Diabetes-associated complications are major causes of morbidity and mortality. The liver plays a unique role in glucose homeostasis. Because of its anatomical location, it is ideally suited to control the systemic supply of absorbed nutrients, and it is one of only two organs that both consume and produce substantial amounts of glucose. The central role of the liver in the maintenance of blood glucose levels is assured by complex regulation by metabolic substrates, insulin and other hormones(Reference Michael, Kulkarni and Postic1). Diabetes-associated hyperglycaemia and hypoinsulinaemia lead to the impairment of hepatic glucose and lipid metabolism. Virtually, the entire spectrum of liver diseases is seen in patients with diabetes, including liver cirrhosis, a significant cause of death in diabetic patients, even more relevant than CVD(Reference de Marco, Locatelli and Zoppini2). Interest in the development of novel antidiabetic agents has been fuelled by the intense complications due to therapeutic treatment of diabetes and associated liver failure(Reference de Marco, Locatelli and Zoppini2, Reference Maritim, Sanders and Watkins3). An increased prevalence of diabetes has been observed in vitamin D-deficient individuals(Reference Chiu, Chu and Go4), and our previous study has shown that vitamin D3 treatment restores blood glucose homeostasis in streptozotocin (STZ)-induced diabetic rats(Reference Kumar, Antony and Nandhu5). Binding of vitamin D3 promotes the vitamin D receptor (VDR) to form a heterodimer with the retinoid X receptor and transactivates vitamin D-responsive elements present in target genes(Reference Kato, Yoshizazawa and Kitanaka6). VDR mRNA and protein have previously been detected and studied in several rat tissues including liver(Reference Mee, Davenport and Hoyland7, Reference Sandgren, Brönnegärd and DeLuca8).
Diabetes-associated liver complications are widely studied using STZ-induced diabetic rats(Reference Thomas9). Net glucose uptake and its metabolism in hepatocytes depend on intracellular metabolic status, which is determined by metabolic enzymes, second messengers and transcription factors. Evidence for the role of free radicals and oxidative stress in diabetes-associated complications has been established. Implication of oxidative stress in the pathogenesis of diabetes mellitus is suggested not only by oxygen free radical generation but also due to non-enzymatic protein glycosylation, auto-oxidation of glucose, impairment of antioxidant enzymes and the formation of peroxides(Reference Pari and Latha10). Free radical-mediated lipid peroxidation (LPO) is a key factor in the development of diabetic liver complications(Reference Chiu, Khan and Farhangkhoee11).
Although vitamin D3 is considered as a potential antidiabetic agent, no studies have reported the effect of vitamin D3 on hepatic LPO, free-radical-scavenging systems, insulin sensitivity, glucose uptake and metabolism at the molecular level. Therefore, the present study was undertaken to investigate the effect of vitamin D3 in the liver of STZ-induced diabetic rats. The therapeutic possibilities of vitamin D3 for diabetes-associated physiological, biochemical and pathological alterations in the liver were assessed in the present study.
Materials and methods
Biochemicals used in the present study were purchased from Sigma Chemical Company. All other reagents of analytical grade were purchased locally. Real-time PCR Taqman probe assays were from Applied Biosystems. d-[14C]Glucose and RIA kits for insulin were purchased from the Bhabha Atomic Research Centre. [3H] inositol triphosphate (IP3), [3H] cyclic GMP (cGMP) and [3H] cyclic AMP (cAMP) Biotrak Assay Systems were purchased from GE Healthcare UK Limited.
Adult male Wistar rats of 180–240 g body weight were used for all experiments. They were housed in separate cages under 12 h light–12 h dark periods. Rats had free access to standard food and water ad libitum. All animal care and procedures were carried out in accordance with the Institutional, National Institute of Health and CPCSEA guidelines. All efforts were made to minimise the number of animals used and their suffering. Diabetes was induced in rats by a single intrafemoral injection of STZ, which was freshly dissolved in 0·1 m-citrate buffer (pH 4·5) under anaesthesia(Reference Junod, Lambert and Staufferacher12). STZ was given at a dose of 55 mg/kg body weight(Reference Hohenegger and Rudas13). Control rats were injected with citrate buffer alone. Diabetes in rats was confirmed on the third day by checking the fasting blood glucose concentration. The animals were divided into the following four groups: (1) control, (2) diabetic, (3) insulin-treated diabetic, (4) vitamin D3-treated diabetic. Each group consisted of six to eight animals. The insulin-treated diabetic group received subcutaneous injections (1 U (45·5 μg)/kg body weight) of Lente and Plain insulin daily during the entire period of the experiment. The last injection was given 24 h before killing the rats. The vitamin D3-treated group received 12 μg cholecalciferol/kg dissolved in 0·3 ml of coconut oil by oral administration. Maximum care was given in order to avoid stress during the oral supplementation of vitamin D3. Rats underwent 14 d of treatment, so that they were habituated to vitamin D3 supplementation(Reference de Souza Santos and Vianna14, Reference Thomsen, Grottick and Menzaghi15). The last dose of vitamin D3 and insulin was given 24 h before killing to minimise stress-induced endocrine changes due to oral administration. Rats were killed on day 15 by decapitation. The liver was quickly dissected out and the tissues collected were stored at − 80°C until the performance of the assay.
Estimation of blood glucose concentration
Blood samples were collected from the tail vein before STZ injection on days 1 and 15 of the treatment. Fasting blood glucose was estimated by a spectrophotometric method using glucose oxidase–peroxidase reactions. The results were expressed in terms of mg/l blood.
Estimation of blood insulin concentration
Blood samples were collected on day 15 of the treatment. The assay was performed according to the procedure of the Bhabha Atomic Research Centre RIA kit. The RIA method was based on the competition of unlabelled insulin in the standard or samples and [125I]insulin for limited binding sites on a specific antibody. At the end of incubation, antibody-bound and free insulin were separated by a second antibody–polyethylene glycol-aided separation method. Measurement of radioactivity associated with the bound fraction of samples and standards allowed the quantification of the insulin concentration of samples.
Determination of hepatic glycogen content and lipid peroxidation
Liver glycogen content was determined according to the method of Hargreaves et al. (Reference Hargreaves, Barrales and Barrales16). This method is based on acid digestion of the liver sample, followed by neutralisation of the solution and subsequent glucose determination. LPO products were measured as thiobarbituric acid-reactive substances and were used to determine the index of LPO(Reference Rajasekaran, Sivagnanam and Subramanian17, Reference Satho18).
Measurement of glucose uptake
Glucose uptake activity was measured using d-[14C]glucose by the modified procedure of Crane & Mandelstam(Reference Crane and Mandelstam19). Liver cells were isolated by perfusion with collagenase according to the modified procedure of Seglen(Reference Seglen20). The isolated cells were resuspended in William's media E and cell viability was determined. For the in vitro study, the liver cells were isolated from normal rats and 106 cells were added to four different culture plates. Together with William's media E, each group of plates contained (1) 4 mm-glucose (control group), (2) 20 mm-glucose (diabetic group), (3) 20 mm-glucose with insulin (insulin-treated diabetic group) and (4) 20 mm-glucose with vitamin D3 (vitamin D3-treated diabetic group). For the in vivo experiments, the liver cells were isolated from the control, diabetic, insulin-treated diabetic and vitamin D3-treated diabetic rats on day 15 of the treatment. The liver cells of the experimental rats were transferred to 3 ml of standard William's media E alone. The isolated liver cells for both in vitro and in vivo experiments were then incubated for 1 h at 37°C with the addition of 20 000 dpm d-[14C]glucose/tube. The gas phase in the flask during the incubation period contained 5 % CO2. After incubation, cells were removed from the culture plates and washed in the incubation medium to remove the unbound d-[14C]glucose. The cells were hydrolysed in 1 m-NaOH (85°C, 1 h) and 14C radioactivity was counted with cocktail in a Wallac 1409 liquid scintillation counter. d-[14C]glucose uptake was expressed as nmol/mg protein.
Second messenger assay
The livers of the experimental rats were homogenised in a polytron homogeniser with twenty volumes of cold 50 mm-Tris–HCl buffer (pH 7·4) containing 1 mm-EDTA. The homogenate was then centrifuged at 30 000 g for 30 min and the supernatant was transferred to fresh tubes for IP3, cAMP and cGMP assays using [3H]IP3, [3H]cAMP and [3H]cGMP Biotrak assay system kits.
Malate dehydrogenase assay
Liver mitochondria were isolated by the modified method of Jonshon & Lardy(Reference Jonshon and Lardy21). Subcellular fractions of the liver were separated by differential centrifugation(Reference Prokhorova22). Mitochondrial and cytosolic fractions were separated and purified in several stages. Total protein concentration was estimated by the method of Lowry et al. (Reference Lowry, Rosenbrough and Farr23) using bovine serum albumin as the standard. Malate dehydrogenase (MDH) was assayed according to the method of Mehler et al. (Reference Mehler, Kornberg and Grisolia24) in the cytoplasmic and mitochondrial fractions, and kinetic parameters were measured. The reaction mixture contained phosphate buffer (pH 7·4), NADH, oxaloacetate and tissue extract. The reaction mixture of 1 ml was assayed at 340 nm in a spectrophotometer by measuring the decrease in optical density due to the oxidation of NADH measured at 15 s intervals for 1 min at room temperature. A unit of enzyme activity is equal to the change in the optical density of 0·1 for 100 s at 334 nm. Kinetic parameters such as V max and K m were calculated from the data of MDH activity measured at substrate concentrations of 0·0125–0·2 mm.
Analysis of gene expression by real-time PCR
RNA was isolated from the liver of the experimental rats using the Tri-reagent (Molecular Research Centre) according to the procedure of Chomczynski & Sacchi(Reference Chomczynski and Sacchi25). Total complementary DNA synthesis was performed using the ABI PRISM complementary DNA archive kit in 0·2 ml microfuge tubes. The reaction mixture of 20 μl contained 0·2 μg total RNA, 10 × RT buffer, 25 × dNTP mixture, 10 × random primers, MultiScribe RT (50 U/μl) and RNase-free water. The complementary DNA synthesis reactions were carried out at 25°C for 10 min and 37°C for 2 h using an Eppendorf Personal Cycler. Real-time PCR assays were performed in ninety-six-well plates in an ABI 7300 Real-time-PCR instrument (Applied Biosystems). The specific primers of GPx (Rn00577994_g1), SOD (Rn01477289_m1), insulin receptor (INSR) (Rn00567070_m1), GLUT2 (Rn00563565_m1), PLC (Rn01647142_m1), CREB (Rn00578826_m1) and VDR (Rn00566976_m1) were purchased from Applied Biosystems. The TaqMan reaction mixture of 20 μl contained 25 ng of total RNA-derived complementary DNA, 200 nm each of the forward primer, reverse primer and TaqMan probe for assay on demand and endogenous control β-actin and 12·5 μl of Taqman 2X Universal PCR Master Mix (Applied Biosystems), and the volume was made up with RNase-free water. The following thermal cycling profile was used (forty cycles): 50°C for 2 min, 95°C for 10 min, 95°C for 15 s and 60°C for 1 min. Fluorescence signals measured during amplification were considered positive if fluorescence intensity was 20-fold greater than the standard deviation of baseline fluorescence. The ΔΔCT method of relative quantification was used to determine the fold change in expression. This was done by normalising the resulting threshold cycle (CT) values of the target mRNA to the CT values of the internal control β-actin in the same samples (ΔCT = CT target − CT β-actin). It was further normalised with the control (ΔΔCT = ΔCT − CT control). The fold change in expression was then obtained as (2− ΔΔCT) and the graph was plotted using log 2− ΔΔCT.
Statistics
Data are presented as means with their standard errors. Statistical evaluations were done by ANOVA using In Stat (version 2.04a; GraphPad Software Inc.). The Student–Newman–Keuls post hoc test was used to compare data among the groups. Relative Quantification Software (Relative Quantification Software) was used for analysing real-time PCR results. In all cases, P < 0·05 was taken to indicate statistical significance.
Results
Fasting glucose and insulin concentrations
Fasting blood glucose concentrations of all rats before STZ administration were within the normal range. STZ administration led to a significant increase in blood glucose and a significant decrease in blood insulin level compared with the non-diabetic controls. In the vitamin D3- and insulin-treated rats, blood glucose and insulin levels were significantly reversed towards control when compared with the diabetic group (Table 1). The body weight of the diabetic group showed a significant decrease and the percentage of liver weight showed a significant increase when compared with the controls. This was significantly reversed in the vitamin D3- and insulin-treated rats (Table 1).
Table 1 Blood glucose, blood insulin, body weight and percentage of liver weight in the experimental rats (Mean values with their standard errors of six to eight separate experiments (n 8–10 animals per group))
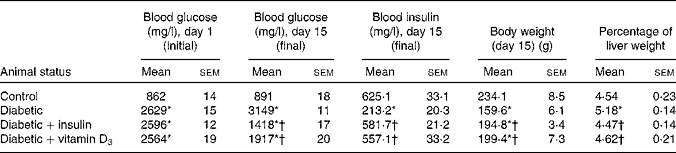
* Mean values were significantly different when compared with the control group (P < 0·05).
† Mean values were significantly different when compared with the diabetic group (P < 0·05).
Malate dehydrogenase activity
Mitochondrial and cytoplasmic MDH activities showed a significant decrease in the liver of diabetic rats. During diabetes, the V max values of MDH showed a significant decrease compared with the control in the mitochondria and cytoplasm. Compared with the diabetic group, the V max of the cytoplasmic fraction showed a significant decrease in the insulin-treated group, but no significant decrease was observed in the vitamin D3-treated group (Table 2). Both vitamin D3 and insulin significantly reversed the V max of the mitochondrial MDH fraction to near control values compared with the diabetic group (Table 2). K m showed no significant change in the mitochondrial fraction but in the cytoplasmic fraction, it showed a significant increase in the diabetic group. The vitamin D3 and insulin treatments significantly reversed K m to near control values (Table 2).
Table 2 Malate dehydrogenase (MDH) activity in the liver of the experimental rats (Mean values with their standard errors of six to eight separate experiments (n 8–10 animals per group))
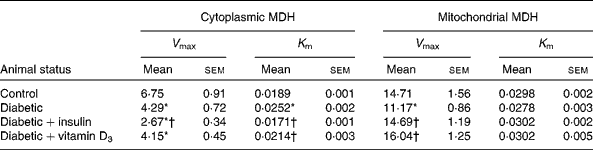
V max, K m, kinetic parameters.
* Mean values were significantly different when compared with the control group (P < 0·05).
† Mean values were significantly different when compared with the diabetic group (P < 0·05).
Real-time PCR analysis of superoxide dismutase, glutathione peroxidase, phospholipase C, cyclic AMP-responsive element-binding protein, vitamin D receptor, insulin receptor and GLUT2
Real-time PCR analysis showed a significant decrease in SOD, GPx, PLC, CREB, VDR and INSR gene expression in the liver of diabetic animals, which was reversed to near control values with the treatment of vitamin D3 or insulin (Figs. 1 and 2). There was a significant increase in GLUT2 gene expression in the liver of the diabetic group; however, it was significantly reversed to near control values in the vitamin D3- and insulin-treated diabetic rats (Fig. 2).

Fig. 1 Real-time amplification of glutathione peroxidase (GPx, □) and superoxide dismutase (SOD, ![]() ) mRNA from the liver of the experimental rats. Values are means of six to eight separate experiments (n 8–10 animals per group), with standard errors of the mean represented by vertical bars. * Mean values were significantly different when compared with the control group (P < 0·05). † Mean values were significantly different when compared with the diabetic group (P < 0·05). RQ, relative quantification; D+I, insulin-treated diabetic rats; D+V, vitamin D3-treated diabetic rats.
) mRNA from the liver of the experimental rats. Values are means of six to eight separate experiments (n 8–10 animals per group), with standard errors of the mean represented by vertical bars. * Mean values were significantly different when compared with the control group (P < 0·05). † Mean values were significantly different when compared with the diabetic group (P < 0·05). RQ, relative quantification; D+I, insulin-treated diabetic rats; D+V, vitamin D3-treated diabetic rats.

Fig. 2 Real-time amplification of insulin receptor (INSR) (□), GLUT2 (![]() ), phospholipase C (PLC,
), phospholipase C (PLC, ![]() ), cyclic AMP-responsive element-binding protein (CREB,
), cyclic AMP-responsive element-binding protein (CREB, ![]() ) and vitamin D receptor (VDR,
) and vitamin D receptor (VDR, ![]() ) mRNA from the liver of the experimental rats. Values are means of six to eight separate experiments (n 8–10 animals per group), with standard errors of the mean represented by vertical bars. * Mean values were significantly different when compared with the control group (P < 0·05). † Mean values were significantly different when compared with the diabetic group. RQ, relative quantification; D+I, insulin-treated diabetic rats; D+V, vitamin D3-treated diabetic rats.
) mRNA from the liver of the experimental rats. Values are means of six to eight separate experiments (n 8–10 animals per group), with standard errors of the mean represented by vertical bars. * Mean values were significantly different when compared with the control group (P < 0·05). † Mean values were significantly different when compared with the diabetic group. RQ, relative quantification; D+I, insulin-treated diabetic rats; D+V, vitamin D3-treated diabetic rats.
Second messenger content in the liver of the experimental rats
IP3 and cGMP contents were significantly decreased in the liver of the diabetic rats, whereas the cAMP level was significantly increased when compared with the control. The vitamin D3 and insulin treatments significantly reversed the IP3, cGMP and cAMP contents near to control values (Table 3).
Table 3 Concentrations of malondialdehyde (MDA), glycogen, second messengers and d-[14C]glucose uptake in the liver of the experimental rats (Mean values with their standard errors of six to eight separate experiments (n 8–10 animals per group))

* Mean values were significantly different when compared with the control group (P < 0·05).
† Mean values were significantly different when compared with the diabetic group (P < 0·05).
Lipid peroxidation in the liver of the experimental rats
There was a significant elevation in thiobarbituric acid-reactive substances in the liver of the diabetic rats when compared with the control. The treatment of vitamin D3 and insulin significantly reversed thiobarbituric acid-reactive substances in the liver of the diabetic rats to near control values (Table 3).
Glycogen content in the liver of the experimental rats
There was a significant decrease in glycogen in the liver of the diabetic rats when compared with the control. The vitamin D3 and insulin treatments significantly reversed the glycogen content in the liver of the diabetic rats to near control values (Table 3).
Glucose uptake activity in the liver of the experimental rats
In the in vivo and in vitro studies, diabetes led to a significant decrease in glucose uptake by hepatocytes when compared with the control. The vitamin D3 and insulin treatments significantly increased glucose uptake to near control values (Table 3).
Discussion
The present study investigated the effects of vitamin D3 treatment on diabetes-associated liver metabolic complications and the possible mechanisms involved. STZ-induced diabetic rats have proved to be useful in trying to determine the underlying cause of liver complications during diabetes(Reference Thomas9). Similar to previous reports, in the present study, STZ-induced diabetes was characterised by hypoinsulinaemia, hyperglycaemia and decreased body weight(Reference Kumar, Antony and Nandhu5, Reference Junod, Lambert and Staufferacher12). Treatment with vitamin D3 significantly improved hypoinsulinaemia and hyperglycaemia and prevented body-weight loss, which indicated amelioration in diabetic complications. Moreover, there were neither significant changes in food intake nor any sign of toxicity. Since STZ is known to destroy pancreatic β-cells, the possible mechanism of action of vitamin D3 is mediated through influencing glucose uptake and utilisation, especially in the liver.
During diabetes, total body weight was significantly reduced, but the percentage of liver weight was significantly increased. This was most probably due to fat accumulation in the liver as a result of the increased release of NEFA from adipose tissue(Reference Rocco, Calevo and Taro'26). Increased accumulation of fat in the form of TAG and reduced protein and stored glycogen contribute to the development of fatty liver disease. Non-alcoholic fatty liver disease is considered as the hepatic manifestation of the metabolic syndrome, and is present in approximately 80 % of type 2 diabetic patients(Reference Farrell and Larter27). The in vitro and in vivo results showed that there was a significant reduction of net glucose uptake into liver cells in diabetic rats. The significant increase in the uptake of glucose in vitro in the presence of vitamin D3 indicates its direct action on hepatocytes. Hepatic glucose uptake was regulated by the glucose load to the liver, arterial–portal glucose gradient and insulin level(Reference Gallán, Carrascosa and Gussinyé28). The present study showed decreased INSR gene expression in the liver of STZ-induced diabetic rats, which subsequently would lead to the deterioration of INSR-mediated cell signalling(Reference Gherzia, Andraghettia and Ferrannini29). This finding is not in accordance with previous results showing increased hepatic INSR levels in a similar diabetic rat model(Reference Calle, Maestro and García-Arencibia30). However, a reduced expression of INSR, a decreased intracellular pool of receptors and diminished insulin-stimulated protein kinase activity have been observed in the liver of diabetic human subjects(Reference Caro, Sinha and Raju31–Reference Taylor, Accili and Imai33). The present findings agree with previous reports that treatment with vitamin D3 causes transcriptional activation of the INSR gene(Reference Maestro, Dávila and Carranza34). Maestro et al. (Reference Maestro, Dávila and Carranza34) observed that vitamin D3 induced a 1·8-fold increase in both INSR gene expression and insulin receptor number, and enhanced cellular responsiveness to insulin in terms of glucose transport (1·3-fold increase) and glucose oxidation (1·6-fold increase) in human cells. Kong et al. (Reference Kong, Zhang and Song35) and Zhang et al. (Reference Zhang, Wei and Xue36) suggested that INSR up-regulators have clinical benefit in improving insulin sensitivity and lowering blood glucose. The vitamin D-responsive element is present in the INSR gene promoter and accounts for the transcriptional induction of the INSR gene by vitamin D3. This vitamin D-responsive element is overlapped by a downstream activator protein 2 (AP-2) sequence. Together, these cis-elements form a locus that can respond to vitamin D3 via the activation of the VDR. This locus could mediate cross-talk between vitamin D and insulin signalling pathways(Reference Maestro, Dávila and Carranza34). Impaired insulin action in the liver resulted in decreased glucose uptake, decreased oxidative and non-oxidative glucose metabolism(Reference Selberg, Burchert and Hoff37). These defects of hepatic glucose uptake and metabolism contribute to the development of diseases such as liver cirrhosis(Reference Imano, Kanda and Nakatani38).
GLUT2 ensures large bidirectional fluxes of glucose in and out of the liver cell due to its low affinity and high capacity(Reference Leturque, Brot-Laroche and Le Gall39). GLUT2 gene expression is regulated at the transcriptional level by its promoter, which is capable of a rapid, dose-dependent glucose response(Reference Rencurel, Waeber and Antoine40). Although GLUT2 mRNA expression was significantly up-regulated in the liver of diabetic rats, it cannot facilitate a net glucose entry into hepatocytes because of diabetes-induced metabolic alterations. The reduced glucose uptake has profound effects on metabolic flux, adenine nucleotide potential ((ATP)/(ADP), (NAD(P)H/(NAD(P)+)), cellular electrical activity and cytosolic (Ca2+), and cells even commit to programmed cell death(Reference Schuit, Flamez and Vos41).
MDH enzyme activity is a useful parameter to evaluate the metabolic status of both the mitochondria and the cytoplasm. Mitochondrial MDH is a tricarboxylic acid cycle marker enzyme. Cytoplasmic MDH plays a crucial role in the malate-aspartate shuttle, lipogenesis and gluconeogenesis. The reduced activity of MDH during diabetes results from reduced glucose uptake, decreased INSR-mediated cell signalling, free radical-induced inactivation and glycation of the enzyme(Reference Rajasekaran, Sivagnanam and Subramanian17). Decreased MDH enzyme activity reflects depression of energy metabolism in the cytoplasm and mitochondria. The reversal of the decreased mitochondrial MDH activity to near control during the vitamin D3 treatment is attributed to the reversal of altered glucose metabolism, increased hepatic antioxidant enzymes and decreased ROS levels. Due to the central role of the liver in glucose homeostasis, impairment in liver glucose transport and metabolism lead to the impairment of blood glucose and fat level. Treatment with vitamin D3 significantly reversed the INRS and GLUT2 mRNA expression levels and glucose uptake to near control to conserve hepatocyte metabolism which would help to maintain blood glucose homeostasis.
Hepatic glycogen storage is promoted by hyperglycaemia and insulin(Reference Peterson, Laurent and Rothma42). In the present study, decreased hepatic glycogen levels during diabetes indicate a decreased ability of the liver to convert glucose to glycogen. Inability to synthesise hepatic glycogen and reduced glucose uptake cause postprandial hyperglycaemia(Reference Weinstein, Correia and Saunders43) and post-absorptive or fasting hyperketonaemia and hypoglycaemia(Reference Féry, Plat and Melot44). Acute therapy of diabetes with insulin causes excessive glycogen storage in the liver and hence can result in hepatomegaly(Reference Tomihira, Kawasaki and Nakajima45). The present results show that treatment with vitamin D3 significantly increased stored glycogen level in the liver of rats. Hence, it facilitated the maintenance of normal blood glucose level in absorptive, post-absorptive and fasting periods. Diabetes-associated reduction in hepatic glucose uptake, metabolism and storage in spite of elevated blood glucose and GLUT expression were reversed by vitamin D3 administration. The potential of vitamin D3 in reversing metabolic alterations during diabetes to near control contributed to its hepatoprotective and anti-diabetic properties.
Liver VDR upon activation induces changes in gene expression via classical transactivation by nuclear receptors and intracellular signalling pathways(Reference Jiang, Miyamoto and Kakizawa46). The vitamin D hormone system is involved in the regulation of more than 800 genes(Reference Johna, Lafage-Proustb and Massyc47). Studies have shown that in the liver, vitamin D3 confers benefits on cholesterol metabolism, bile acid metabolism and Ca2+ homeostasis(Reference Jiang, Miyamoto and Kakizawa46). The present study showed that VDR gene expression was modulated by diabetes, confirming a role in the development of diabetes-associated complications. Therefore, the present results provide the first demonstration of an in vivo tissue-specific regulation of VDR gene expression levels by its ligand, vitamin D3, in STZ-induced diabetic rats. Vitamin D3-induced changes in gene expression through the VDR seemed to have a role in reversing impaired hepatic metabolism during diabetes to near control. These vitamin D3-induced changes in VDR expression are suggested to contribute to its hepatoprotective and antidiabetic effects.
Several pathways are thought to be impaired in the pathogenesis of hyperglycaemia-induced liver complications. Our studies showed that the gene expression of CREB and cAMP levels was significantly increased in the liver of diabetic rats, which indicates the stimulated cAMP transduction pathway due to hyperglucagonaemia, a common hormonal perturbation during diabetes(48). Increased cAMP-mediated cell signalling resulted in cholangiocyte proliferation and fluid secretion, which led to the development of polycystic liver diseases(Reference Masyuk, Masyuk and Torres49). Insulin and vitamin D3 treatments significantly reversed the changes in cAMP transduction pathway, which in turn contributed to the normalisation of liver metabolic alterations. Impaired Ca2+ signalling plays an important role in the long-term complications observed in the diabetic liver(Reference Chan and Junger50). Decreased IP3 and PLC levels in diabetic rats resulted in poor cell signalling due to the impaired Ca2+ release from intracellular stores. STZ-induced diabetic rats were associated with a decreased Ca-dependent regulator activity which played a major role in the distortion of cAMP metabolism(Reference Smoake and Solomon51). Del Pino-Montes et al. (Reference Del Pino-Montes, Benito and Fernández-Salazar52) showed that vitamin D3 treatment did not change serum Ca levels in STZ-induced diabetic rats. During diabetes, increased reactive oxygen species levels resulted in the down-regulation of guanylate cyclase expression and activity by tyrosine phosphorylation, leading to a reduction in apoptotic inhibition mediated by cGMP(Reference Gerassimou, Kotanidou and Zhou53). Treatment with vitamin D3 reverses the cGMP content to near control and inhibited NADPH oxidase activity, mitochondrial permeability transition and cytochrome c release through the activation of the cGMP-dependent protein kinase pathway, and thus reduced TNF-α-induced apoptosis in hepatocytes(Reference Calafell, Boada and Santidrian54). The differential regulation of cAMP and cGMP observed during diabetes is through the action of phosphodiesterases. The phosphodiesterases allow cross-talk between the cGMP and cAMP signalling pathways because they cause the concentration of one cyclic nucleotide to influence the degradation of the other(Reference Calafell, Boada and Santidrian54). Treatment with vitamin D3 and insulin significantly reversed IP3 and cGMP contents in the liver of diabetic rats to near control.
The present study showed that peroxidation of lipids was significantly increased in the liver of diabetic rats, which in turn resulted in elevated production of other free radicals that are harmful to the cell(Reference Sathishsekar and Subramanian55). Hypoinsulinaemia during diabetes increased the oxidation of fatty acids and resulted in decreased membrane fluidity, changed the activity of membrane-bound enzymes and receptors, impaired Ca metabolism(Reference Lehotsky, Kaplan and Racay56), elevated the level of TAG and LDL-cholesterol and reduced the level of HDL-cholesterol, which led to liver dysfunction(Reference Kundsen, Ericksson and Lahdenpera57). Antioxidant enzymes, SOD and GPx, have an important role in maintaining physiological levels of free radicals by hastening the dismutation of free radicals and eliminating organic peroxides and H2O2(Reference Pari and Latha10). The observed decrease in SOD activity in the liver of diabetic rats is suggested to result from the inactivation by free radicals or by glycosylation of the enzyme, which have been reported to occur during diabetes(Reference Ravi, Ramachandran and Subramanian58). Reduced gene expression of GPx and SOD during diabetes suggests impairment of hepatic cell signalling, and is responsible for the inadequacy of antioxidant defence in combating free radical-mediated damage(Reference Pari and Latha10). GPx has been regarded as a major determinant of hepatic antioxidant status, and it protects the tissue from highly reactive hydroxyl radicals by catalysing the reduction of H2O2. In the present study, lower levels of hepatic antioxidant enzymes and increased LPO in the liver of diabetic rats were observed when compared with the control. Vitamin D3 and insulin treatments reduced hepatic oxidative stress by minimising the imbalance between the rate of reactive oxygen species generation and activity of antioxidant enzymes. An increase in GPx and SOD expression protects cellular proteins against inactivation by free radicals(Reference Selvam and Anuradha59). Therefore, treatment with vitamin D3 could reduce free radical generation and increase the activity of antioxidant enzymes, and hence help to control free radical-mediated hepatic complications.
The major strengths of the present study include its prospective design and the range of parameters that have been evaluated regarding cellular metabolism, cell signalling and oxidative stress, thus promoting a better overview of the effects of diabetes, insulin and vitamin D3. However, one limitation of the study is that the dosage of vitamin D3 used is difficult to extrapolate to the human setting because humans have multiple sources of vitamin D.
In conclusion, the present study suggests that vitamin D3 treatment ameliorates impaired liver metabolism during diabetes by regulating glucose uptake, storage and metabolism. Vitamin D3 acts via VDR in the liver of diabetic rats, which in turn normalises signal transduction by improving the expression of IP3, cAMP, cGMP, PLC and CREB. Vitamin D3 treatment reduced diabetes-associated hepatic LPO and oxidative stress. Thus, the results provide evidence for antidiabetic and hepatoprotective properties of vitamin D3. Considering that the treatment of diabetes is hampered by the well-known ability of insulin to induce profound and life-threatening hypoglycaemia, vitamin D3 represents a therapeutic possibility for the better management of diabetes-mediated hepatic complications and other liver diseases. Our investigations add to the understanding of the mechanisms of the antidiabetic effects of vitamin D3. The effect of vitamin D3 pretreatment should be evaluated and further studies should focus on signal transduction to elucidate the detailed mechanisms of the antidiabetic effect of vitamin D3.
Acknowledgements
This study was supported by research grants from the DBT, DST, ICMR, Government of India and KSCSTE, Government of Kerala to C. S. P. N. G. thanks the CSIR, India for a Junior Research Fellowship. The contributions of each author are as follows: N. G., T. P. K. and C. S. P. designed the research; N. G. and T. P. K. conducted the research and analysed the data; S. A. and S. J. provided technical support and performed the statistical analysis; N. G. was primarily responsible for the writing of the manuscript; T. P. K., S. A., S. J. and C. S. P. contributed to the editing of the manuscript. The authors have no conflicts of interest.







