reference Pizzagalli19 highlight the importance of the subgenual ACC area in treatment prediction. Extensive PET and functional MRI (fMRI) data also support an important role for the amygdala, particularly in the maintenance of a depressed state. Reference Canli, Cooney, Goldin, Shah, Sivers and Thomason8,Reference Arnone, McKie, Elliott, Thomas, Downey and Juhasz20 This role is likely associated with the abnormal emotional reactivity and the negative emotional bias that are core features of depression. Reference Drevets, Price and Furey12
Recent work using diffusion tensor imaging (DTI) has shown that the structural white matter connectivity of this same neural circuitry distinguishes people with MDD from healthy peers Reference Korgaonkar, Cooper, Williams and Grieve21-Reference Cole, Chaddock, Farmer, Aitchison, Simmons and McGuffin23 (see Murphy & Frodl Reference Murphy and Frodl24 for a detailed review of DTI findings in MDD). It has yet to be established whether structural connectivity based on DTI is a predictor of treatment outcomes in MDD. Given the centrality of subgenual ACC and the amygdala, we proposed to examine the DTI measures of white matters tracts associated with this structure. In order to maximise the translational potential we used a technique called tract-based spatial statistical analysis (TBSS), which is a highly reproducible, extremely robust and easily automated method that can generate simple metrics of white matter tracts. Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols and Mackay25 Based on prior data from our laboratory and others, in this study we selected, a priori, five frontolimbic white matter fibre tracts that relate to the amygdala and subgenual ACC as potential predictors of treatment outcomes. These were: the cingulum bundle - an association pathway innervated by the cingulate gyrus and connected to the hippocampus (subdivided into cingulate (CgC) and hippocampal (CgH) parts); the stria terminalis and the fornix - which comprise axonal projections from the hippocampus and the amygdala respectively, and connect to the hypothalamus and rest of the limbic system; and the uncinate fasciculus - connecting the anterior temporal lobe (hippocampus and amygdala) with the medial and lateral orbitofrontal cortex.
Here we present the first planned analysis to evaluate the predictive utility of DTI using data from the International Study to Predict Optimized Treatment for Depression (iSPOT-D, trial registered at ClinicalTrials.gov: NCT00693849). This is a large multicentre trial designed to identify pre-treatment measures that predict or moderate MDD treatment outcomes Reference Grieve, Korgaonkar, Etkin, Harris, Koslow and Wisniewski26,Reference Williams, Rush, Koslow, Wisniewski, Cooper and Nemeroff27 We also tested whether these tracts were significantly different between people with MDD (MDD group) and controls (control group). We hypothesised that: (a) for these selected five fibre tracts (CgC, CgH, stria terminalis, fornix and uncinate fasciculus), the fractional anisotropy index of white matter connectivity from DTI predicts outcome remission following 8 weeks of treatment with antidepressants (hypothesis 1); and (b) the tracts that predict outcome are also altered in comparison with healthy controls at pre-treatment baseline (hypothesis 2). As part of hypothesis testing, we used cross-validation methods to test the generalisability of prediction models.
Method
This study was conducted according to the principles of the Declaration of Helsinki 2008. After the study procedures were fully explained in accordance with the ethical guidelines of the institutional review board (Western Sydney Local Health District Human Research Ethics Committee), participants provided written informed consent.
iSPOT-D enrolment and protocol
A complete description of the multisite randomised iSPOT-D study protocol, clinical assessments, inclusion/exclusion criteria and diagnosis procedures is provided in Williams et al. Reference Williams, Rush, Koslow, Wisniewski, Cooper and Nemeroff27 In short, the Mini-International Neuropsychiatric Interview, Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller28 according to DSM-IV criteria, and a 17-item Hamilton Rating Scale for Depression (HRSD17) Reference Hamilton29 score ⩾16 confirmed the primary diagnosis of MDD. All the MDD group were either antidepressant-naive or had undergone a wash-out period of at least five half-lives of a previously prescribed antidepressant.
Participants were randomised to receive one of the three commonly prescribed antidepressants: two selective serotonin reuptake inhibitors - escitalopram or sertraline, or a serotonin-noradrenaline reuptake inhibitor - extended-release venlafaxine (venlafaxine-XR). Investigators/raters and participants were not masked to treatment assignment. Antidepressants were prescribed and doses adjusted by each participant’s treating clinician according to routine clinical practice, but following the recommended dose ranges. Our study recruited from a community setting and hence closely reflects the bulk of the target population for antidepressants. The approach described above was used to replicate a ‘real-world’ approach to treating depression. Participants returned for their follow-up visit at the end of 8 weeks. The HRSD17 was used to assess depression severity at week 8. A total of 22 participants exited the study before the week 8 follow-up and did not have HRSD17 scores at week 8 and hence were excluded from this analysis.
The primary outcome was remission as determined by a clinician-rated HRSD17 score ⩽7. Response rate was also analysed and was defined as a ⩾50% decrease in HRSD17 score from baseline to week 8. The results for response are presented in the online supplement. In addition, self-reported age at onset and disease duration (years) were documented for analyses.
Participants in the present study
Diffusion tensor imaging is a non-invasive MRI technique that measures the connectivity within white matter tracts in vivo by calculating the direction and magnitude of water diffusion within the brain. Data on DTI were drawn from the first 102 participants with MDD and 34 age- and gender-matched healthy participants who provided MRI data at Westmead Hospital (University of Sydney Medical School) as part of baseline data collection for iSPOT-D. These represent the first half of the planned imaging cohort for the study (i.e. 50% out of the total 200 patients with MDD, ages 18-65, estimated to be tested at this site). Reference Grieve, Korgaonkar, Etkin, Harris, Koslow and Wisniewski26 Of the 80 participants in the MDD group who returned for their week 8 follow-up visit, DTI data for 6 participants had not been collected at baseline because of time constraints, resulting in data on 74 individuals with MDD being suitable for analysis (Fig. 1).
Image acquisition
Magnetic resonance images were acquired using a 3.0 Tesla GE Signa HDx scanner (GE Healthcare, Milwaukee, Wisconsin). Acquisition was performed using an 8-channel head coil. Diffusion tensor images were acquired using a spin-echo DTI-echo planar imaging sequence. Seventy contiguous 2.5 mm slices were acquired in an axial orientation with an in-plane resolution of 1.72 mm × 1.72 mm and a 128 × 128 matrix (repetition time (TR) = 17000 ms; echo time (TE) = 95 ms; fat saturation: on; number of excitations (NEX) = 1; frequency direction: right/left). A baseline image (b = 0) and 42 different diffusion orientations were acquired with a b-value of 1250. Total acquisition time for the DTI protocol was 13 min 36 s.
Tract-based spatial statistical analysis of DTI data
Diffusion tensor imaging data-processing and analytic methods have been described in detail. Reference Korgaonkar, Cooper, Williams and Grieve21,Reference Korgaonkar, Grieve, Koslow, Gabrieli, Gordon and Williams22 The DTI data was preprocessed and analysed using the Oxford Centre for Functional MRI of the Brain (FMRIB) diffusion toolbox and TBSS software tools as part of the FMRIB Software Library release 4.1.3 (http://www.fmrib.ox.ac.uk/fsl). Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols and Mackay25 Diffusion tensor models were fitted and images of fractional anisotropy were generated for each participant. An average fractional anisotropy image was generated and thinned to create a white matter skeleton representing the centres of all white matter tracts common to all participants. This fractional anisotropy skeleton was then thresholded to fractional anisotropy ⩾0.2 to include the major white matter pathways but avoid peripheral tracts. The Johns Hopkins University International Consortium for Brain Mapping (JHU ICBM) DTI-81 white matter labels atlas was used to identify parts of the tract skeleton for the hypothesised frontolimbic white matter tracts (Fig. 2). Reference Mori, Oishi, Jiang, Jiang, Li and Akhter30 The mean fractional anisotropy for each tract was calculated and used for further analyses.

Fig. 1 Flow diagram of participant progress through the study.
Venlafaxine-XR, extended-release venlafaxine; iSPOT-D, International Study to Predict Optimized Treatment for Depression.
Statistical analyses
Prediction models for treatment outcome
Logistic regression statistics were used to test the prognostic utility of DTI fractional anisotropy measures for antidepressant treatment outcome. Age, baseline depressive severity, age at onset and duration of illness were all found to be associated with treatment outcome (Table 1). To remove any proxy effects of these measures via white matter fractional anisotropy, residual fractional anisotropy values were calculated controlling for these measures by regression. These residuals were then used for all further analyses. The MDD group were characterised with regard to: (a) remission with treatment and (b) response to treatment based on the criteria outlined above. These outcomes were tested in separate analyses as dependent variables. To test hypothesis 1, all of the hypothesised white matter tracts (CgC, CgH, stria terminalis, fornix, uncinate fasciculus) were tested using a backward stepwise logistic regression model. This analysis performs a backward stepwise elimination of candidate independent variables until there is no further improvement in model estimation. The model was assessed using a significance level of P<0.05 and the accuracy in prediction was reported. In order to confirm the validity of findings for the preselected white matter tracts at the whole brain level, we analysed all the JHU ICBM-DTI-81 white-matter-atlas-labelled tracts in a single backward stepwise logistic regression model.
Cross-validation of prediction models
To test the generalisability of prediction models, a stepwise linear discriminant analysis (LDA) with an inbuilt K-fold cross validation was performed using the klaR package in R. In this cross-validation method, a random subset according to the ratio K:1 is partitioned out and used as a validation data-set. The remaining subset serves as a training data-set. The prediction models were derived using the training data-set and predictive accuracy was evaluated on the validation data-set. The cross-validated accuracy is the average of the accuracies of all randomisations. The cross-validation analysis was run for five different values of K: 2, 3, 5, 10 and 15, which correspond to training sets of the sizes 50%, 66.6%, 80%, 90% and 95%, respectively. Each analysis was performed 20 times and model accuracies (and the selected predictors) were combined across all 100 runs of stepwise LDA. Testing with different subsets helps to develop more replicable models, and it avoids over-fitting to the data-set. Reference Zhang31
Two approaches were used to cross-validate the model. First, the five white matter tracts were ranked based on the analysis described above. For each of the white matter predictors, a summary prediction score of model accuracy was derived for the models (out of all 100 runs) of which that predictor was a member. The score for each run is the total accuracy of the model containing that predictor. A predictor gets a higher score based on the frequency that it appears in models, and on the accuracy of these models. The score is scaled such that the maximum possible score for a predictor is 1.0. The second measure of cross-validation was the average accuracy of models containing the best set of predictors for a subset of 100 trials; also provided is a range of the highest and lowest model accuracy for models containing that predictor set.
MDD v. control group comparisons
To test hypothesis 2, two sample t-tests were used to evaluate significant pre-treatment differences in fractional anisotropy between the MDD and control groups for our five preselected white matter tracts (i.e., CgC, CgH, stria terminalis, fornix, uncinate fasciculus).
Results
Participant characteristics
The clinical and demographic features and the remission rates for participants with MDD are presented in Table 1 (online Table DS1 for response rates). The average dose for the three treatment arms were as follows: escitalopram 12 mg (s.d. = 7); sertraline 53 mg (s.d. = 28); and venlafaxine-XR 86 mg (s.d. = 32). There was a significant difference in baseline severity between the treatment arms (HRSD17 baseline - F(2,72) = 3.19, P = 0.047; escitalopram >venlafaxine-XR; no difference between sertraline and escitalopram or venlafaxine-XR). Demographic and other clinical measures were similar across the three treatment arms. Of the 80 participants in the MDD group who completed the week 8 follow-up, 60% were responders and 46.3% remitters based on our predefined criteria. These response and remission rates were similar across treatment arms and match those from the global iSPOT-D sample (i.e. response 60% and remission 45.4% for 1008 participants with MDD tested at the time of analysis as part of the overall study) (unpublished, details available from the author on request). Remitters were characterised by a younger age at MDD onset and had experienced depression for a shorter period of time (Table 1). Responders were younger, had a higher depressive severity at the baseline visit and had developed depression at a younger age than non-responders.
Table 1 Demographics and clinical measures summary
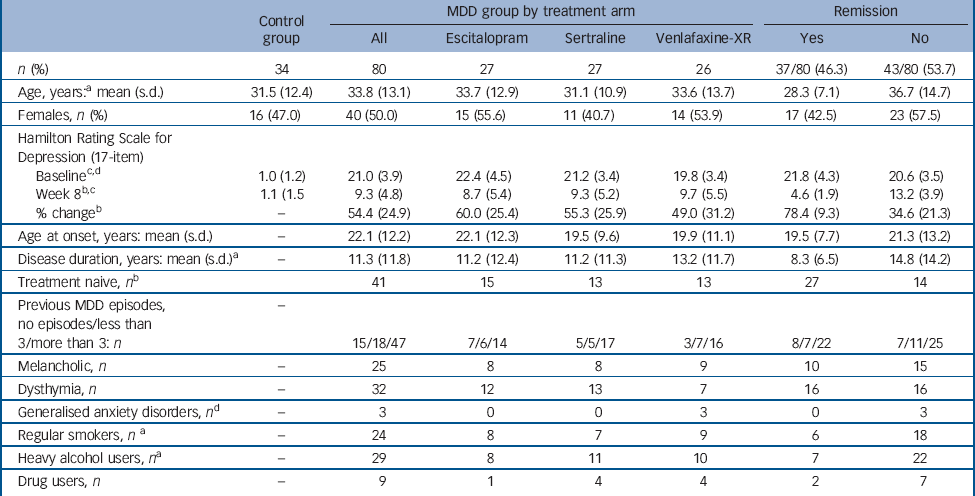
| Control group |
MDD group by treatment arm | Remission | |||||
|---|---|---|---|---|---|---|---|
| All | Escitalopram | Sertraline | Venlafaxine-XR | Yes | No | ||
| n (%) | 34 | 80 | 27 | 27 | 26 | 37/80 (46.3) | 43/80 (53.7) |
| Age, years:Footnote a mean (s.d.) | 31.5 (12.4) | 33.8 (13.1) | 33.7 (12.9) | 31.1 (10.9) | 33.6 (13.7) | 28.3 (7.1) | 36.7 (14.7) |
| Females, n (%) | 16 (47.0) | 40 (50.0) | 15 (55.6) | 11 (40.7) | 14 (53.9) | 17 (42.5) | 23 (57.5) |
| Hamilton Rating Scale for Depression (17-item) | |||||||
| BaselineFootnote c , Footnote d | 1.0 (1.2) | 21.0 (3.9) | 22.4 (4.5) | 21.2 (3.4) | 19.8 (3.4) | 21.8 (4.3) | 20.6 (3.5) |
| Week 8Footnote b , Footnote c | 1.1 (1.5 | 9.3 (4.8) | 8.7 (5.4) | 9.3 (5.2) | 9.7 (5.5) | 4.6 (1.9) | 13.2 (3.9) |
| % changeFootnote b | - | 54.4 (24.9) | 60.0 (25.4) | 55.3 (25.9) | 49.0 (31.2) | 78.4 (9.3) | 34.6 (21.3) |
| Age at onset, years: mean (s.d.) | - | 22.1 (12.2) | 22.1 (12.3) | 19.5 (9.6) | 19.9 (11.1) | 19.5 (7.7) | 21.3 (13.2) |
| Disease duration, years: mean (s.d.)Footnote a | - | 11.3 (11.8) | 11.2 (12.4) | 11.2 (11.3) | 13.2 (11.7) | 8.3 (6.5) | 14.8 (14.2) |
| Treatment naive, n Footnote b | 41 | 15 | 13 | 13 | 27 | 14 | |
| Previous MDD episodes, no episodes/less than 3/more than 3: n | - | 15/18/47 | 7/6/14 | 5/5/17 | 3/7/16 | 8/7/22 | 7/11/25 |
| Melancholic, n | - | 25 | 8 | 8 | 9 | 10 | 15 |
| Dysthymia, n | - | 32 | 12 | 13 | 7 | 16 | 16 |
| Generalised anxiety disorders, n Footnote d | - | 3 | 0 | 0 | 3 | 0 | 3 |
| Regular smokers, n Footnote a | - | 24 | 8 | 7 | 9 | 6 | 18 |
| Heavy alcohol users, n Footnote a | - | 29 | 8 | 11 | 10 | 7 | 22 |
| Drug users, n | - | 9 | 1 | 4 | 4 | 2 | 7 |
MDD, major depressive disorder.
a. Difference between remitters and non-remitters at P<0.05.
b. Difference between remitters and non-remitters at P<0.001.
c. Difference between MDD and control group at P<0.001.
d. Difference between treatment arms at P<0.05.
Predictors of treatment remission
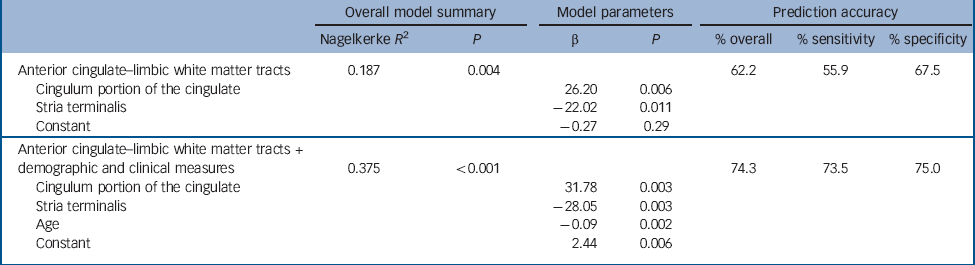
The backward logistic regression analysis converged on a model containing only the CgC and stria terminalis white matter tracts (P = 0.004, Table 2). Overall accuracy in predicting remission was 62.2% (sensitivity: 55.9%, specificity: 67.5%). Fractional anisotropy values for the CgC and stria terminalis bundle were significant predictors of remission status in the optimal model, with those in the MDD group who had a higher fractional anisotropy in the CgC (fractional anisotropy CgC remitters: 0.603 (s.d. = 0.006); fractional anisotropy CgC non-remitters: 0.587 (s.d. = 0.005)), and a lower fractional anisotropy in the stria terminalis being more likely to achieve remission (fractional anisotropy stria terminalis remitters: 0.556 (s.d. = 0.006); fractional anisotropy stria terminalis non-remitters: 0.573 (s.d. = 0.006)). Both the CgC and stria terminalis tracts were also selected in the most parsimonious model at a whole brain level (P<0.001), thus validating their role in prediction of remission when assessed at a whole brain level. Figure 2 shows a comparison of the fractional anisotropy between remitters and non-remitters for the five white matter tracts.
The LDA cross-validation analysis confirmed the logistic regression modelling, but also highlighted the contribution of the fornix to prediction. The weighted prediction scores for each predefined white matter tract were: CgC, 0.658; CgH, 0.113; stria terminalis, 0.291; fornix, 0.567; and uncinate fasciculus, 0.220. The CgC scored the highest, showing the most reliable prediction of remission status. The CgC was selected as a predictor in 99/100 LDA stepwise runs. The fornix and stria terminalis were the next best reliable predictors in the cross-validation analysis. The stria terminalis was selected in 44/100 models; however, 34 of these 44 models also included the fornix. Note that although the fornix was not identified in the most significant model from the logistic regression analysis, the fornix, CgC and stria terminalis were identified as the second best model in the analysis (P = 0.005, overall accuracy 68.9%, with 61.8% sensitivity and 75% specificity). The significance value for the fornix did not meet our set criteria (P<0.05) for prediction variables to be retained in the logistic regression analysis and hence was excluded in the final step. The two sets of predictors most often chosen were the CgC and fornix (32/100), and the CgC, stria terminalis and fornix (23/100). The average cross-validated prediction accuracy for both models was similar: for CgC and fornix it was 66.7% (range: 64.6-69.2%) and for the CgC, stria terminalis and fornix it was 66.2% (range: 63.4-70.3%).

Fig. 2 Fractional anisotropy differences in preselected white matter tracts between the participants in the major depressive disorder group who reached remission (remission subgroup) and those that did not (non-remission subgroup).
The upper panel shows the five preselected white matter tracts (in red) and the white matter skeleton representing the centre of all white matter tracts (in green) overlaid on a standard brain. The lower panel shows fractional anisotropy differences between participants in the remission and non-remission subgroups. Fractional anisotropy for the stria terminalis and cingulate portion of the cingulum bundle was identified as the most significant predictors of remission (**).
Table 2 Prediction models of remission
| Overall model summary | Model parameters | Prediction accuracy | |||||
|---|---|---|---|---|---|---|---|
| Nagelkerke R 2 | P | β | P | % overall | % sensitivity | % specificity | |
| Anterior cingulate-limbic white matter tracts | 0.187 | 0.004 | 62.2 | 55.9 | 67.5 | ||
| Cingulum portion of the cingulate | 26.20 | 0.006 | |||||
| Stria terminalis | –22.02 | 0.011 | |||||
| Constant | –0.27 | 0.29 | |||||
| Anterior cingulate-limbic white matter tracts + demographic and clinical measures | 0.375 | <0.001 | 74.3 | 73.5 | 75.0 | ||
| Cingulum portion of the cingulate | 31.78 | 0.003 | |||||
| Stria terminalis | –28.05 | 0.003 | |||||
| Age | –0.09 | 0.002 | |||||
| Constant | 2.44 | 0.006 | |||||
Additional effect of demographic and clinical variables in treatment prediction
The previous analysis excluded the effects of age, baseline depressive severity, age at onset and duration of illness on white matter fractional anisotropy measures prior to analysis (see Method). We therefore investigated the additional effect of adding these clinical and demographic variables for the predictive model (Table 2). Only age was a significant additional element (with no change in the significance of the existing fractional anisotropy measures) for the remission prediction model, with an improvement in overall accuracy to 74.3% (sensitivity: 73.5%, specificity: 75.0%). Age at onset, duration of MDD and baseline severity were not significant elements of the model.
Pre-treatment differences between MDD and control groups
For the five hypothesised white matter tracts, significant baseline differences in fractional anisotropy between the MDD and control groups were found only for the fornix (MDD group: 0.525 (s.d. = 0.008), control group: 0.559 (s.d. = 0.011), P = 0.038) and the CgC bundle (MDD group: 0.591 (s.d. = 0.003), control group: 0.606 (s.d. = 0.006), P = 0.022). The MDD group had a lower fractional anisotropy in comparison with the control group in both of these white matter tracts.
Discussion
Main findings
The purpose of this study was to evaluate DTI measures of brain white matter as a prognostic biomarker in MDD. Extending from previous data that show DTI evidence of baseline differences in white matter integrity between participants with MDD and unaffected individuals, Reference Korgaonkar, Cooper, Williams and Grieve21-Reference Cole, Chaddock, Farmer, Aitchison, Simmons and McGuffin23 our results demonstrate for the first time that DTI measures of white matter integrity can contribute to treatment prediction in MDD (hypothesis 1). Of our five hypothesised regions, the stria terminalis fractional anisotropy and the CgC fractional anisotropy were shown to predict remission. These two tracts predicted remission with an overall accuracy of 62%, with a sensitivity of 56% and a specificity of 68%. The addition of age to this model improved prediction accuracy overall to 74%, with a sensitivity of 74% and a specificity of 75%. We suggest that the fractional anisotropy of these two tracts could constitute an important biomarker of treatment response (stria terminalis-CgC fractional anisotropy).
At baseline, the MDD group had significant alterations in the fornix and CgC (and not the stria terminalis) in comparison with the control group (hypothesis 2). These results confirm our previous findings, and again highlight the central role of the fornix and the CgC in MDD. Reference Korgaonkar, Cooper, Williams and Grieve21,Reference Korgaonkar, Grieve, Koslow, Gabrieli, Gordon and Williams22 The CgC collects projections from the rostral prefrontal/anterior cingulate cortices to the posterior cingulate, whereas the fornix and stria terminalis are comprised of axonal projections from the hippocampus and the amygdala, respectively, and connect to the hypothalamus and the rest of the limbic system. Reference Wakana, Jiang, Nagae-Poetscher, van Zijl and Mori32 The fornix was the only significant predictor of response (see online supplement).
Our MDD group showed a 60% response rate, with 39% of these participants going on to achieve remission. These results are in line with the poor rate of remission achieved across numerous comparable cohorts in the published literature. Because our participant population has been chosen to be as realistic as possible, this rate of remission is likely to be reflective of that seen in the community. Predicting remission is important for a number of reasons: (a) identifying individuals who may need greater multidisciplinary care because of an increased risk of pharmacological treatment resistance; (b) potentially assisting the effective and rational targeting of treatment, thereby reducing waste and improving the speed of effective treatment; and (c) an improved understanding of the mechanisms that underpin remission. Using DTI, we are able to capture aspects of the neural circuits that govern remission, a finding that is particularly critical for understanding the key components of the circuits that underpin MDD.
In addition to the main aim of the study to test whether DTI measures of these circuits are related to treatment outcomes in MDD, we also tested improvement in the prediction of outcome by including demographic and clinical features that are also associated with treatment outcome. In our cohort, we found that age and duration of illness were associated with remission, and age, severity and age at onset of MDD were associated with treatment response. Using these measures, only age was found to significantly improve the DTI prediction accuracy for remission (described above), whereas age at onset, duration and baseline severity improved prediction of response (described in the online supplement). These data suggest that a combination of these easily assessable demographic and clinical measures with the imaging measures may aid clinical application.
Significance of our findings
Our data highlight three of our hypothesised regions: the CgC (predicts remission and baseline difference between the MDD and control groups), the stria terminalis (predicts remission) and the fornix (predicts response and baseline difference). The direction of the predictive relationship of the CgC and stria terminalis tracts is particularly interesting, with the CgC fractional anisotropy being positively related to remission, and the stria terminalis negatively related to remission. Functional signals from the subgenual ACC (area 25) and amygdala limbic regions that are associated with these white matter tracts have been linked to treatment outcome following antidepressant medication and cognitive-behavioural therapy. Reference Mayberg, Brannan, Mahurin, Jerabek, Brickman and Tekell7,Reference Siegle, Thompson, Collier, Berman, Feldmiller and Thase18,Reference Arnone, McKie, Elliott, Thomas, Downey and Juhasz20 Our data extend these findings, providing new evidence that the structural white matter connections for these same regions may have a role in the prediction of treatment outcomes. Preserved connectivity in the CgC (i.e. a positive relationship between CgC fractional anisotropy and the probability of remission) may be a prerequisite for sustained response to medication for MDD because of the importance of frontolimbic modulation in the maintenance of a depressive state. Similarly, a negative relationship between the stria terminalis fractional anisotropy and remission probability (i.e. lower fractional anisotropy predicts remission) may be a reflection of an important deleterious role of the amygdala in the maintenance of the remission state. Although our data clearly show that the integrity of these two regions are key factors in remission, it is clear that a combination of functional and structural information will be required to properly understand the dynamic mechanisms via which the CgC and stria terminalis influence remission. Our data also highlighted the possible role of the fornix in relation to prediction of remission. The fornix, CgC and stria terminalis were identified as the second best model for remission prediction in the logistic regression analysis, with slightly better accuracy in prediction of remission than the final step model (i.e. CgC and stria terminalis only). However, the fornix did not meet our set retention criteria in the logistic regression analysis and hence was dropped in the final prediction model. The possible importance of the fornix in remission was also reflected in the cross-validation analysis. As expected, the direction of the predictive relationship of the fornix was the same as that for the stria terminalis tract, given the role of both these connections with the amygdala.
Treatment studies targeting the subgenual ACC region using deep brain stimulation and repetitive transcranial magnetic stimulation demonstrate considerable efficacy in the treatment of major depression. Reference Mayberg, Lozano, Voon, McNeely, Seminowicz and Hamani33,Reference Mottaghy, Keller, Gangitano, Ly, Thall and Parker34 The robust involvement of the CgC in our study, the literature support for a central involvement of the subgenual ACC and the treatment data targeting this region all provide converging evidence that the projections of the ACC hold the key to effective treatment prediction in MDD. A limitation of our current data is that the anterior projections of the subgenual ACC are not adequately isolated using TBSS. Accordingly, we are currently focusing on using tractography and network-based approaches to better describe the abnormal ACC connectivity that provides the signal for the predictive power seen in our study.
Limitations
Despite the robustness of the predictive results, there are also limitations to be considered. Although the sample size of this study is larger than those of most previous studies, replication in an independent cohort and also at a different imaging site is crucial to establish generalisability. Our cross-validation analysis presented here establishes that the stria terminalis-CgC fractional anisotropy biomarker is a stable predictor of remission within our sample. The tracts we targeted in this study could be further characterised at higher anatomical resolution using DTI tractography analysis. TBSS may have some limitations that relate to a failure to characterise all the voxels that are specific to a tract, and may also dilute the number of streamlines or fibres for that particular tract. However, DTI tractography is a user-intensive method, and therefore in view of our intended application in a clinical setting, TBSS was selected as our preferred method of analysis because of its robust nature, ease of automation and speed of calculation - major practical advantages for routine use. Also, we used fractional anisotropy instead of number of streamlines in our analysis. The sensitivity of the TBSS approach to examine white matter integrity using scalar measures such as fractional anisotropy has also been well established across a number of psychiatric and neurological conditions. We consider these factors to be important to aid in the future clinical application of these findings.
Implications
The results of this study have translational implications. They point to the promise of DTI from structural imaging for the development of a decision-support tool for guiding prospective treatment choices based on the patient’s individual neurobiology. This interesting proposition, however, requires further studies to compare sensitivity across different types of antidepressants and alternative treatment options. Our MDD cohort was equally randomised to three commonly prescribed antidepressants in current clinical settings; however, low number of participants in treatment outcome groups for each individual treatment arm precluded us from evaluating whether DTI prediction is specific for one type of medication over another. The future availability of the whole iSPOT-D imaging cohort should provide sufficient power to test this and is a planned analysis of the trial. Reference Grieve, Korgaonkar, Etkin, Harris, Koslow and Wisniewski26 Nevertheless, the results of the present study provide the first evidence that DTI-assessed white matter connectivity of the cingulate-limbic regions predicts a general response to antidepressants. We anticipate that augmentation of this biomarker with additional data (both imaging and non-imaging) from iSPOT-D will offer further improvements in sensitivity and specificity as the nature of the abnormal predictive neural circuits are more completely captured by additional independent measures.
Acknowledgements
We acknowledge the iSPOT-D Investigators Group, the contributions of principal investigators at each site and the central management team (global coordinator Mrs Claire Day). We acknowledge the iSPOT-D Publication Committee: Dr A John Rush (Chair), Dr Leanne Williams, Dr Evian Gordon, Dr Steve Koslow, Dr Steve Wisniewski and Dr Jayashri Kulkarni for their useful discussions on the draft. We acknowledge the editorial support of Jon Kilner, MS, MA (Pittsburgh, PA, USA) for this manuscript. We would also like to acknowledge the support of Mrs Claire Day and Mrs Angela Robl in the recruitment and data collection of the participants in the study; Dr Lavier Gomes from the Department of Radiology at Westmead Hospital; and the help of Sheryl Foster and the other radiographers at Westmead Hospital, Sydney with the MRI data collection.







eLetters
No eLetters have been published for this article.