LEARNING OBJECTIVES
After reading this article you will be able to:
• demonstrate an understanding of problems caused by high prolactin levels
• discuss options for investigation
• demonstrate an understanding of available hyperprolactinaemia management strategies.
There has been a drive to improve the physical healthcare of those within mental health services. This is of importance following the emergence of data suggesting that people with schizophrenia are 2–3 times more likely to die early from preventable causes (World Health Organization Reference Wang, Wu and Chai2018).
An example of the overlap between psychiatry and endocrinology is hyperprolactinaemia, a common problem for those on antipsychotic medications (Cookson Reference Cookson, Hodgson and Wildgust2012). The National Institute for Health and Care Excellence (NICE) (Reference Milano, Colletti and Capasso2014) suggests that, before deciding on and commencing a specific antipsychotic, the patient should be given information on the benefits and side-effects, including the risk of hyperprolactinaemia. To best inform those due to commence antipsychotics, it is therefore important that clinicians keep up to date with this topic.
This review offers updated knowledge on prolactin and hyperprolactinaemia and considers investigative strategies. Management options are identified, with a focus on antipsychotic-induced hyperprolactinaemia and prolactinoma.
Prolactin
Prolactin is a polypeptide hormone produced primarily in the anterior pituitary gland. It has been further identified as having a role in the central nervous system (CNS) and immune system (Milano Reference McCabe and Priebe2017). Secretion of prolactin is primarily controlled through a negative feedback loop, via hypothalamic dopamine. The dopamine receptors involved in this loop belong to the D2 class and are located on the membranes of the lactotrophic (prolactin-producing) cells of the anterior pituitary gland. Three major forms of prolactin can be found, namely ‘free prolactin’, ‘big prolactin’ and ‘big big prolactin’ (or macroprolactin), with the last two forms being inactive (Saleem Reference Rand, Kink and Sator2018).
The functions of prolactin are divided into five categories: lactation, reproduction, osmoregulation, metabolism and regulation of the immune system (Riecher-Rössler Reference Pérez-Iglesias, Mata and Martinez-Garcia2013; Milano Reference McCabe and Priebe2017).
Hyperprolactinaemia
The prevalence of hyperprolactinaemia in the general adult population is approximately 0.4% (Majumdar Reference Kyritsi, Dimitriadis and Angelousi2013). Although definitions of hyperprolactinaemia and preferred units of measurement differ across literature and laboratories, for the purposes of this review, all levels have been reported in SI units (μg/L ). A prolactin level can be considered to be raised if it is above 20 μg/L in men and above 25 μg/L in women (Milano Reference McCabe and Priebe2017). Symptoms of hyperprolactinaemia may be present at even moderately raised levels, but levels above 70.5 μg/L, and especially levels over 141 μg/L, are likely to result in symptoms becoming evident. At levels of over 100 μg/L, the specialist involvement of an endocrinologist should be considered (Cookson Reference Cookson, Hodgson and Wildgust2012; Grigg Reference Grigg, Worsley and Thew2017).
Hyperprolactinaemia subtypes
Hyperprolactinaemia can be divided into three subtypes, related to the underlying cause: functional or physiological; factitious or analytical; and pathological (Box 1).
BOX 1 Hyperprolactinaemia subtypes and common causes
Functional/physiological hyperprolactinaemia
• Pregnancy
• Lactation
Factitious/analytical hyperprolactinaemia
• Macroprolactin
Pathological hyperprolactinaemia
• Idiopathic effects
• Pituitary stalk damage
• Hypersecretion
• Systemic disorder
• Medication side-effect
Functional or physiological hyperprolactinaemia (with prolactin levels usually in the range of approximately 18.8–28.2 μg/L) primarily occurs as a result of prolactin's physiological functions related to pregnancy and lactation, hence prolactin may be above normal levels and not be a sign of any underlying pathology (Majumdar Reference Kyritsi, Dimitriadis and Angelousi2013; Saleem Reference Rand, Kink and Sator2018).
Factitious or analytical causes of hyperprolactinaemia are secondary to macroprolactinaemia (Majumdar Reference Kyritsi, Dimitriadis and Angelousi2013; Saleem Reference Rand, Kink and Sator2018). In macroprolactinaemia, high levels of biologically inactive macroprolactin molecules can be detected in immunoassay testing. These macromolecules are inactive owing to their inability to bind to receptors (Milano Reference McCabe and Priebe2017).
Pathological causes of hyperprolactinaemia can further be divided into five subtypes: idiopathic; secondary to hypothalamic pituitary stalk damage; pituitary hypersecretion; as a by product of systemic disorders (such as chronic renal failure, hypothyroidism, liver cirrhosis, epilepsy); or drug/medication induced (Majumdar Reference Kyritsi, Dimitriadis and Angelousi2013; Saleem Reference Rand, Kink and Sator2018). It can occur through various mechanisms. For example, in hypothyroidism hyperprolactinaemia is due to an increase in thyrotropin-releasing hormone (TRH), which increases both thyroid-stimulating hormone (TSH) and prolactin (Bahar Reference Bahar, Akha and Vesgari2011). Comparatively, in liver disease there is alteration in the type of amino acids entering the nervous system, inhibiting dopamine release (dopamine helps regulate prolactin) (Jha Reference Jha and Kannan2016).
Most importantly in the context of psychiatry, hyperprolactinaemia can be drug/medication induced. Hyperprolactinaemia has long been known as a possible side-effect related to the use of antipsychotics and this is one of the most commonly reported adverse effects in clinical trials (Bushe Reference Bushe, Shaw and Peveler2008; Milano Reference McCabe and Priebe2017).
As prolactin release is mediated via hypothalamic dopamine, antagonism due to antipsychotics results in the interruption of negative feedback pathways, which leads to hyperprolactinaemia (Saleem Reference Rand, Kink and Sator2018). Individuals with psychosis who are being treated with long-term antipsychotic medication are therefore at an increased risk of the long-term consequences of hyperprolactinaemia.
Prolactinoma
Another important cause of hyperprolactinaemia that must be considered are prolactin-secreting tumours called prolactinomas, which account for 40–66% of functional pituitary tumours and represent the most common organic cause of hyperprolactinaemia (Saleem Reference Rand, Kink and Sator2018; Olarescu 2019). Majumdar & Mangal (Reference Kyritsi, Dimitriadis and Angelousi2013) report that a prolactinoma is likely if the prolactin level is 250 μg/L and note that some medications, such as risperidone, can cause levels over 200 μg/L.
Prolactinomas can be broken down into adenomas and carcinomas. Adenomas can be subclassified by size as microadenomas (<10 mm), macroadenomas (≥10 mm) and giant prolactinomas (>40 mm). Carcinomas are defined by the presence and location of metastatic processes. Carcinomas are very rare among prolactinomas (approximately 0.2% of operated adenohypophyseal tumours). However, up to 15% of pituitary tumours may be defined as clinically aggressive because of rapid growth, invasive properties or continued growth despite treatment (Olarescu 2019).
In the case of a prolactinoma, symptoms may include features related to the compressive effect of the tumour. These could include headache, visual disturbances and hypopituitarism (most commonly with deficiencies in follicle-stimulating hormone (FSH) and luteinising hormones – with symptoms including fertility problems and deficiencies of oestrogen and testosterone) (Olarescu 2019). If the prolactinoma extends into surrounding cerebral structures there may be atypical symptoms such as reversible dementia, disorientation, olfactory hallucinations, temporal lobe epilepsy, hemiparesis and cranial nerve palsies (Shimon Reference Sethi, Chanukya and Nagesh2019).
Hyperprolactinaemia symptoms
Clinical symptoms are related to prolactin's physiological functions and are most commonly related to either hypogonadism or lactation. Women often present earlier, with abnormal menstruation (oligomenorrhoea or amenorrhoea), galactorrhoea, sexual dysfunction and infertility. Post-menopausal women may notice only a decreased frequency of hot flushes and therefore may not report symptoms. Men may present with reduced sexual function and loss of libido, as well as infertility and gynaecomastia (Haddad Reference Haddad and Wieck2004; Milano Reference McCabe and Priebe2017; Saleem Reference Rand, Kink and Sator2018). Early symptoms are summarised in Fig. 1.
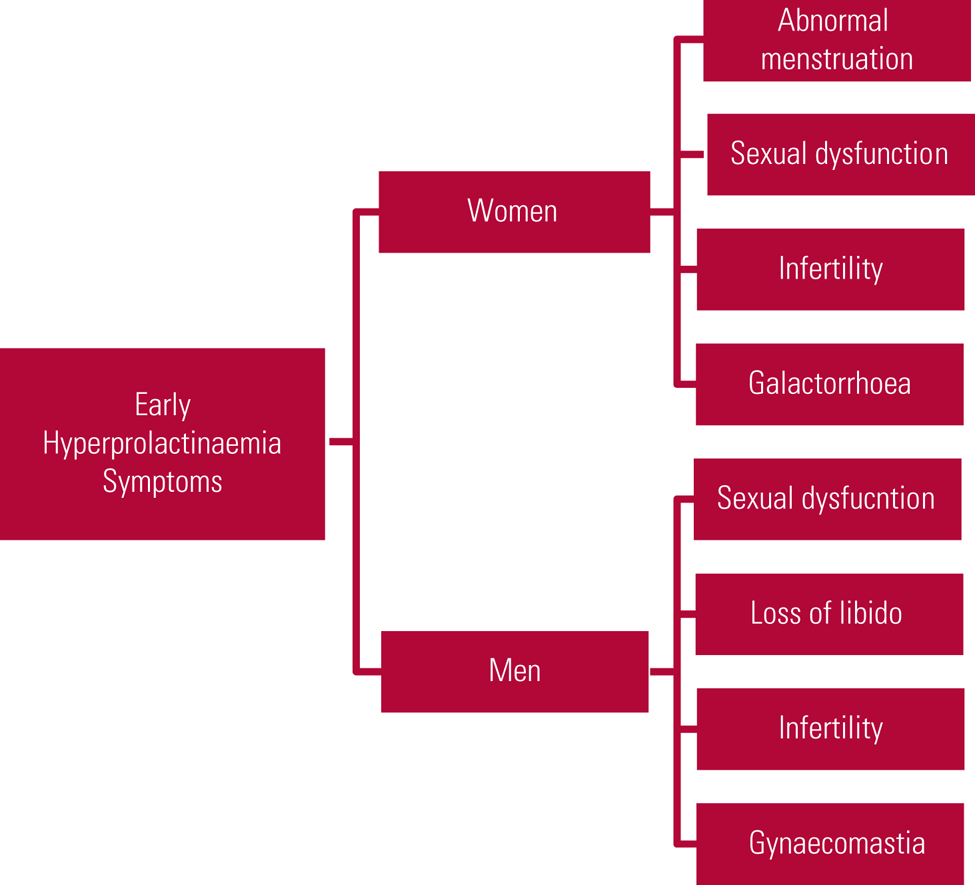
FIG 1 Early symptoms of hyperprolactinaemia (Haddad Reference Haddad and Wieck2004; Milano Reference McCabe and Priebe2017; Saleem Reference Rand, Kink and Sator2018).
Longer-term consequences of hyperprolactinaemia include altered bone mineral density and are summarised in Fig. 2. Importantly, an association has been identified between hip fracture and schizophrenia (OR = 1.73, 95% CI 1.32–2.28) as well as prolactin-raising antipsychotics (OR = 2.6, 95% CI 2.43–2.87). This study considered more than ten antipsychotics, including haloperidol, zuclopenthixol and sulpiride, and included both oral and depot preparations (Howard Reference Howard, Kirkwood and Leese2007).

FIG 2 Long-term consequences of hyperprolactinaemia (Haddad Reference Haddad and Wieck2004; Howard Reference Howard, Kirkwood and Leese2007; Majumdar Reference Kyritsi, Dimitriadis and Angelousi2013; González-Blanc Reference González-Blanco, Greenhalgh and Garcia-Rizo2016; Saleem Reference Rand, Kink and Sator2018).
Osteopenia resulting from hypoestrogenaemia occurs more frequently in women, and it can result in decreased spinal bone mineral density. Other potential long-term effects of hyperprolactinaemia in women include features of hyperandrogenism such as acne, increased facial and body hair growth and abnormal libido; these symptoms are due in part to increased endogenous steroid hormones, dehydroepiandrosterone and free testosterone (Majumdar Reference Kyritsi, Dimitriadis and Angelousi2013; González-Blanco Reference González-Blanco, Greenhalgh and Garcia-Rizo2016; Saleem Reference Rand, Kink and Sator2018).
In men with chronic hyperprolactinaemia, long-term consequences again include osteopenia, alongside lower energy levels and reduced muscle mass. Osteopenia leads to an increased risk of pathological fractures due to altered bone structure. Furthermore, progressive atherosclerosis occurs in both genders, related to decreased oestrogen as a result of hypogonadism secondary to hyperprolactinaemia (Haddad Reference Haddad and Wieck2004; Majumdar Reference Kyritsi, Dimitriadis and Angelousi2013; González-Blanco Reference González-Blanco, Greenhalgh and Garcia-Rizo2016; Saleem Reference Rand, Kink and Sator2018).
Malignancy
Raised prolactin has been implicated in the pathogenesis of upper gastrointestinal and haematopoietic cancers (Sethi Reference Saleem, Martin and Coates2012). In addition to this, prolactin receptor pathways have been associated with the development of resistance to chemotherapy (Sethi Reference Saleem, Martin and Coates2012). In both cases there is insufficient evidence at this time to suggest causation.
The link with breast cancer has been more substantiated, with Johnston et al (Reference Johnston, Bu and Hein2018) demonstrating in animal models that hyperprolactinaemia-inducing antipsychotics, in this case risperidone and pimozide, caused pre-existing premalignant cells to progress to breast cancer by supressing apoptosis. However, this was not supported by a cohort study and meta-analysis by Dekkers et al (Reference Dekkers, Ehrenstein and Bengtsen2015), who concluded that there was no increased risk of breast cancer in people (men and women) with hyperprolactinaemia (RR = 1.04, 95% CI 0.75–1.43). A subsequent meta-analysis by Wang et al (Reference Trimble2016) that included seven case–control and cohort studies of the association between prolactin and breast cancer in women found a positive association (RR = 1.16, 95% CI 1.04–1.29).
Most recently, a meta-analysis by Ni et al (Reference Miyamoto, Galecki and Francois2019) attempted to quantify malignancy and mortality risks of site-specific cancer in people with schizophrenia. They found an increased risk of mortality in breast cancer (RR = 1.97, 95% CI 1.38–2.83), with lung and colon cancer also showing significantly increased risk (RR = 1.93, 95% CI 1.46–2.54 and RR = 1.69, 95% CI 1.60–1.80 respectively). They agreed that hyperprolactinaemia could be linked to increased breast cancer risk but felt that overall associations between cancer incidence and schizophrenia were unclear owing to many potential confounding factors, including gender, genetic background and antipsychotic medication use. They noted that further studies are necessary to identify a more robust association.
Investigations
What to monitor and when
Both the Maudsley Prescribing Guidelines (Taylor Reference Shim, Shin and Kelly2018) and the NICE Clinical Guideline (National Institute for Health and Care Excellence Reference Milano, Colletti and Capasso2014) suggest that baseline prolactin levels should be measured routinely if a patient is due to commence antipsychotic therapy. However, other reviewers go further, suggesting that a sexual and menstrual history should be completed at the time of antipsychotic initiation to help clinicians identify symptom changes (Grigg Reference Grigg, Worsley and Thew2017).
The Maudsley guidelines suggest that reassessment should then take place following 3 months of antipsychotic treatment, when a further prolactin level should be obtained if there are suggestive symptoms or the medication is known to be prolactin-elevating (Taylor Reference Shim, Shin and Kelly2018). If antipsychotic medication is altered with the goal of reducing prolactin, levels should be remeasured after 3 days to allow for response (Melmed Reference McCabe and Healey2011).
In their treatment algorithms, Grigg et al (Reference Grigg, Worsley and Thew2017) provide recommendations for later monitoring of prolactin levels. They advise that prolactin should be rechecked annually if the level is between 16.5 and 50 μg/L, every 6 months if over 50 μg/L and 3 months after any antipsychotic medication change.
Further indications for testing prolactin levels include clinical symptoms of hyperprolactinaemia such as galactorrhoea, amenorrhoea and sexual dysfunction (Cookson Reference Cookson, Hodgson and Wildgust2012). Discussing symptoms of high prolactin with individuals currently exhibiting symptoms of psychosis is challenging in itself. McCabe & Priebe (Reference Majumdar and Mangal2008) considered the difficulties of communication and building an effective therapeutic relationship in these cases and reflected that this is a difficult area to research effectively, with little in the way of established guidance or training. More recently, McCabe & Healey (Reference Lieberman, Stroup and McEvoy2018) compared two studies looking to use the conversation analysis ‘repair’ framework to reduce potential miscommunication with patients with psychosis. Although work such as this is in its early stages, with development needed to support its clinical utility, the key message is that clinicians should continue to work to understand the person's perspective and build effective therapeutic relationships.
Historically some evidence has supported checking prolactin levels following seizures to assist in differentiating epileptic activity from other seizure causes (Trimble Reference Taylor, Barnes and Young1978). This was reinforced more recently by Ahmad et al (Reference Ahmad, Ijaz and Tauseef2019) in a 2-year cross-sectional study involving 90 participants, which found that prolactin levels were higher in those with tonic–clonic epileptic seizures than those with non-epileptic seizures. However, the evidence is not always consistent: in a 4-year retrospective study of 218 participants Abubakr & Wambacq (Reference Abubakr and Wambacq2016) reported that prolactin did not provide additional support when differentiating between epileptic and psychogenic non-epileptic seizures and concluded that it should not be routinely tested. A summary of when prolactin should be tested is contained in Box 2.
BOX 2 When to check prolactin levels in patients prescribed antipsychotics
• Before commencing antipsychotics
• 3 months after commencing antipsychotics and after medication changes
• If there are symptoms suggestive of hyperprolactinaemia
• 3 days after a change in antipsychotic medication aimed at prolactin reduction
• During regular monitoring: once a year if level is <50 μg/L, or every 6 months if level is higher
Checking prolactin
The Endocrine Society recommends that, if hyperprolactinaemia is suspected, a single blood test for serum prolactin is needed. If this is above the upper level of normal a diagnosis of hyperprolactinaemia can be made. These guidelines suggest that the sample should be obtained without significant venepuncture stress (Melmed Reference McCabe and Healey2011). However, it should be noted that other authors found no significant effect of stress on prolactin levels (Ferriani Reference Ferriani and Silva de Sá1985), and others suggest testing serum levels twice before confirming the diagnosis (De Hert Reference De Hert, Detraux and Peuskens2014). If the diagnosis is in doubt, a repeat test may therefore be appropriate.
For those with asymptomatic hyperprolactinaemia, Melmed et al (Reference McCabe and Healey2011) suggest assessment of macroprolactin. Macroprolactin has a lower biological effect, so fewer investigation and management changes are needed as it is unlikely to cause symptoms. Hyperprolactinaemia caused by raised macroprolactin can be detected by polyethylene glycol (PEG) precipitation, which is the most common method for assessing for macroprolactin levels in clinical laboratories (Chen Reference Chen, Wang and Yang2016). Some laboratories automatically test macroprolactin levels following a high prolactin result.
Other investigations
Further investigations are then dependent on the suspected cause. They involve close focus on the broader history and assessment for temporal links or disease-specific symptoms (De Hert Reference De Hert, Detraux and Peuskens2014). This is likely to include blood tests for alternative causes, such as renal or thyroid dysfunction, and, in some cases, ordering magnetic resonance imaging (MRI). The Endocrine Society advises a head MRI to assess for prolactinoma if it is not practicable to alter the antipsychotics or if the high prolactin level does not appear to be linked to the medication. The aim is to further investigate whether there is a pituitary or hypothalamic mass (Melmed Reference McCabe and Healey2011). Similarly, De Hert et al (Reference De Hert, Detraux and Peuskens2014) in their comprehensive review suggest an MRI of the pituitary when hyperprolactinaemia onset does not coincide with antipsychotic use.
Symptoms that may suggest that high prolactin is caused not by antipsychotics but by prolactinoma are those consistent with a space-occupying lesion, for example headache or visual field defects, the presence of which would warrant an MRI head scan (Miyamoto Reference Melmed, Casanueva and Hoffman2015).
Setting a prolactin level at which an MRI should be conducted is challenging. Grigg et al (Reference Grigg, Worsley and Thew2017) collated practice guidelines and concluded that computed tomography (CT) or MRI should be completed when prolactin levels reach 150 μg/L. However, Rand et al (Reference Olarescu, Perez-Rivas and Gatto1996), Kyritsi et al (2018) and Miyamoto et al (Reference Melmed, Casanueva and Hoffman2015) suggest that lower levels – between 85.2 and 100 μg/L – require testing and provide evidence that the probability of adenoma increases as the prolactin level rises. Given that the 85.2 μg/L cut-off was suggested by work in a specific non-psychiatric population, we suggest that prolactin levels of over 100 μg/L warrant an MRI, particularly in the absence of other physiological explanations.
A summary of indications for MRI is contained in Box 3.
BOX 3 Indications for magnetic resonance imaging (MRI) of the head in hyperprolactinaemia
• High prolactin level does not appear to be linked to medication
• If it is not practicable to alter antipsychotics to assess prolactin level change (in which case, MRI should be done to rule out other causes)
• There are symptoms suggestive of a prolactinoma, e.g. visual disturbance and headache
• To monitor prolactinoma treatment
• Prolactin level is 100 μg/L or higher
Management of antipsychotic-related hyperprolactinaemia
Link between antipsychotics and prolactin
Melmed et al (Reference McCabe and Healey2011) explain that the most frequent cause of hyperprolactinaemia in the absence of a tumour is medication, further identifying antipsychotics as the most common culprit. Alongside this they report anticonvulsants, antihistamines and opiates among other causative agents.
The Maudsley Prescribing Guidelines suggest those at higher risk of significant adverse effects of hyperprolactinaemia to be people under 25 years of age (as this is before peak bone mass is achieved), those with osteoporosis and people with a history of hormone-dependent breast cancer (Taylor Reference Shim, Shin and Kelly2018). Serum prolactin levels associated with antipsychotics are typically 25–100 μg/L, although some can produce levels in excess of 200 μg/L (Grigg Reference Grigg, Worsley and Thew2017).
Antipsychotics particularly associated with hyperprolactinaemia include amisulpride, risperidone, paliperidone, sulpiride, haloperidol and chlorpromazine (Bushe Reference Bushe, Shaw and Peveler2008; Melmed Reference McCabe and Healey2011; Cookson Reference Cookson, Hodgson and Wildgust2012; Taylor Reference Shim, Shin and Kelly2018; Samperi Reference Riecher-Rössler, Rybakowski and Pflueger2019). The comparative risk of hyperprolactinaemia for various antipsychotics is shown in Table 1.
TABLE 1 Comparative risk of hyperprolactinaemia for various antipsychotics

Source: Samperi et al, Reference Riecher-Rössler, Rybakowski and Pflueger2019.
When considering the effect of these medications it is worth noting evidence that some antipsychotic-naive people have raised prolactin at baseline (Pérez-Iglesias Reference Ni, Wu and Long2012; González-Blanco Reference González-Blanco, Greenhalgh and Garcia-Rizo2016). This finding is supported by a meta-analysis by González-Blanco et al (Reference González-Blanco, Greenhalgh and Garcia-Rizo2016), who found significantly raised prolactin levels in both male and female antipsychotic-naive patients with schizophrenia and related disorders. This was considered to be related to stress response, and Reference González-Blanco, Greenhalgh and Garcia-RizoGonzález-Blanco et al further linked raised prolactin to biochemical alterations, including TRH, ghrelin and interleukins.
Rates of hyperprolactinaemia vary, with reports of 40–90% for typical antipsychotics (Melmed Reference McCabe and Healey2011), 40–93% for any antipsychotic (Shim Reference Samperi, Lithgow and Karavitaki2007; Cookson Reference Cookson, Hodgson and Wildgust2012) and 50–100% for risperidone specifically (Melmed Reference McCabe and Healey2011; Pérez-Iglesias Reference Ni, Wu and Long2012). There are a variety of reasons underlying the varied effect of antipsychotics on prolactin levels, as summarised in Fig. 3. It is therefore not surprising that there are differences in effect size noted across the literature.
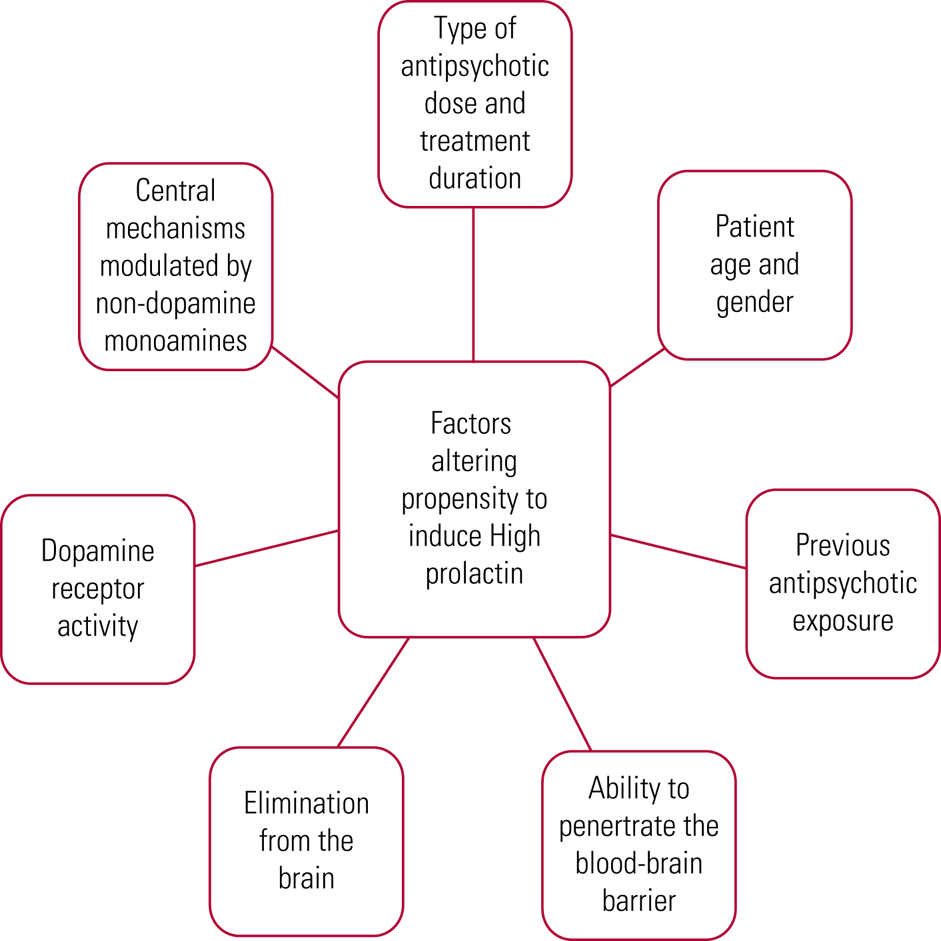
FIG 3 Differences in antipsychotic effect on prolactin (Cookson Reference Cookson, Hodgson and Wildgust2012; Pérez-Iglesias Reference Ni, Wu and Long2012).
Discontinuation risks
The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study involved 1493 participants with schizophrenia. In phase 1, participants were randomly assigned to receive olanzapine, perphenazine, quetiapine, risperidone or ziprasidone. Overall, 74% discontinued their medication within 18 months owing to inefficacy or intolerable side-effects. Interestingly, despite risperidone being the only drug in this trial associated with a substantial increase in prolactin it had the lowest rate of discontinuation because of intolerable side-effects (10%, compared with the highest rate of 18% with olanzapine) (Lieberman 2005).
Prolactin-sparing antipsychotics similarly differ in effect. For example, Crespo-Facorro et al (Reference Crespo-Facorro, Ortiz-Garcia de la Foz and Suarez-Pinilla2017) demonstrated that aripiprazole had a lower risk of prolactin excess (14.3% showed mild excess, 5.4% marked) compared with two other prolactin-sparing antipsychotics: quetiapine (36.1% mild, 5.6% marked) and ziprasidone (18.4% mild, 8.2% marked) after 1 year of treatment. Over the year, participants on aripiprazole showed an overall decrease in prolactin levels, whereas a progressive increase was seen in those on quetiapine and ziprasidone.
Adaptation
Eberhard et al (Reference Eberhard, Lindström and Holstad2007) focused on risperidone therapy in a longitudinal study over 5 years, finding that the increase in prolactin was linked to the metabolite 9-OH risperidone and that this prolactin effect was more pronounced in women. However, in those who took risperidone monotherapy over the 5 years a linear reduction in prolactin was noted later on, suggesting this adaptation over time. Corroborating this was work by Pérez-Iglesias et al (Reference Ni, Wu and Long2012), who found a tendency for prolactin levels to decrease at 1 year for those on risperidone. However, they noted that 71% of values remained above the normal range.
Confounders
When considering medication-induced hyperprolactinaemia, it is important to remember that medications other than antipsychotics can be a potential cause. Other psychotropics, including tricyclic antidepressants and monoamine oxidase inhibitors, as well as a number of physical health medications, such as anti-emetics, opiates and antihypertensives, potentially cause or worsen hyperprolactinaemia. Some key examples are listed in Box 4.
BOX 4 Common medications that can cause hyperprolactinaemia
• Antipsychotics: amisulpride, risperidone, paliperidone, sulpiride, haloperidol and chlorpromazine
• Tricyclic antidepressants: amitriptyline, clomipramine
• Monoamine oxidase inhibitors
• Anti-emetics: metoclopramide
• Opiates: morphine
• Antihypertensives: methyldopa, verapamil
Treatment of antipsychotic-induced hyperprolactinaemia
Once high prolactin has been established, alternatives have been ruled out and symptoms assessed, if the antipsychotic is identified as the cause, treatment must be reviewed (Grigg Reference Grigg, Worsley and Thew2017). Options given in the Maudsley Prescribing Guidelines (Taylor Reference Shim, Shin and Kelly2018) and the Endocrine Society's clinical practice guideline (Melmed Reference McCabe and Healey2011) include: continuing the antipsychotic and monitoring; stopping/reducing the antipsychotic; switching to a prolactin-sparing antipsychotic; or considering adjunctive therapy (Fig. 4). Prolactin-sparing antipsychotics include aripiprazole, asenapine, quetiapine and clozapine (Haddad Reference Haddad and Wieck2004; Taylor Reference Shim, Shin and Kelly2018).
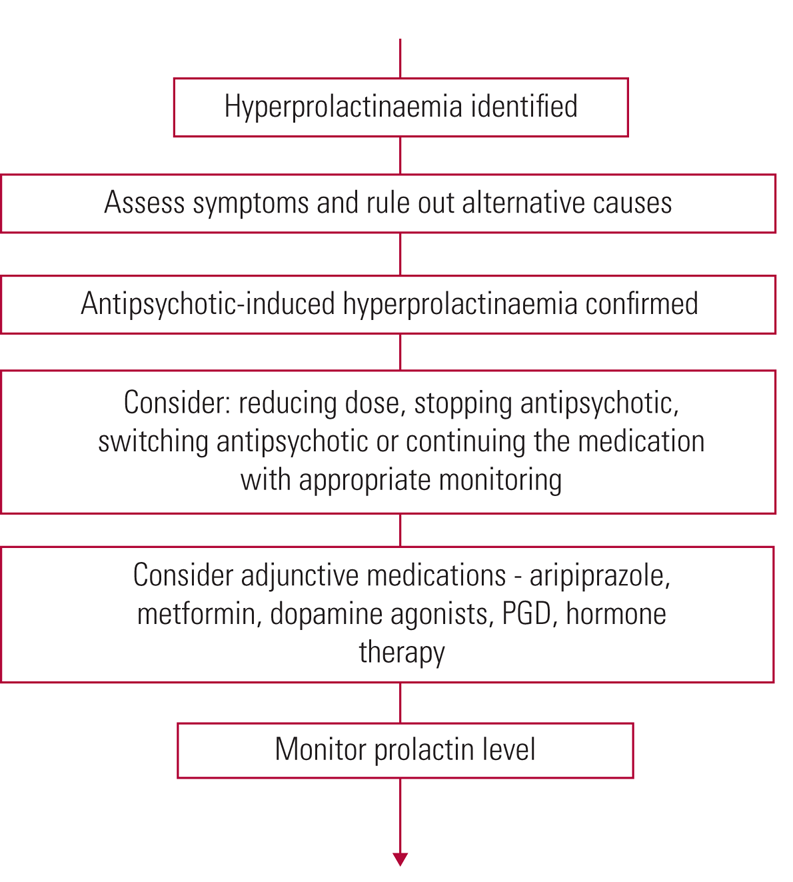
FIG 4 Summary of treatment of antipsychotic-induced hyperprolactinaemia (Haddad Reference Haddad and Wieck2004; Melmed Reference McCabe and Healey2011; Cookson Reference Cookson, Hodgson and Wildgust2012; Grigg Reference Grigg, Worsley and Thew2017; Taylor Reference Shim, Shin and Kelly2018). PGD, peony-glycyrrhiza decoction.
When considering these options, it is important to balance carefully the benefits of antipsychotic medication against the risk of relapse if altered, as well as the distress reported regarding side-effects (De Hert Reference De Hert, Detraux and Peuskens2014; Grigg Reference Grigg, Worsley and Thew2017). Prolactin-sparing antipsychotics will have their own side-effect profiles and so it may instead be preferable to decrease the current antipsychotic rather than attempting a switch (De Hert Reference De Hert, Detraux and Peuskens2014).
If the patient is asymptomatic, the Maudsley guidelines suggest that a joint decision should be taken as to whether to treat the high prolactin or continue the current antipsychotic regimen with monitoring. Strategies such as stopping smoking and ensuring adequate calcium and vitamin D intake can be used to help with the risk of altered bone mineral density if this is a concern (Taylor Reference Shim, Shin and Kelly2018).
Switching antipsychotics
Given the evidence showing a decrease in prolactin with aripiprazole it is logical to switch to this medication when prolactin presents a problem. However, Grigg et al (Reference Grigg, Worsley and Thew2017) advise that switching those already stabilised on antipsychotics should be done with extreme caution and only if symptoms of hyperprolactinaemia are present and other interventions are inappropriate or unsuccessful.
Byerly et al (Reference Byerly, Marcus and Tran2009) studied switching to aripiprazole from risperidone or olanzapine. They investigated three methods: aripiprazole initiation with immediate olanzapine/risperidone discontinuation; initiating aripiprazole while tapering off olanzapine/risperidone; and titrating up aripiprazole while tapering off olanzapine/risperidone. When switching either olanzapine or risperidone to aripiprazole, prolactin significantly decreased in all groups at 1 week and no switching strategy was evidently superior.
Adjunctive aripiprazole
If switching the antipsychotic to a prolactin-sparing form is not appropriate or is not successful, adjunctive aripiprazole can be considered, since adjunctive aripiprazole has been shown to be successful in reducing prolactin to normal levels (Cookson Reference Cookson, Hodgson and Wildgust2012; Taylor Reference Shim, Shin and Kelly2018).
Shim et al (Reference Samperi, Lithgow and Karavitaki2007) demonstrated that when aripiprazole was added to haloperidol, prolactin was reduced without significant effects on psychopathology, although it is worth noting that the study was conducted in a stable clinical group. Prolactin levels decreased by 76.5% at 4 weeks and 84.2% at 8 weeks of adjunctive aripiprazole. Furthermore, 84.6% of participants had normal prolactin ranges at 8 weeks of aripiprazole treatment, compared with only 3.6% in the placebo group (Shim Reference Samperi, Lithgow and Karavitaki2007). Interestingly, Hoffer et al (Reference Hoffer, Roth and Mathews2009) concluded in their review that aripiprazole can lower prolactin in those with both iatrogenic and tumorigenic hyperprolactinaemia.
It is important to consider the effect of adding aripiprazole on the initial antipsychotic. It is possible that it could displace other antipsychotics from the dopamine D2 receptor, altering their effect (Hoffer Reference Hoffer, Roth and Mathews2009; Grigg Reference Grigg, Worsley and Thew2017). Furthermore, aripiprazole itself is still linked with symptoms of sexual dysfunction, despite having the most favourable prolactin profile, and there are concerns about potential adverse effects of antipsychotic polytherapy (De Hert Reference De Hert, Detraux and Peuskens2014; Grigg Reference Grigg, Worsley and Thew2017). Comparatively, of the second-generation antipsychotics the one thought to have the lowest impact on sexual function is quetiapine (De Hert Reference De Hert, Detraux and Peuskens2014).
Alternative adjuncts
If aripiprazole is not tolerated, alternative adjuncts could be discussed. The Endocrine Society explains that cautious administration of a dopamine agonist can be considered. In the case of antipsychotic use, adding a dopamine agonist may help with high prolactin effects by normalising levels (in around 75% of individuals) but this risks worsening any existing psychotic symptoms and precipitating other side-effects (Melmed Reference McCabe and Healey2011). These are further considered below. Grigg et al (Reference Grigg, Worsley and Thew2017) reviewed multiple global guidelines and similarly express a concern that dopamine agonists could worsen psychotic symptoms, and currently do not endorse this approach.
Other options reported in the Maudsley guidance include adjunctive metformin or peony-glycyrrhiza decoction (PGD) (Taylor Reference Shim, Shin and Kelly2018). PGD, a herbal remedy, has been compared with dopamine agonists and was found to have a similar effect on prolactin as bromocriptine but without exacerbating psychosis. Interestingly, a greater proportion of those on PGD showed improvement in prolactin-related side-effects (56% v. 17%) (De Hert Reference De Hert, Detraux and Peuskens2014). Metformin is an emerging possibility, with some evidence suggesting a role in reducing effects such as weight gain, insulin resistance and prolactin levels; however, these emerging treatments need further study (Grigg Reference Grigg, Worsley and Thew2017).
Grigg et al (Reference Grigg, Worsley and Thew2017) further recognise the importance of ensuring hormone balance in antipsychotic-related hyperprolactinaemia and suggest testosterone therapy to protect bone health in men who are persistently hypogonadal. In premenopausal women they suggest the oral contraceptive pill to help regulate menstrual cycles. In postmenopausal women they suggest that there may be a role for selective oestrogen-receptor modulators, again to help protect bone health.
The various adjunct options are summarised in Fig. 5.

FIG 5 Summary of possible adjuncts to address hyperprolactinaemia (Shim Reference Samperi, Lithgow and Karavitaki2007; Melmed Reference McCabe and Healey2011; Cookson Reference Cookson, Hodgson and Wildgust2012; Grigg Reference Grigg, Worsley and Thew2017; Taylor Reference Shim, Shin and Kelly2018).
Management of prolactinoma
Prolactinoma management does not fall naturally within the remit of psychiatrists, but the impacts of treatment on psychiatric patients are of significant importance – thus an awareness of treatments is helpful.
Dopamine agonists are the first-line treatment for prolactinoma, resulting in prolactin normalisation and symptom suppression in 90% of cases, thankfully with low doses required: as much as ten times lower than usually required in the management of Parkinson's disease (Webster Reference Wang, Mullan and Lane1994; Melmed Reference McCabe and Healey2011; Wang Reference Tirosh and Shimon2012). Monitoring includes periodic prolactin measurements, repeated MRI, visual field checks and comorbidity assessment. There is also the possibility of tapering and stopping the dopamine agonists after 2 years, if prolactin levels and MRI scans remain normal (Melmed Reference McCabe and Healey2011).
Other management options include surgical interventions and radiotherapy. However, these have comparatively poorer outcomes, carry more risk and are less cost-effective (Bloomgarden Reference Bloomgarden and Molitch2014). They may be used in specific circumstances, such as when dopamine agonists are contraindicated, there is treatment resistance to less invasive methods or in malignancy (Casanueava Reference Casanueva, Molitch and Schlechte2006; Melmed Reference McCabe and Healey2011; Tirosh Reference Shimon2015). Unfortunately, there are no biomarkers to predict whether a benign prolactinoma will progress to a malignancy. Carcinomas or aggressive pituitary adenomas should be treated at a tertiary centre by a multidisciplinary team, but these are comparatively rare diagnoses. Treatment options include radiation therapy or the chemotherapeutic alkylating agent temozolomide, particularly if conventional medical or surgical treatment has not controlled the tumour (Olarescu 2019).
Effects of prolactinoma treatments relevant to mental health
Although the adverse psychiatric effects of dopamine agonists are infrequent, especially in the low doses recommended for prolactinoma treatment, there are several that can be severe, and an awareness of their impact is crucial (Melmed Reference McCabe and Healey2011; Wang Reference Tirosh and Shimon2012). The key adverse effects relevant to mental health (Box 5) are as follows.
BOX 5 Potential effects of dopamine agonists relevant to mental health
• Exacerbation of psychosis
• Impulse control disorders
• Sleep attacks
• Metabolic effects
• Valvular heart disease
Exacerbation of psychosis
In the British National Formulary (BNF), serious mental health problems – particularly psychosis – are listed as cautions for cabergoline and bromocriptine, the two most commonly used dopamine agonists (Joint Formulary Committee Reference Bushe, Shaw and Peveler2019a, Reference Byerly, Marcus and Tran2019b). Common side-effects of cabergoline include hallucinations and delusions, with psychotic disorder noted as a less common effect (Joint Formulary Committee Reference Bushe, Shaw and Peveler2019a). Contrasting this, bromocriptine also has hallucinations listed as a common side-effect, but psychotic disorder and neuroleptic malignant-like syndrome are listed as rare effects (Joint Formulary Committee Reference Byerly, Marcus and Tran2019b). Importantly, neuroleptic malignant syndrome is linked with withdrawal of bromocriptine rather than commencement (electronic medicines compendium 2018). Contextualising these risks in psychiatric patients is difficult, as many studies into dopamine agonists involve patients being treated for Parkinson's disease rather than a general population.
Impulse control disorders
Evidence suggests that people may exhibit hypersexuality, pathological gambling, compulsive overspending and compulsive eating with dopamine agonist treatment (electronic medicines compendium 2016; Grall-Bronnec Reference Grall-Bronnec, Victorri-Vigneau and Donnio2018; Shimon Reference Sethi, Chanukya and Nagesh2019). Grall-Bronnec et al (Reference Grall-Bronnec, Victorri-Vigneau and Donnio2018) conducted a large systematic review that reported a prevalence of 10–25% for impulse control disorders in people being treated with dopamine agonists for prolactinoma.
From a psychiatric viewpoint it is important to note that mental illness – particularly depression with anxiety and traits of impulsivity and neuroticism – correlates with development of impulse control disorders in those being treated with dopamine agonists for Parkinson's disease or restless legs syndrome. Mixed data were also reported regarding pre-existing addiction disorders and development of impulse control disorders with dopamine agonist therapy (Grall-Bronnec Reference Grall-Bronnec, Victorri-Vigneau and Donnio2018).
Sleep attacks
A potential side-effect of dopamine agonist treatment is the development of sleep attacks – sudden, irresistible urges to sleep – found to occur in around 6.6% of people with Parkinson's disease taking dopamine agonists (Homann Reference Homann, Wenzel and Suppan2002; electronic medicines compendium 2016). These effects occurred at both high and low doses, with different durations of treatment and across the medications in this drug class, making this side-effect difficult to predict.
Metabolic effects
In a non-psychiatric population, Auriemma et al (Reference Auriemma, Granieri and Galdiero2013) concluded that treatment of a prolactinoma with cabergoline significantly reduced the prevalence of metabolic syndrome. This effect is believed to be due to hyperprolactinaemia being linked to glucose intolerance and the development of an impaired metabolic profile. This is positive news, as not only could psychiatrists use cabergoline to relieve troubling symptoms in patients prescribed psychotropics, but they could also potentially reduce metabolic risk factors by responding to raised prolactin appropriately. Conversely, however, it is possible for cabergoline to cause compulsive eating, linking back to impulse control disorders (electronic medicines compendium 2016).
Valvular heart disease
A possible effect of cabergoline is the development of valvular heart disease, particularly regurgitation, with some evidence demonstrating this effect in both Parkinson's and hyperprolactinaemia patient populations (De Vecchis Reference De Vecchis, Esposito and Ariano2013). However, cabergoline is used in much lower doses in treating prolactinomas, and contrasting evidence reported by Melmed et al (Reference McCabe and Healey2011) and Shimon (Reference Sethi, Chanukya and Nagesh2019) did not suggest an association with increased risk of valvular disease in this group. Therefore, the evidence is not yet conclusive in this regard.
Conclusions
Throughout this narrative review we have considered the underlying physiology of prolactin and hyperprolactinaemia and given an overview of investigation and management options. On the basis of this we have devised the key practice points contained in Box 6. Overall, hyperprolactinaemia is a significant risk to both patient well-being and concordance with antipsychotic medication; and it is hoped that the key practice points described will help clinicians to best identify and treat abnormal prolactin levels and take a collaborative approach when making treatment decisions (Grigg Reference Grigg, Worsley and Thew2017).
BOX 6 Key practice points for mental health clinicians
• Baseline prolactin level should be measured on commencement of antipsychotics
• Where possible, choose a prolactin-sparing medication
• Where possible, take a collaborative treatment planning approach and discuss with the patient the potential side-effects of antipsychotics, including risk of hyperprolactinaemia
• Ensure adequate medication and prolactin-level monitoring to reduce physical health risks
• When hyperprolactinaemia is evident, consider physiological, analytical and pathological differentials
• For those receiving dopamine agonist treatment, monitor for psychiatric side-effects
Acknowledgements
We thank the Coventry and Warwickshire Partnership NHS Trust library team for their assistance in obtaining the necessary literature for this review.
Author contributions
K.R. was the lead author on this project. Otherwise, equal contributions to the manuscript content were made by all authors.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bja.2020.36.
MCQs
Select the single best option for each question stem
1 The most common neuropsychiatric effect of the dopamine agonists cabergoline and bromocriptine is:
a delusions
b pathological gambling
c hallucinations
d neuroleptic malignant syndrome
e psychotic disorder.
2 Which of the following is not a pathological cause of hyperprolactinaemia?
a pituitary stalk damage
b pregnancy
c pituitary hypersecretion
d antipsychotic medication
e systemic disorder.
3 The population thought to be at higher risk of significant adverse effects of hyperprolactinaemia is:
a people under 25
b people under 35
c people under 45
d people under 55
e people under 65.
4 An indication for head MRI would be:
a mildly raised asymptomatic hyperprolactinaemia
b if high prolactin does not appear linked with medication
c physiological hyperprolactinaemia in pregnancy
d prescription review for antipsychotic therapy
e prescription review for antidepressant therapy.
5 Of the following medications, the one most likely to raise prolactin levels is:
a aripiprazole
b quetiapine
c asenapine
d risperidone
e clozapine.
MCQ answers
1 c 2 b 3 a 4 b 5 d










eLetters
No eLetters have been published for this article.