The emergence of the novel influenza A(H1N1) virus in the Americas during March/April 2009 led to the first influenza pandemic of this century, and the first the world has experienced for over 40 years [Reference Jennings1].
The rapid spread of pandemic influenza infection across the world illustrated the need for robust surveillance systems to track the activity of the virus across countries, nations and continents. These surveillance systems facilitate early warning of community outbreaks, which enables the prompt recognition of sustained community activity thus guiding subsequent public health decisions at both national and local levels.
We describe the spread of the first wave of the influenza A(H1N1) pandemic across England using the Health Protection Agency (HPA)/QSurveillance national surveillance system. We also discuss the development of the surveillance system, its added value, and use thus far for the 2009 pandemic and for surveillance of other health protection incidents.
The first cases of pandemic (H1N1) 2009 infection in the UK were reported on 27 April 2009. During the first wave of pandemic (H1N1) 2009 in England it is estimated that between 144 000 and 670 000 people (mid-estimate of 320 000) fell ill with influenza [2]. The initial response in England involved a containment strategy where each suspected clinical case was investigated microbiologically and treated with antivirals; close contacts of each case were followed up and offered antiviral prophylaxis. Thus, surveillance focused exclusively on virologically confirmed cases. As the number of cases increased a national ‘treatment-only’ (with no prophylaxis for contacts) phase was initiated on 2 July 2009 to manage the pandemic, whereby emphasis of surveillance changed to population-based systems capable of monitoring the cases presenting clinically with symptoms suggestive of influenza infection.
In recent years, syndromic surveillance has become a more timely way of tracking influenza activity, as data are collected in near ‘real-time’ [Reference Cooper3–Reference Josseran6]. In England, the HPA utilize a range of syndromic surveillance systems to monitor and track the activity of seasonal influenza; these systems having been used to monitor the impact of the pandemic since the initial UK cases were detected in April 2009 [Reference Elliot7]. The HPA monitor data from two general practice-based morbidity reporting schemes: the Royal College of General Practitioners Weekly Returns Service (RCGP WRS) and the HPA/QSurveillance national surveillance system [8]. The RCGP WRS has provided continuous weekly reporting of a range of commonly diagnosed problems in England and Wales since 1966 and is considered the gold standard of sentinel GP reporting across Europe [Reference Fleming9].
Since 2004 the HPA, Nottingham University Division of Primary Care and Egton Medical Information Systems Ltd (EMIS) have worked closely together to develop a national health protection surveillance system using general practice-derived data extracted from the QSurveillance® database. This system collects, analyses, interprets and reports on a set of key syndromic indicators used for health protection. General practices using the EMIS general practice computer system are invited to contribute aggregated anonymized consultation data to the QSurveillance database. The data extraction and reporting processes are automated from participating EMIS practices on a daily and weekly basis. The QSurveillance database extracts anonymized summary data which are aggregated by age, sex and condition (i.e. counts of patients who have the conditions of interest in a given time period). No identifiable patient data are extracted and there is no risk to patient confidentiality. Summary data are supplied to the HPA in tabular and graphical form where they are interpreted by the HPA Real-Time Syndromic Surveillance Team (ReSST) and published in the HPA/QSurveillance national surveillance system routine weekly bulletins distributed by the HPA and made available from the HPA Health Protection Report [8]. Low counts are suppressed in any publications. The Team monitor the data for any unexpected increases in consultation rates both nationally and locally [to Primary Care Trust (PCT) level – the lowest designated level of healthcare provision in England with an average population size of 350 000]. The weekly bulletins aim to provide a summary of the recent trends of a range of syndromic indicators with added interpretation putting these findings in context with other relevant evidence [8].
The QSurveillance database collects data from general practices across the UK with good coverage and representation across England. Coverage in Wales and Northern Ireland is much lower and currently no practices are able to report from Scotland. There are about 3400 EMIS practices that contribute to the database with a current UK patient list population in excess of 23 million (about 38% of the UK population).
A series of syndromic indicators, based upon aggregations of clinical diagnoses coded by GPs during the course of their routine consultations (using the Read code system [Reference Benson10]) are monitored routinely in the HPA/QSurveillance national surveillance system. These syndromic indicators were selected to provide health protection practitioners with general practice-derived information on respiratory infections, gastrointestinal infections, common infections and symptoms that could be the result of a deliberate release of a chemical, biological or radiological agent, and on prescribing for antivirals, antibiotics and other treatments. Data for each syndromic indicator are presented in the HPA/QSurveillance national surveillance system weekly bulletin as consultation rates per 100 000 practice population at UK, country, Strategic Health Authority (SHA) (the equivalent of English regions which manage the NHS locally and provide a link between the Department of Health and the NHS) and PCT level [8]. In order to determine the statistical significance of GP consultation data recorded, standardized incidence ratios (SIR) are calculated with upper and lower 95% confidence intervals using indirect standardization with the UK consultation rate as the comparator. These are used to identify areas with high consultation rates which may warrant further investigation [Reference Breslow and Day11].
For monitoring of the pandemic, the clinical indicators influenza-like illness (ILI), upper respiratory tract infections (URTI) and lower respiratory tract infections (LRTI), pneumonia, ILI with antivirals prescribed and pneumonia with antibiotics prescribed were analysed on a daily and weekly basis (from 27 April 2009 to 28 January 2010) and summary, daily bulletins were produced to give a timely overview for the HPA, Department of Health, NHS and the Government.
The HPA/QSurveillance national surveillance system is able to provide data at both SHA and PCT level, from a denominator population large enough to make analysis of these geographical denominations useful. During the weeks following the initial UK cases, there was a small rise in the daily and weekly rate of ILI (Fig. 1 a). This was followed by a large increase in consultation rates which peaked at 225·6/100 000 during week 30 (Fig. 1 a). Data analysis at SHA level demonstrated that the focus of early influenza activity in England was in the West Midlands and London SHAs, coinciding with an unprecedented number of school outbreaks in these regions at this time (Fig. 1 b) [12,Reference Calatayud13].
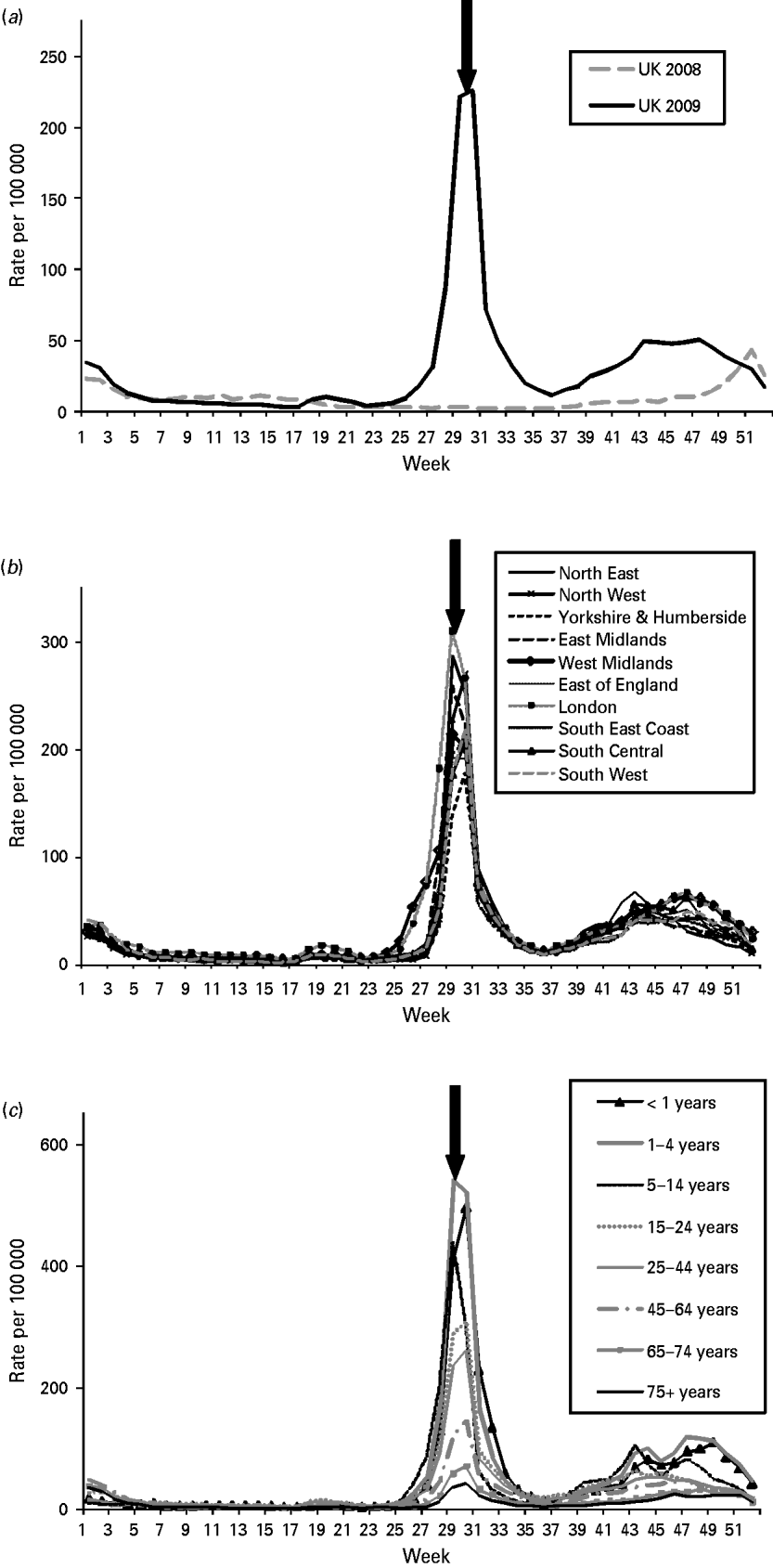
Fig. 1. (a) Influenza-like illness (ILI) per 100 000 UK population. (b) QSurveillance ILI consultation rates per 100 000 population by Strategic Health Authority (SHA). (c) QSurveillance weekly age-specific ILI rates per 100 000 in UK. Arrows indicate the point at which the National Pandemic Flu Service (NPFS) was introduced (during week 30, 2009).
In advance of this pandemic, it was not necessarily anticipated that the fine granularity of data to PCT level would be needed for monitoring the progression of the pandemic. However, in the early phases of the 2009 pandemic in England, there were several areas particularly affected, Birmingham (in the West Midlands) and London and the ability of the HPA/QSurveillance system to monitor disease incidence at PCT level provided the opportunity to identify these areas of particularly high influenza activity and rapid rates of increase (‘hot spots’) to enable local response to change the local management of the pandemic [14].
The clinical incidence data monitored by the HPA/QSurveillance system are routinely analysed by eight age bands: <1, 1–4, 5–14, 15–24, 25–44, 45–64, 65–74, ⩾75 years. The influenza A(H1N1) pandemic 2009 virus predominantly affected younger age groups. School-aged children (5–14 years) and pre-school children (1–4 years) were particularly affected during June and July, although the onset of the school summer holidays in late July seemed to interrupt the transmission in the 5–14 years age group resulting in a fall in incidence (Fig. 1 c) [14]. On 23 July 2009 the National Pandemic Flu Service (NPFS) was launched by the Department of Health to provide a new self-care service to people with pandemic swine flu symptoms providing fast access to information and antivirals. Following the launch of the NPFS, the incidence of ILI in young children aged <1 year recorded by the HPA/QSurveillance national surveillance system became predominant compared to other age groups (Fig. 1 c). Children aged <1 year were not authorized antivirals through the NPFS, and were referred to GP services and therefore the higher ILI consultation rates in young children reflect this policy. Throughout the pandemic, GP incidence rates for ILI in the elderly population (patients aged ⩾65 years) were particularly low, consistent with the available evidence that individuals within this age group had pre-existing immunity through previous exposure to an antigenically related influenza H1N1 virus [Reference Miller15].
Thresholds were generated to describe and contextualize the level of influenza circulating in the community and to help designate when influenza levels reached epidemic proportions. The RCGP WRS has established thresholds for seasonal influenza [Reference Goddard16,17]; however, historical experience has shown that these RCGP thresholds are not suitable for use with QSurveillance ILI data. Although the peaks in ILI in both systems coincide, the rates at the peak of the influenza season normally recorded in the HPA/QSurveillance system are about two thirds of those recorded by the RCGP. Using the RCGP thresholds as a guide, equivalent thresholds for weekly UK level data from the HPA/QSurveillance system were estimated to be about two thirds of those for the RCGP system (baseline influenza activity, less than 20/100 000; normal activity, 21–70/100 000; above average activity, 71–130/100 000; exceptional activity, above 130/100 000). The calculation of these thresholds was based on this observation and supported statistically by using inverse regression analysis of historical data where the exact thresholds calculated were 20·6 (95% CI 18·5–22·8), 65·2 (95% CI 62·5–68·1) and 129·0 (95% CI 124·7–133·7), respectively.
The HPA/QSurveillance thresholds may be used as a guide when interpreting weekly QSurveillance data at SHA or PCT level, but should be interpreted with caution as the peak ILI rate recorded by QSurveillance during the pandemic was higher than the rate recorded by the RCGP system [14]. Indicative estimated thresholds for daily UK level QSurveillance data are estimated to be about one fifth of the weekly thresholds as almost all of the data are recorded on weekdays when GP surgeries are open.
The HPA/QSurveillance system is the only UK ‘real-time’ surveillance system that is currently able to link consultation rates to prescribing rates. These morbidity-linked-to-prescribing indicators have been valuable in being able to monitor the use of antivirals prescribed for ILI presenting to general practice. On 23 July 2009 (during week 30) the NPFS was initiated and patients with influenza-like symptoms were encouraged to contact the NPFS for advice and for antiviral treatment. This introduction of the NPFS was associated with a marked fall in consultations for ILI with antivirals prescribed. There are also a variety of indicators where different antibiotics being used for the treatment of pneumonia are monitored but in this pandemic secondary bacterial infections were not a predominant feature, and there was little impact on these indicators. However the HPA/QSurveillance system data provides both the ability to alert about acute changes in prescribing and also to monitor changes if antibiotic advice is amended during the course of a pandemic.
The advent of the influenza A(H1N1) pandemic 2009 has provided new challenges for surveillance, requiring systems to be timely and able to report at both national and local level, providing relevant data to inform and guide local response. Syndromic surveillance systems provide the ability to respond to major public health incidents and the HPA/QSurveillance national surveillance system was specifically planned and designed to respond to an influenza pandemic. To our knowledge, the system is one of the largest of its kind in the world and provides good representation of national populations across England, and other parts of the UK. Importantly, such systems do not require clinicians to record any additional information to that which they would normally record in routine clinical practice.
During the pandemic the HPA/QSurveillance system, in conjunction with other surveillance sources such as the RCGP WRS and the NHS Direct syndromic surveillance system, provided data in ‘real-time’ relating to the overall impact of the pandemic on the community, and providing an estimate of the burden on primary-care services both at national and local level. During the first wave of pandemic (H1N1) 2009 in England it is estimated that between 144 000 and 670 000 people (mid-estimate of 320 000) fell ill with influenza; QSurveillance consultation data for ILI were used along with other data sources in the construction of this estimate [2].
In addition to providing helpful information to monitor the extent of the pandemic, the PCT-level data were used, with other indicators of influenza activity, to declare certain areas of the country as having high ILI activity and ‘areas of sustained community transmission’. For such areas the national containment strategy was changed to a local outbreak management approach, where not all cases needed to be confirmed microbiologically before being treated [14].
The HPA/QSurveillance national surveillance system has demonstrated its usefulness during both the surveillance of the 2009 pandemic, and also through routine surveillance of other seasonal infections. There are, however, limitations which need to be considered. QSurveillance only extracts data from EMIS practices and data from general practices that use other general practice systems are not included. Other surveillance systems, including the RCGP WRS, utilize multiple providers; however, the overall trends remain very similar to those reported by the HPA/QSurveillance system suggesting that only using data from one provider is not significantly detrimental.
Although QSurveillance has excellent coverage with about one third of UK general practices contributing data to the database, population coverage in Wales and Northern Ireland is much lower than in England. Currently, there are no practices reporting from Scotland, which has an alternative GP-based surveillance system. However, QSurveillance coverage of Scotland is likely to increase in 2010–2011 with the roll out of EMIS Web thus enabling capture of data from these practices.
QSurveillance provides daily data from ‘in-hours’, or scheduled GP activity. Even though the system extracts data from each practice on every day of the week, the closure of GP services over the weekend results in a deficiency of data at weekends and evenings/nights. We are currently identifying a system that will capture the unscheduled (out-of-hours) activity that should complement the HPA/QSurveillance system.
The introduction of the NPFS on 23 July 2009 significantly affected the consultations for ILI recorded by the HPA/QSurveillance system and demonstrates how the system can be affected by national policy changes as well as disease trends.
In summary, the HPA/QSurveillance system has provided ‘real-time’ and useful morbidity and prescribing data to monitor the course of the pandemic on a population of over 23 million patients, this degree of coverage being a ‘world first’ for such a timely system. Data from the system have also been used to monitor the effects of other health protection incidents such as the eruption and subsequent ash plume of the Eyjafjallajökull volcano in Iceland on 14 April 2010 when the QSurveillance data revealed that there were no particularly unusual increases in any of the monitored conditions [Reference Elliot18]. The 2012 London Olympics will provide another surveillance challenge, for which the HPA/QSurveillance scheme will play an important role in providing timely data at national and local level to provide ‘real time’ support for incidents with potential public health impact.
ACKNOWLEDGEMENTS
We are grateful to EMIS and the EMIS practices who voluntarily contribute their data to the QSurveillance® database and to the QSurveillance team in the Division of Primary Care, Nottingham University and EMIS for the provision of the data. We are grateful to HPA stakeholders for their support for the development of the HPA/QSurveillance system.
QSurveillance receives funding from the HPA for the HPA/QSurveillance national syndromic surveillance system.
Trent MREC indicated that ethical approval was not required for this descriptive paper.
DECLARATION OF INTEREST
J.H.C. is director of QResearch which is a not-for-profit venture between the University of Nottingham and EMIS which is a commercial supplier of GP clinical computer systems. J.H.C. is director of QSurveillance® and also clinical director of ClinRisk Ltd which provides commercial software and services together with EMIS.



