Introduction
Mosasaur (Lepidosauria, Squamata) fossils have revealed several of their physiological and behavioral traits in the last decades: evidence concerning their endothermy (Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010; Motani Reference Motani2010), diving (Schulp et al. Reference Schulp, Vonhof, van der Lubbe, Janssen and van Baal2013), and migration behavior (Lindgren and Siverson Reference Lindgren and Siverson2005) has been presented based on their stable isotope signature. These traits add to environmental drivers that can influence the carbon and oxygen isotope compositions of mosasaur bioapatite, such as the climate, the food source, their wide latitudinal distribution (see Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010), and the euryhalinity of some of the taxa (Kocsis et al. Reference Kocsis, Ősi, Vennemann, Trueman and Palmer2009). To better understand the relative importance of each of these drivers, we compare the oxygen and carbon stable isotope compositions (δ18OPO4, δ18OCO3, and δ13C) of fish (actinopterygians and chondrichthyans) and plesiosaur bioapatite with those of mosasaurs from two latest Maastrichtian sites of different paleolatitudes, namely the Isla Marambio (Seymour Island), Antarctic Peninsula (64°S), and Los Bajos de Trapalcó and Santa Rosa, Argentine Patagonia (45°S).
The δ13C composition mainly depends on the food source, which links to dissolved organic and inorganic carbon, both of which vary with latitude, water depth, and distance from the shore (e.g., Goericke and Fry Reference Goericke and Fry1994). Differences in the carbon fraction are thus expected between the two sites due to different paleolatitudes, and intertaxonomic differences relating to different habitats might also appear. In addition to diet, other factors may influence the carbon isotope composition of bioapatite: breath holding is thought to lower the δ13C values of pulmonate diving animals such as mosasaurs (Robbins et al. Reference Robbins, Ferguson, Polcyn, Jacobs and Everhart2008; Schulp et al. Reference Schulp, Vonhof, van der Lubbe, Janssen and van Baal2013, Reference Schulp, Janssen, van Baal, Jagt, Mulder and Vonhof2017; van Baal et al. Reference Baal, van, Janssen, van der Lubbe, Schulp, Jagt and Vonhof2013), while sharks are known to show particularly high δ13C values in their enameloid (Vennemann et al. Reference Vennemann, Hegner, Cliff and Benz2001; van Baal et al. Reference Baal, van, Janssen, van der Lubbe, Schulp, Jagt and Vonhof2013; Kocsis et al. Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Yans and Amaghzaz2014).
Because δ18OPO4 values depend strongly on the precipitation temperature of bioapatite (Kolodny et al. Reference Kolodny, Luz and Navon1983), we expect the latitudinal thermal gradient to have a marked influence on the δ18OPO4 values of thermoconforming taxa (i.e., with a body temperature varying according to the ambient temperature; e.g., Withers Reference Withers1992), while the δ18OPO4 values of thermoregulating taxa such as mosasaurs and plesiosaurs (Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010; Harrell et al. Reference Harrell, Pérez-Huerta and Suarez2016) should be more stable across latitudes. A thorough literature search revealed that stable isotope data of Late Cretaceous marine vertebrates from sites beyond 52° paleolatitude are absent so far, limiting the elaboration of a complete latitudinal thermal gradient based on thermoconforming vertebrates (e.g., Pucéat et al. Reference Pucéat, Lécuyer, Donnadieu, Naveau, Cappetta, Ramstein, Huber and Kriwet2007). Our fish values greatly extend the latitudinal range of marine vertebrate δ18OPO4 data, and we explore here their implication for global latitudinal gradients elaborated so far.
Geographic, Geological, and Climatic Background
The material studied comes from two geographic areas: Río Negro Province, northern Argentine Patagonia; and Marambio (i.e., Seymour) Island, Antarctic Peninsula (Fig. 1). Photographs of the material are available in the Supplementary Material.
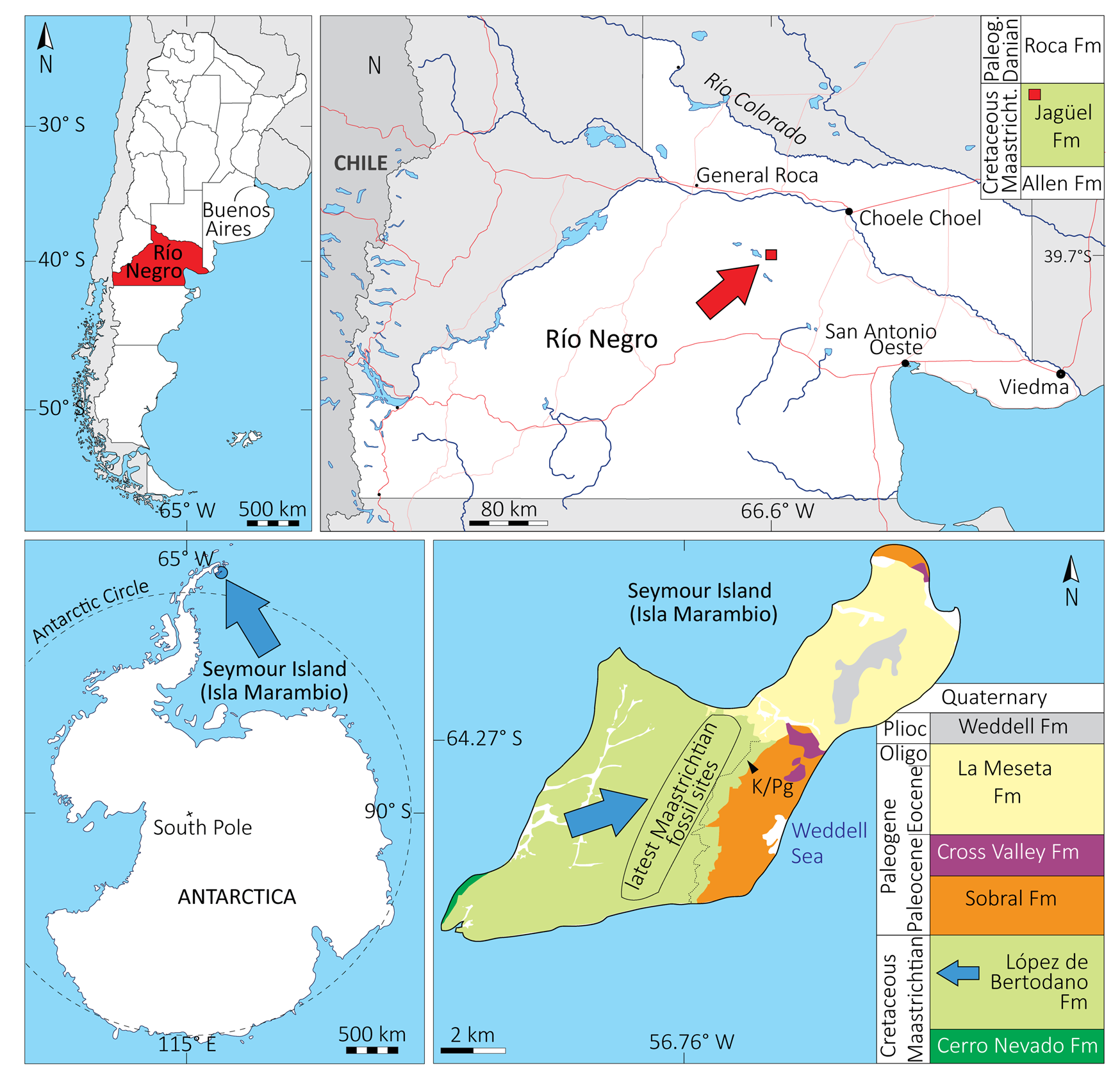
Figure 1. Geographic and stratigraphic setting of the study sites. A, Los Bajos de Trapalcó and Santa Rosa, Río Negro Province, Argentine Patagonia. B, Isla Marambio (Seymour Island), Antarctic Peninsula. Based on Montes et al. (Reference Montes, Nozal, Santillana, Marenssi and Olivero2007), Fernández et al. (Reference Fernández, Martin and Casadío2008), and Fernández and Gasparini (Reference Fernández and Gasparini2012). Oligo, Oligocene; Paleog, Paleogene; Plioc, Pliocene.
Río Negro Province, Argentine Patagonia
The Patagonian material was recovered from the sites of Los Bajos de Trapalcó and Santa Rosa, Río Negro Province (Argentina), and is assigned to the latest Cretaceous Jagüel Formation, which is part of the Malargüe Group. During the Campanian–Maastrichtian, northern Patagonia was an archipelago largely flooded by the Atlantic Ocean as a consequence of sediment load and thermal subsidence of the lithosphere (Uliana and Biddle Reference Uliana and Biddle1988; Gasparini et al. Reference Gasparini, Casadio, Fernández and Salgado2001). During that period, the volcanic arc related to the Andean orogeny was developing to the west. In the study area, the Jagüel Formation deposited under marine settings at the southern margin of the Neuquén Basin (Gasparini et al. Reference Gasparini, Salgado and Casadío2003; O'Gorman et al. Reference O'Gorman, Gasparini and Salgado2014) and mostly consists of olive-green mudstones, occasionally laminated (Gasparini et al. Reference Gasparini, Salgado and Casadío2003; Fernández et al. Reference Fernández, Martin and Casadío2008). The Cretaceous/Paleogene boundary (K/Pg) is contained within the Jagüel Formation, as evidenced by the presence of late Maastrichtian nannofossils followed by Biantholithus sparsus, an index nannofossil for the early Danian (Fernández et al. Reference Fernández, Martin and Casadío2008).
Isla Marambio, Antarctic Peninsula
The Antarctic material comes from a unit of the López de Bertodano Formation (LBF) (Rinaldi et al. Reference Rinaldi, Massabie, Morelli, Rosenman and Del Valle1978), a homoclinal sequence dipping 8° to 10° toward the east (Macellari Reference Macellari1988) and extending SSW-NNE at the center of Isla Marambio, itself located east of the Antarctic Peninsula. The LBF constitutes the uppermost part of the Santonian–Danian Marambio Group (Olivero Reference Olivero2012), and it is Maastrichtian to Danian in age (González Ruiz et al. Reference González Ruiz, Fernández, Talevi, Leardi and Reguero2019). It was deposited in the back-arc Larsen Basin (James Ross subbasin), east of the active arc of the Antarctic Peninsula generated by the subduction of the proto-Pacific oceanic crust (Ditchfield et al. Reference Ditchfield, Marshall and Pirrie1994). The LBF is divided into two sections, the “lower Rotularia units” and the “upper molluscan units,” and 10 informal units (LB1 to LB10) defined by Macellari (Reference Macellari1988), the first of which has since been reassigned to an older formation (see González Ruiz et al. Reference González Ruiz, Fernández, Talevi, Leardi and Reguero2019). The study material comes from the ninth unit (Unit 9), which includes a glauconite-rich interval that contains the K/Pg boundary, characterized by a reduction of the number of invertebrate fossils, a sudden lack of ammonites, and an iridium anomaly (Elliot et al. Reference Elliot, Askin, Kyte and Zinsmeister1994). According to sedimentological studies, the sandy siltstones of Unit 9 were deposited in a shallow outer shelf under low-energy settings and below the wave base (Macellari Reference Macellari1988; Elliot et al. Reference Elliot, Askin, Kyte and Zinsmeister1994). An upward-increasing abundance of glauconite across Unit 9 suggests an increase in water depth with time. This unit reflects a marine transgression and represents the deepest, most offshore facies of the LBF (Macellari Reference Macellari1988; O'Gorman et al. Reference O'Gorman, Bona, de los Reyes, Raffi and Reguero2020), in the general context of a prograding shelf that characterizes the Marambio Group (Olivero Reference Olivero2012).
Climatic Context
The Cretaceous Period is considered one of the warmest of Earth's history, although numerous studies point to a global cooling by the Campanian based on different paleothermometers (δ18O of foraminifera, TEX86; Li and Keller Reference Li and Keller1998; Linnert et al. Reference Linnert, Robinson, Lees, Bown, Pérez-Rodríguez, Petrizzo, Falzoni, Littler, Arz and Russell2014). The last 200 kyr of the Maastrichtian in particular appear to have been a relatively cooler period within the Cretaceous, still with no evidence of permanent ice sheets near the poles. At a smaller spatial and temporal scale, a multi-proxy analysis has suggested a gradual warming at the end of the Maastrichtian in the South Atlantic region (Woelders et al. Reference Woelders, Vellekoop, Kroon, Smit, Casadío, Prámparo, Dinarès-Turell, Peterse, Sluijs, Lenaerts and Speijer2017). The absence of large ice masses resulted in seawater δ18O estimated to be equal to −1‰ (Shackleton and Kennett Reference Shackleton and Kennett1976), that is, lower than the present value (0‰ on average). A seawater δ18O value of −1.25‰ is also reported for the Late Cretaceous in particular (Pucéat et al. Reference Pucéat, Lécuyer, Donnadieu, Naveau, Cappetta, Ramstein, Huber and Kriwet2007). The thermal latitudinal gradient, closely related to the atmospheric pCO2, was shallower during the Cretaceous than it is today (present gradient = 0.56°C/1° of latitude; Zhang et al. Reference Zhang, Hay, Wang and Gu2019); however, it became steeper by the end of the period due to a decrease of pCO2 in the atmosphere (Linnert et al. Reference Linnert, Robinson, Lees, Bown, Pérez-Rodríguez, Petrizzo, Falzoni, Littler, Arz and Russell2014). For the last period of the Maastrichtian, Zhang et al. (Reference Zhang, Hay, Wang and Gu2019) published a marine latitudinal gradient of 0.41°C/1° of latitude, similar to the terrestrial air temperature gradient of 0.4°C/1° of latitude calculated by Amiot et al. (Reference Amiot, Lécuyer, Buffetaut, Fluteau, Legendre and Martineau2004) based on δ18O values of continental vertebrate bioapatite. Pucéat et al. (Reference Pucéat, Lécuyer, Donnadieu, Naveau, Cappetta, Ramstein, Huber and Kriwet2007) also reported a marine latitudinal gradient similar to the present-day gradient for paleolatitudes between 1° and 50°, based on δ18O values of Late Cretaceous fish teeth. This shallower gradient implies that the latitudinal limits of the climate zones were shifted poleward in comparison with present-day zones, and according to Zhang et al. (Reference Zhang, Hay, Wang and Gu2019), both of the sites analyzed in our study were located in the temperate climate zone. The paleolatitudes were approximately 45°S for Patagonian study sites and 64°S for the Antarctic locality based on the work of van Hinsbergen et al. (Reference Hinsbergen, de Groot, van Schaik, Spakman, Bijl, Sluijs, Langereis and Brinkhuis2015).
Material and Methods
Material
The study material consists of 29 fossil specimens, mainly teeth of mosasaurs, as well as plesiosaurs, bony fish, and sharks from Argentine Patagonia (Los Bajos de Trapalcó and Santa Rosa localities, Río Negro Province) and Antarctica (Unit 9, LBF, Isla Marambio). Among these, 2 mosasaur and 4 shark teeth are from Patagonia, while 15 mosasaur teeth (for one specimen, an associated bone fragment was also studied), 5 plesiosaur teeth, and 3 bony fish teeth come from Antarctica. The mosasaur material is assigned to Mosasauridae, although some specimens permit a higher degree of taxonomic assignment, such as cf. Liodon, Globidensisni, ?Plioplatecarpinae and ?Mosasaurus (Table 1). The plesiosaur specimens are referred to the family Elasmosauridae or Aristonectidae. The bony fish material is likely referable to Ichthyodectiformes or Aulopiformes, showing affinities to Enchodus. The shark teeth are assigned to the order Lamniformes and probably to the family Odontaspididae.
Table 1. Oxygen and carbon stable isotope compositions of the study material, measured in phosphate and structural carbonate of bioapatite. bo, bone; td, tooth dentine; te, tooth enamel; VPDB, Vienna Pee Dee Belemnite; VSMOW, Vienna Standard Mean Ocean Water.
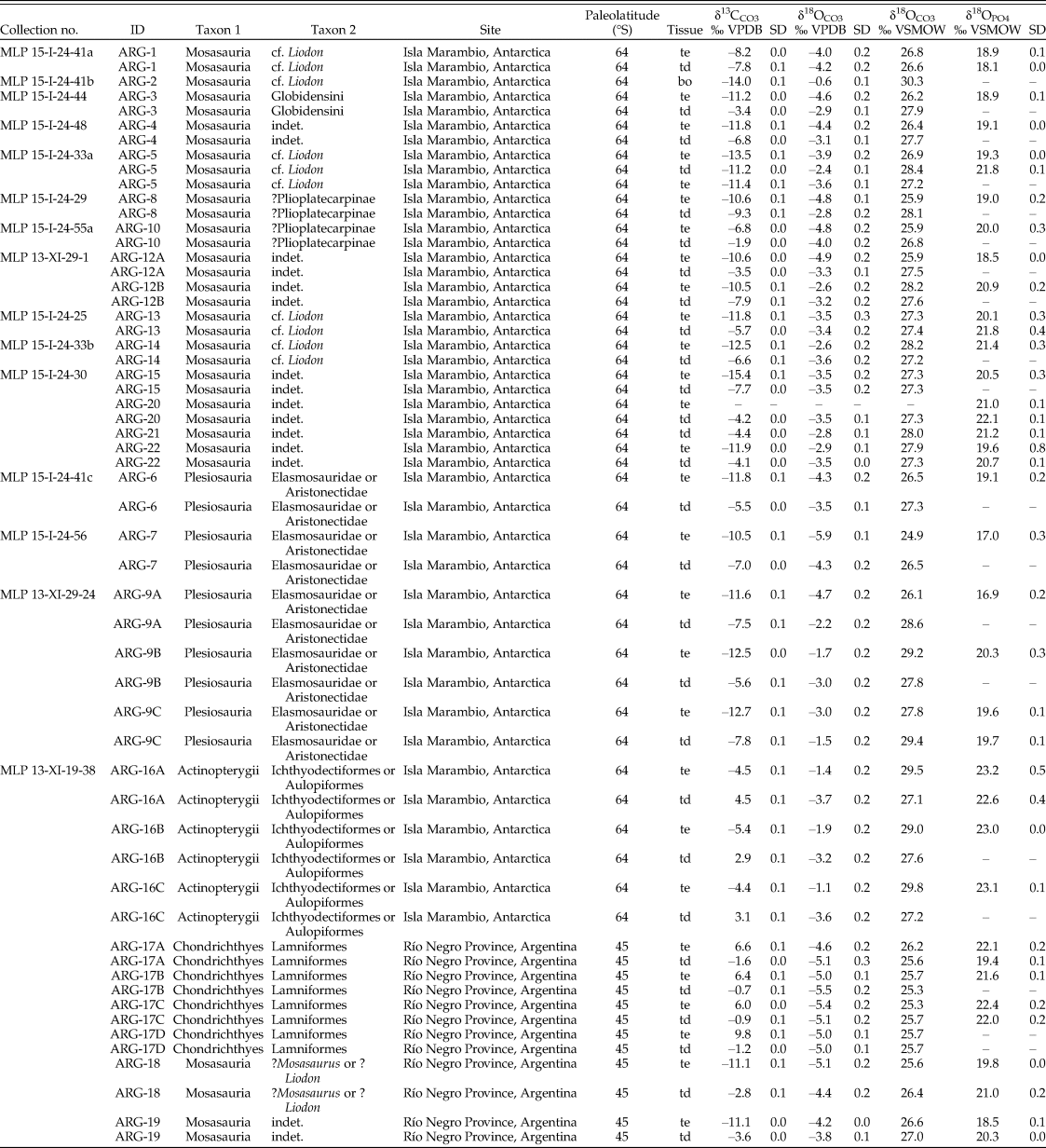
When possible, we sampled both enamel(oid) and dentine from the same tooth to determine the degree of preservation. In one case (ARG-2, mosasaur), we additionally sampled the bone in which the tooth was still implanted. Every sample was analyzed for the stable isotope composition of its bioapatite carbonate fraction (δ13C, δ18OCO3), and most were analyzed for their phosphate (δ18OPO4) fraction as well. A selection of samples were also analyzed to determine their rare earth element (REE) concentration. The material from Antarctica was found isolated and loose in the sediment, with the single exception of ARG-1, which was found embedded in a rock concretion. Most of the material used in this study was either completely used up for the analyses, or a small amount of sample powder and/or tiny fragments kept in epoxy remained. However, many fossils from the same sample lot related to the field surveys are kept under the same collection number at the Museo de La Plata in Argentina. These “MLP” catalogue numbers have been provided in Table 1 to allow scientists to examine and resample these faunas for possible future studies.
Stable Isotope Analyses
We performed carbon and oxygen stable isotope analyses on both the phosphate and structural carbonate fractions of bioapatite at the Stable Isotope Lab of the Institute of Earth Surface Dynamics, University of Lausanne (UNIL), Switzerland. For the analysis of the phosphate fraction (δ18OPO4), we pretreated at least 2 mg of each sample according to the procedure described in Koch et al. (Reference Koch, Tuross and Fogel1997), and we separated the PO43− ion from the apatite to precipitate it as silver phosphate (Kocsis Reference Kocsis2011; O'Neil et al. Reference O'Neil, Roe, Reinhard and Blake1994). We prepared in parallel the internationally accepted and frequently analyzed NBS-120c phosphorite reference material. A combination of two USGS standards (USGS 80: 12.50‰; and USGS 81: 34.71‰) and two in-house phosphate standards (LK-2L: 12.1‰; and LK-3L: 17.9‰) were used for data reduction. Note that the values were derived by analyzing the standards with a fluorination method (see Vennemann et al. Reference Vennemann, Fricke, Blake, O'Neil and Colman2002) and/or through calibration with other silver-phosphate standards with a high-temperature conversion elemental analyzer (TC/EA). When possible, we measured triplicates of each sample. The measurement was achieved through a TC/EA coupled to a Finningan MAT Delta Plus XL mass spectrometer (see Vennemann et al. Reference Vennemann, Fricke, Blake, O'Neil and Colman2002). The results are expressed in per mille and reported as δ18OPO4 on the Vienna Standard Mean Ocean Water (VSMOW) scale. The overall analytical error is considered to be 0.3‰; however, most samples often reproduced better, while some others present a higher standard deviation. The NBS-120c yielded an average value of 22.0 ± 0.2‰ (n = 6), slightly higher than the value of 21.7‰ generally considered (e.g., O'Neil et al. Reference O'Neil, Roe, Reinhard and Blake1994), but within the analytical error of the method.
The measurement of the carbon and oxygen isotope compositions in the carbonate fraction (δ13C and δ18OCO3) was achieved using a Gasbench II coupled to a Finnigan MAT Delta Plus XL mass spectrometer at UNIL. The equivalent of 10–15 μg of carbonate powder was weighed from each sample. For the normalization of the results, we used an in-house Carrara calcite marble standard calibrated against NBS-19. The δ13C values are expressed relative to the Vienna Pee Dee Belemnite (VPDB), while the δ18OCO3 values are expressed against the VSMOW scale. The analytical precision for this method was better than ±0.07‰ for C isotopes and ±0.09‰ for O isotopes.
Expected differences in the isotopic compositions between taxa and sites are used to evaluate the preservation state of the original isotopic composition of our material. Additionally, the sampling is designed to allow the comparison between tissues or ions of differential resistance to diagenesis (dentine vs. enamel/enameloid, respective carbonate vs. phosphate) and to help detect diagenetic trends.
REE Analyses
Selected samples were analyzed for trace element concentrations by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) using a Perkin Elmer ELAN DRC II ICP-MS mass spectrometer at the Institute of Earth Sciences at UNIL, according to the method detailed in Günther et al. (Reference Günther, Frischknecht, Heinrich and Kahlert1997). Fragments of fossil teeth and bone were embedded in resin and polished before measurements were made. Each sample was ablated at three to seven spots (diameter = 50–80 μm). Standard reference material (NIST612) was used for external standardization (Pearce et al. Reference Pearce, Perkins, Westgate, Gorton, Jackson, Neal and Chenery1997), while CaO was used as an internal standard with values of 54 and 50 wt.%, respectively, for enameloid and for dentine and bone (e.g., Kocsis et al. Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Yans and Amaghzaz2014). Though CaO content may vary within the remains with small deviation from these internal values, the data still allow relative comparison between samples in their normalized REE distributions and elemental ratios. The analytical reproducibility was generally better than ±5% SE.
We focused on elements that typically have an early diagenetic origin, such as REE and uranium (e.g., Trueman and Tuross Reference Trueman and Tuross2002) in order to test depositional/burial conditions. However, concentration of other elements such as Cu, Zn, Sr, and Ba are reported as well (see Supplementary Table 1). The data are separated based on the different histological parts such as enamel/enameloid, dentine, and in one case also bone. The REE data are reported normalized to Post Archean Australian Shale (PAAS); and the REE series is subdivided into light (LREE), middle (MREE), and heavy (HREE) elements as follows: LREE (La, Pr, Ce, Nd), MREE (Eu, Gd, Tb, Dy), and HREE (Er, Tm, Yb, Lu). MREE* is defined by the average of LREE and HREE.
Results
Oxygen Isotope in Phosphate (δ18OPO4)
The mosasaur tooth samples from Antarctica have an average δ18OPO4 value of 19.8 ± 0.9‰ for their enamel (n = 13) and 20.9 ± 1.5‰ for their dentine (n = 6). The two mosasaur teeth from Patagonia have an average δ18OPO4 value of 19.2 ± 0.9‰ and 20.6 ± 0.4‰ for their enamel and dentine tissues, respectively. Plesiosaur tooth enamel from Antarctica (n = 5) have δ18OPO4 values of 18.6 ± 1.6‰, while a single dentine sample resulted in a δ18OPO4 value of 19.7‰. The δ18OPO4 value of the bony fish tooth enameloid (n = 3) from Antarctica is 23.1 ± 0.1‰, and that of a single dentine sample is 22.6‰. Shark teeth from Patagonia have an average δ18OPO4 value of 22.0 ± 0.4‰ for their enameloid (n = 3) and 20.7 ± 1.8‰ for their dentine (n = 2).
Oxygen and Carbon Isotope Composition in Structural Carbonate (δ18OCO3 and δ13C)
Mosasaur tooth enamel from Antarctica (n = 13) has an average δ13C value of −11.3 ± 2.2‰ and a δ18OCO3 value of 26.9 ± 0.8‰, while dentine (n = 14) has a δ13C value of −6.0 ± 2.6‰ and a δ18OCO3 value of 27.5 ± 0.5‰. In one instance (ARG-2), we analyzed associated bone, which has δ13CCO3 and δ18OCO3 values of −14.0‰ and 30.3‰, respectively. Two mosasaur tooth enamel samples from Patagonia have respective mean δ13CCO3 and δ18OCO3 values of −11.1 ± 0.0‰ and 26.1 ± 0.7‰, while the dentine values are −3.2 ± 0.5‰ and 26.7 ± 0.4‰, respectively.
The plesiosaur tooth enamel (n = 5) from Antarctica yielded an average δ13CCO3 value of −11.8 ± 0.9‰ and a δ18OCO3 value of 26.9 ± 1.7‰. The plesiosaur dentine (n = 5) produced a δ13C value of −6.7 ± 1.1‰ and a δ18OCO3 value of 27.9 ± 1.1‰.
Bony fish tooth enameloid (n = 3) from Antarctica has an average δ13C of −4.8 ± 0.6‰ and δ18OCO3 of 29.4 ± 0.4‰, while their dentine (n = 3) yielded δ13C and δ18OCO3 values that averaged 3.5 ± 0.9‰ and 27.3 ± 0.2‰, respectively.
Finally, shark tooth enameloid (n = 4) has an average δ13C value of +7.2 ± 1.8‰ and a δ18OCO3 value of 25.7 ± 0.3‰, while the isotopic values of corresponding dentine (n = 4) are δ13C = −1.1 ± 0.4‰ and δ18OCO3 = 25.6 ± 0.2‰.
Trace Elements Composition
Six tooth specimens from Antarctica (three mosasaurs, two plesiosaurs, and one fish) and four from Patagonia (one mosasaur and three sharks) were measured for trace element concentrations (see Supplementary Table 1). For Antarctica, the total REE and U content varies between 1.3 and 3553 μg/g and 0.2 and 6.1 μg/g, respectively, except two remains that had exceptionally high total REE (>4000 μg/g): the enameloid of the bony fish (ARG-16) and part of the enamel of a mosasaur tooth (ARG-12A). All other Antarctic samples have REE content lower than 200 μg/g, but it must be mentioned that, in two cases (ARG-1 and ARG-4), the REE content of enamel was too low to obtain a consistent signal. The REE values in the Patagonian samples range from 27 to 6899 μg/g, while the U varies between 0.4 and 15.3 μg/g. The dentine of the mosasaur tooth (ARG-18) had the highest concentrations in our entire dataset.
A concentration offset between enamel and dentine is evident; however, it is not consistent, and in many cases enamel yielded higher total REE concentration than dentine, especially in the Antarctic sample set. Despite the large variation in REE concentration, the REE distributions within the same specimen fluctuate less, and the majority of the analyses yielded slight MREE enrichment or flat patterns, while others displayed slight HREE enrichment (Fig. 2). The LREE/HREE versus MREE/MREE* graph expresses variation among the samples. Most of the Antarctic fossils cluster together with a somewhat larger variation in their LREE/HREE ratios than the Patagonian samples, with the exception of the dentine and bone of a single Antarctic specimen (ARG-1 and 2) that plot away from the rest with relatively higher HREE content. In the Patagonian assemblage, the shark teeth and the mosasaur tooth show two discrete clusters, with more HREE enrichment in the shark teeth.
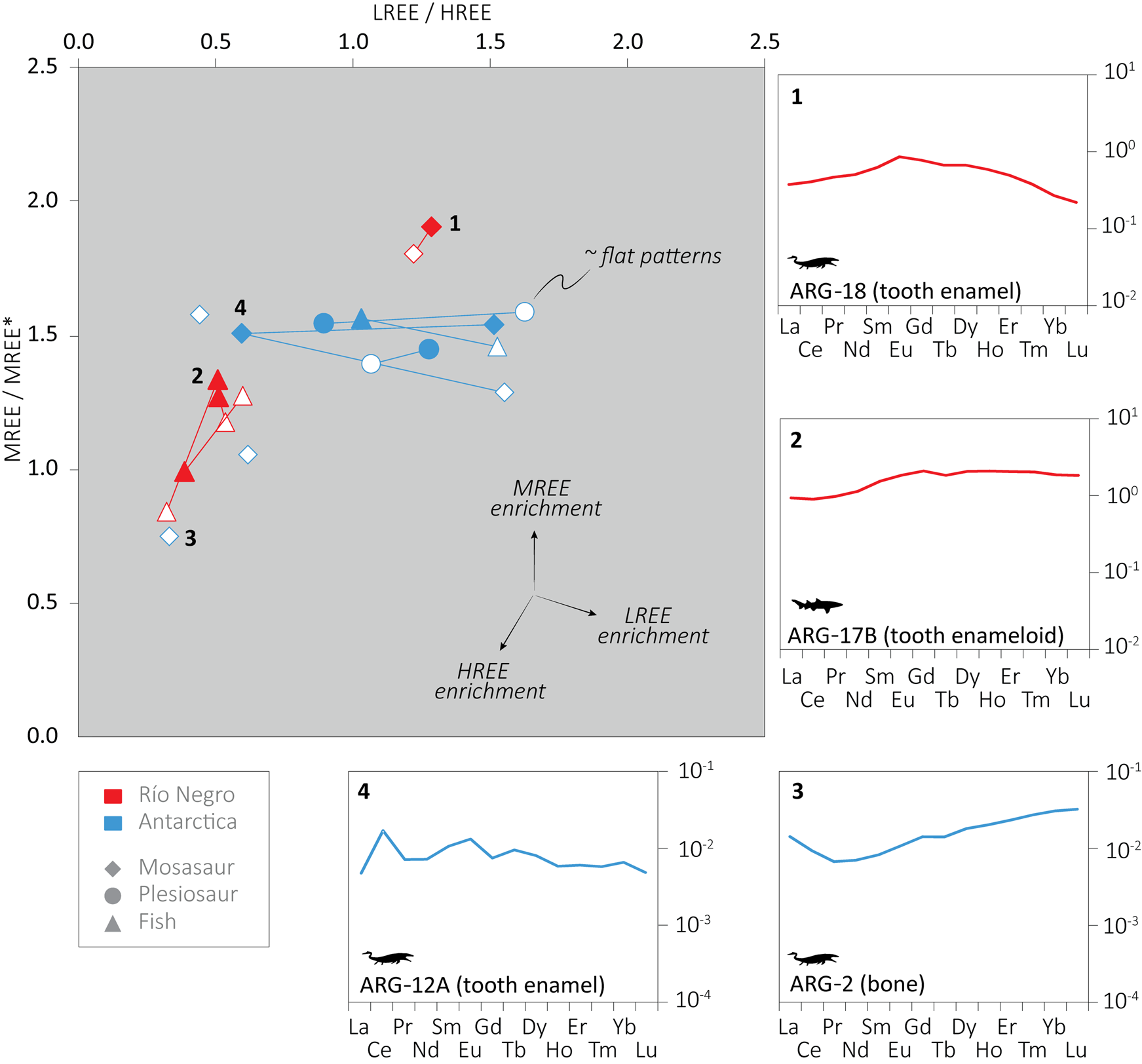
Figure 2. Post Archean Australian Shale (PAAS) shale-normalized rare earth element (REE) ratios and REE patterns. Light (LREE) = La, Pr, Ce, Nd; middle (MREE) = Eu, Gd, Tb, Dy; heavy (HREE) = Er, Tm, Yb, Lu; MREE* = average LREE+HREE; ![]() = mosasaur;
= mosasaur; ![]() = shark; open symbols = dentine; solid symbols = enamel(oid).
= shark; open symbols = dentine; solid symbols = enamel(oid).
Discussion
Preservation of the Isotopic Signature
Comparison of Isotopic Compositions between Tissues and Ions
The pristine state of the original isotopic signature of fossil material cannot be established with certainty (e.g., Kolodny et al. Reference Kolodny, Luz, Sander and Clemens1996; Fricke and Rogers Reference Fricke and Rogers2000; Pucéat et al. Reference Pucéat, Lécuyer, Sheppard, Dromart, Reboulet and Grandjean2003; Zazzo et al. Reference Zazzo, Lécuyer, Sheppard, Grandjean and Mariotti2004). However, a good evaluation of the preservation can be achieved in vertebrate remains by comparing the isotopic composition of tissues that react differently to alteration, due to differing microstructure and organic content. Fossils exposed to similar diagenetic conditions should have their densest, most mineralized hard parts (i.e., enamel/enameloid) less altered than more porous tissues such as dentine or bone. In case of an extensive diagenetic alteration, the isotopic composition of all tissues may be homogenized and almost entirely secondary, reflecting the diagenetic fluids rather than the ambient water or ingested food of the studied organism. A consistent offset between enamel(oid) and dentine or bone in all samples of a single site suggests that at least part of the original signal is preserved in the most resistant fraction (e.g., Zazzo et al. Reference Zazzo, Lécuyer, Sheppard, Grandjean and Mariotti2004; Amiot et al. Reference Amiot, Wang, Zhou, Wang, Buffetaut, Lécuyer, Ding, Fluteau, Hibino, Kusuhashi, Mo, Suteethorn, Wang, Xu and Zhang2011; Leuzinger et al. Reference Leuzinger, Kocsis, Billon-Bruyat, Spezzaferri and Vennemann2015). Such an offset also reveals whether the diagenetic alteration shifts the original isotopic composition toward lower or higher values, which helps to characterize the nature of the diagenetic fluids. For marine organisms, however, early diagenesis is often driven by marine pore fluids that might result in secondary isotopic values that differ only slightly—if at all—from those of the pristine material.
The phosphate ion is especially resistant to diagenetic alteration, and the δ18OPO4 values measured in enamel(oid) are particularly suited for paleoenvironmental studies (Iacumin et al. Reference Iacumin, Bocherens, Mariotti and Longinelli1996; Zazzo et al. Reference Zazzo, Lécuyer, Sheppard, Grandjean and Mariotti2004). Regarding the structural carbonate of bioapatite and its isotopic values (δ18OCO3 and δ13C) and related ecological applications, the oxygen isotope composition has a lower chance of being preserved compared with carbon. Isotopic exchange is indeed more likely to occur between the oxygen of the diagenetic fluids and the biomineralized tissues, simply because the fluids contain much more oxygen than carbon (Wang and Cerling Reference Wang and Cerling1994).
In our results, all enamel(oid)–dentine pairs of single teeth show an offset in both their δ18OPO4 and δ13C values (Table 1), as well as a clear difference between fish and marine reptiles, which we take as a good sign of enamel preservation. Overall, the enamel δ18OPO4 values are lower than their dentine counterparts in mosasaurs (by 1.6‰ on average, n = 8). This 18O enrichment of dentine was likely driven by alteration through pore water colder than the reptiles’ body temperature, resulting in higher δ18OPO4 values than the original ones (e.g., Lécuyer et al. Reference Lécuyer, Amiot, Touzeau and Trotter2013). On the other hand, fish teeth result in values higher in enameloid than in dentine (by 1.2‰ on average, n = 3); however, in two of the three enameloid–dentine pairs analyzed (ARG-16A and ARG-17C), this difference falls within the standard deviation. This could reflect the fact that marine diagenesis only generates small changes for organisms that have body temperature and body water δ18O values close to those of the ambient water.
As for the carbon isotope composition, there is a clear pattern of the tooth enamel(oid) δ13C values being lower than the dentine counterpart (by 5.6‰ on average in marine reptiles and 8.3‰ in bony fish) in all taxa but sharks, for which the enameloid δ13C values are higher than the dentine values by 8.3‰ on average (Fig. 3A). Especially high δ13C values for shark enameloid have already been observed (e.g., Vennemann et al. Reference Vennemann, Hegner, Cliff and Benz2001; van Baal et al. Reference Baal, van, Janssen, van der Lubbe, Schulp, Jagt and Vonhof2013; Kocsis et al. Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Yans and Amaghzaz2014), but have not yet been entirely explained (see “δ13C Values of Shark Enameloid: Driven by Biomineralization Rather Than Diet”). In the case of the other taxa, the higher δ13C values in dentine than in enamel(oid) point to a participation of dissolved inorganic carbon (DIC) from the diagenetic fluids that altered the original isotopic signature of the least-resistant tissues (i.e., dentine) and pushed their δ13C values toward more positive values. In the Patagonian material (again, except in sharks), dentine samples are merging toward common δ13C values (−3.6 to −0.7‰) that are likely close to the isotopic composition of the diagenetic fluids. We could not recognize any trend between the δ18OCO3 values of the different tissues, which suggest a certain degree of alteration.
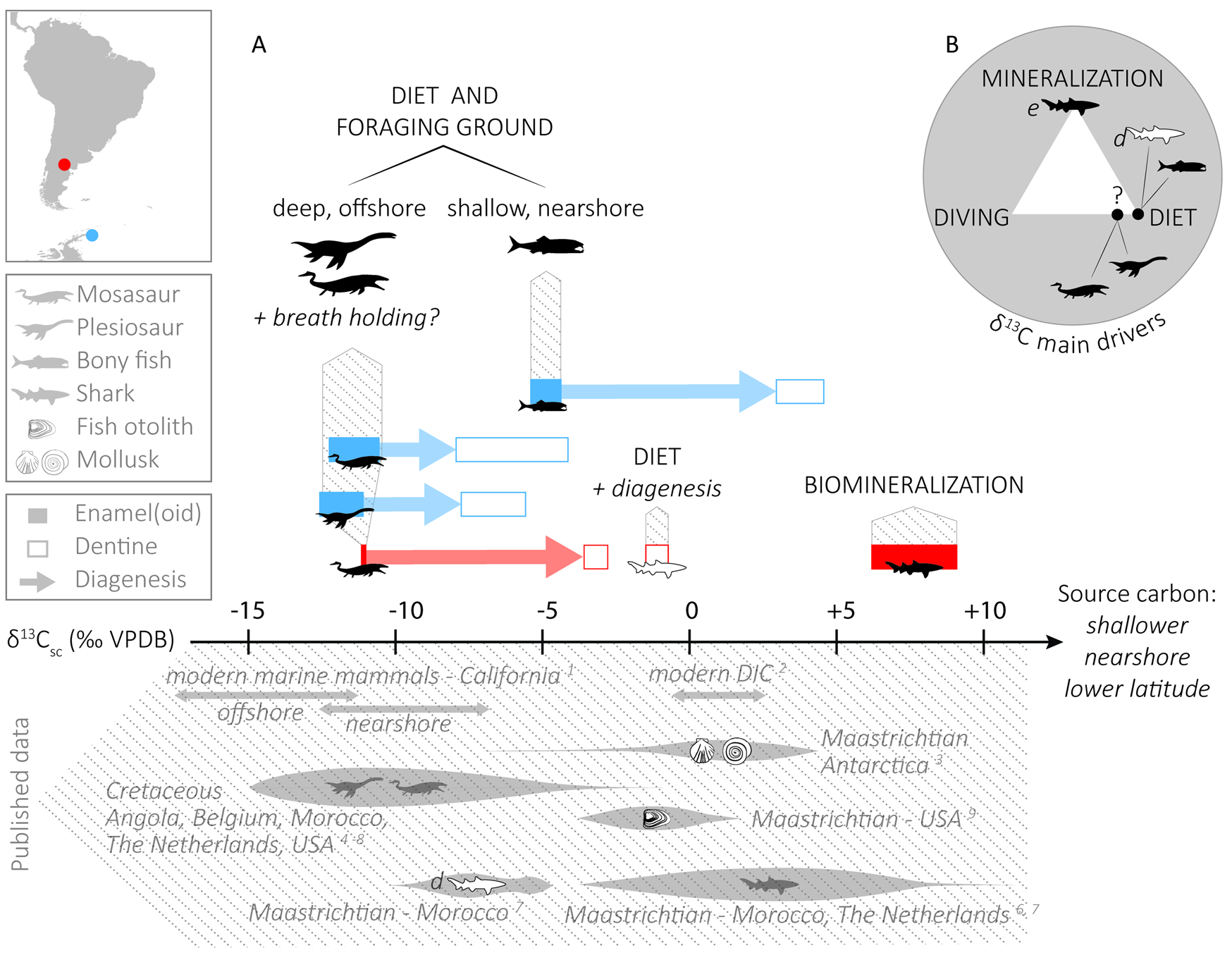
Figure 3. A, Top, δ13C ranges for each taxon of this study, and the main parameters that influence their isotopic composition. Diagenetic alteration pushes the δ13C of reptiles and bony fish toward higher values. For sharks, note that unlike dentine, enameloid is not linked to diet, therefore the offset between these tissues is no indicator of diagenetic alteration. Bottom, Literature δ13C data in carbonate from modern and Cretaceous organisms, as well as dissolved inorganic carbon (DIC) values. VPDB, Vienna Pee Dee Belemnite. B, Main drivers of δ13C compositions in pristine material. The “diet” extreme refers to the food source and foraging ground, and hence regroups DIC, latitude, distance from the shore, and depth, whereas “diving” refers to the physiological effect of breath holding. e, enamel(oid); d, dentine. References: 1, Clementz and Koch Reference Clementz and Koch2001; 2, Coplen et al. Reference Coplen, Hopple, Böhlke, Peiser, Rieder, Krouse, Rosman, Vocke, Révész, Lamberty, Taylor and De Bièvre2002; 3, Tobin and Ward Reference Tobin and Ward2015; 4, Robbins et al. Reference Robbins, Ferguson, Polcyn, Jacobs and Everhart2008; 5, Schulp et al. Reference Schulp, Vonhof, van der Lubbe, Janssen and van Baal2013; 6, van Baal et al. Reference Baal, van, Janssen, van der Lubbe, Schulp, Jagt and Vonhof2013; 7, Kocsis et al. Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Yans and Amaghzaz2014; 8, Strganac et al. Reference Strganac, Jacobs, Polcyn, Ferguson, Mateus, Olimpio Gonçalves, Morais and Tavares2015; 9, Carpenter et al. Reference Carpenter, Erickson and Holland2003.
Finally, the δ18O of extant homeothermic vertebrate bioapatite has been shown to present a systematic difference of 7‰ to 9‰ between its carbonate (δ18OCO3) and phosphate (δ18OPO4) fractions, resulting from a different fractionation factor between water and each of these ions (Bryant et al. Reference Bryant, Koch, Froelich, Showers and Genna1996; Iacumin et al. Reference Iacumin, Bocherens, Mariotti and Longinelli1996) at a constant body temperature. This offset has been repetitively used as a proxy for the preservation of the original isotopic signal (e.g., Zazzo et al. Reference Zazzo, Lécuyer, Sheppard, Grandjean and Mariotti2004; Lécuyer et al. Reference Lécuyer, Balter, Martineau, Fourel, Bernard, Amiot, Gardien, Otero, Legendre, Panczer, Simon and Martini2010; Rey et al. Reference Rey, Amiot, Fourel, Abdala, Fluteau, Jalil, Rubidge, Smith, Steyer, Viglietti, Wang and Lécuyer2017). In several instances, our marine reptile data show a smaller δ18OCO3-PO4 offset comprised between 6‰ and 7‰, further supporting a partial alteration of one of the fractions, most probably the carbonate. Unsurprisingly, all fish show a lower δ18OCO3-PO4 offset (down to 3‰), which is expected, given their poikilothermic–ectothermic nature.
δ13C Values of Shark Enameloid: Driven by Biomineralization Rather Than Diet
Although natural material usually has negative δ13C values (Kelly Reference Kelly2000), positive values of shark enameloid as reported here have readily been observed in other datasets (van Baal et al. Reference Baal, van, Janssen, van der Lubbe, Schulp, Jagt and Vonhof2013; Kocsis et al. Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Yans and Amaghzaz2014). Teeth of extant chondrichthyans also have enameloid with δ13C values typically 6‰ to 8‰ higher than dentine δ13C values (Vennemann et al. Reference Vennemann, Hegner, Cliff and Benz2001); the reason for this is so far unknown. A secondary, diffusional isotopic exchange with ambient water DIC was suggested (Vennemann et al. Reference Vennemann, Hegner, Cliff and Benz2001), which would be stronger in the part of the tooth directly exposed to seawater (i.e., mostly enameloid). However, some of our shark values are too high (up to 9.8‰) to result from this process alone; indeed, modern DIC in seawater usually does not exceed 3‰ (e.g., Böhm et al. Reference Böhm, Joachimski, Lehnert, Morgenroth, Kretschmer, Vacelet and Dullo1996; Coplen et al. Reference Coplen, Hopple, Böhlke, Peiser, Rieder, Krouse, Rosman, Vocke, Révész, Lamberty, Taylor and De Bièvre2002; Mackensen and Schmiedl Reference Mackensen and Schmiedl2019). The mode of enameloid formation in sharks may also play a role via a process of organic matter removal enhanced by enzymes at a late stage of enamel mineralization (Kocsis et al. Reference Kocsis, Vennemann, Ulianov and Brunnschweiler2015). A similar enzymatic process has been observed in mammals, with the involvement of the element zinc (e.g., Goettig et al. Reference Goettig, Magdolen and Brandstetter2010), and high Zn concentration was also reported in modern shark enameloid (Kocsis et al. Reference Kocsis, Vennemann, Ulianov and Brunnschweiler2015). Such an enzymatic reaction may result in carbon isotope fractionation between the newly formed dental tissue and the removed organic matter. Finally, it is noteworthy that shark tooth dentine and enameloid have different origins (odontoblastic vs. ameloblastic, respectively; Kemp Reference Kemp1985; Vennemann et al. Reference Vennemann, Hegner, Cliff and Benz2001).
Even though the mechanism behind high δ13C shark enameloid values has not yet been fully explained, such values are expected and considered to be a further sign of the good preservation of shark enameloid. Furthermore, alteration is hardly considered to be the trigger for high δ13C values, because diagenetic fluids and DIC would cause such high original δ13C values to be lowered. Here, we calculated an offset of 18.3‰ between shark and mosasaur enamel(oid) from Río Negro, comparable in magnitude to the value of around 17‰ reported by Schulp et al. (Reference Schulp, Vonhof, van der Lubbe, Janssen and van Baal2013). These authors, however, interpreted the shark enameloid δ13C values as a diet indicator, whereas we refrain from doing so. We argue that in the case of sharks, dentine is probably a better diet indicator, if unaltered, than enameloid. This also means that the δ13C offset between these tissues does not strictly reflect the direction of the diagenetic alteration (see Fig. 3A).
REE: Complex Diagenetic History
Bioapatite of modern teeth and bones has very low REE and U contents (<10 ng/g); however, fossils are often enriched severalfold (Trueman and Tuross Reference Trueman and Tuross2002). Therefore, these elements unambiguously represent a diagenetic signal that links to early depositional conditions (e.g., pore fluid chemistry). As these conditions can vary with time and also spatially, REE chemistry of fossil vertebrate remains is often used to assess paleoenvironmental conditions, highlight taphonomic processes, trace reworked specimens, or infer the provenance of specimens of unknown stratigraphic origin (e.g., Elderfield and Pagett Reference Elderfield and Pagett1986; Wright et al. Reference Wright, Schrader and Holser1987; Trueman and Benton Reference Trueman and Benton1997; Lécuyer et al. Reference Lécuyer, Reynard and Grandjean2004; Shields and Webb Reference Shields and Webb2004; Trueman et al. Reference Trueman, Behrensmeyer, Potts and Tuross2006; Tütken et al. Reference Tütken, Vennemann and Pfretzschner2008; Kocsis et al. Reference Kocsis, Ősi, Vennemann, Trueman and Palmer2009, Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Ulianov, Chiaradia and Bardet2016; Rogers et al. Reference Rogers, Fricke, Addona, Canavan, Dwyer, Harwood, Koenig, Murray, Thole and Williams2010; Suarez et al. Reference Suarez, Macpherson, González and Grandstaff2010). Ideally, when such applications are targeted, relatively thick bone samples should be tested for fractionation, as a separation of REE due to different diffusion rate related to different ion radii can occur (see Trueman et al. Reference Trueman, Kocsis, Palmer and Dewdney2011; Herwartz et al. Reference Herwartz, Tütken, Jochum and Sander2013), and a prolonged, late diagenetic overprint might also occur for older remains, which can alter early diagenetic signal (Kocsis et al. Reference Kocsis, Trueman and Palmer2010; Herwartz et al. Reference Herwartz, Tütken, Münker, Jochum, Stoll and Sander2011; Kowal-Linka et al. Reference Kowal-Linka, Jochum and Surmik2014).
Generally, higher REE content is expected in the more porous dentine and bone, which also have smaller crystal sizes and originally higher organic content than enamel/enameloid (Trueman and Tuross Reference Trueman and Tuross2002). Surprisingly, many of the Antarctic samples show an opposite trend, with some enamel samples being more than 700 or 1200 times more enriched than dentine. In contrast, some samples hardly yielded any REE in their enamel (ARG-1 and ARG-4). Considerable variations in the REE concentrations were also observed within a single sample, as in the mosasaur tooth enamel ARG-12A, with more than 2600 μg/g on one side (n = 2) and only 51 μg/g on the other (n = 2) (Supplementary Table 1). These observations indicate a complex diagenetic history in the Antarctic assemblage. Enamel with extremely high REE content compared with dentine may have sheltered the internal part of the teeth. Samples with low REE content in their enamel might have been embedded in rock that quickly cemented, blocking migration of pore fluid. One specimen (fragment with tooth and bone: ARG-1 and ARG-2) was indeed found embedded in strongly cemented sedimentary rock, and the different tissues yielded very low REE content (maximal total REE concentration = 19 μg/g, in dentine), supporting this interpretation. Although we observed some variation in the REE patterns of the Antarctic samples, most of them cluster together, which supports a common diagenetic history with similar REE source in the burial environment (see Fig. 2). The outliers correspond to the remains found embedded in the sedimentary rock (ARG-1 and ARG-2). Interestingly, REE patterns similar to those of these outliers (i.e., with HREE-enrichment) were reported by Patrick et al. (Reference Patrick, Martin, Parris and Grandstaff2004, Reference Patrick, Martin, Parris, Grandstaff, Martin and Parris2007) for mosasaurs thought to have suffered diagenesis in presence of oxic, open-marine water. The generally low REE content and the slight HREE enrichment of these samples may reflect fractionation along the REE series when compared with other patterns from Antarctica (Trueman et al. Reference Trueman, Kocsis, Palmer and Dewdney2011). A broader study with more samples is necessary to get more insight into the REE chemistry of these Antarctic remains.
Most of the Patagonian samples fit the expected differences between enamel/enameloid and dentine, except one shark tooth for which the enameloid is five times more enriched than the dentine. The REE composition of that tooth might also partially relate to the sheltering effect of enameloid on the internal part of the tooth. Interestingly, the shark teeth yielded somewhat different REE patterns from the mosasaur tooth (see Fig. 2), which indicates that these remains probably originated from different beds with different REE composition in the burial fluid. The MREE-enriched mosasaur tooth might indicate somewhat lower redox conditions than the shark teeth in their respective depositional beds (e.g., Ounis et al. Reference Ounis, Kocsis, Chaabani and Pfeifer2008). Still, the observed differences possibly link to a broader range of variation in REE chemistry within the Jagüel Formation, but the few samples obtained do not allow decipherment of spatial and temporal details.
δ13C, Diet Source, and Feeding Ground
The stable carbon isotope composition of vertebrate bioapatite mainly derives from ingested food (except in chondrichthyan enameloid as detailed in “δ13C Values of Shark Enameloid: Driven by Biomineralization Rather Than Diet”), and it is therefore commonly used to study diet (e.g., DeNiro and Epstein Reference DeNiro and Epstein1978; Hobson and Welch Reference Hobson and Welch1992; Bocherens et al. Reference Bocherens, Fizet and Mariotti1994). In the case of marine vertebrate macrofauna, however, the bioapatite carbon isotope composition serves as a general indicator of the source diet rather than giving clues on high-resolution trophic relations as stable calcium isotopes would (Rau et al. Reference Rau, Mearns, Young, Olson, Schafer and Kaplan1983; Wada et al. Reference Wada, Terazaki, Kabaya and Nemoto1987; Fry Reference Fry1988; Hobson and Welch Reference Hobson and Welch1992; Hobson Reference Hobson1993; Hobson et al. Reference Hobson, Piatt and Pitocchelli1994; Kelly Reference Kelly2000; Clementz et al. Reference Clementz, Holden and Koch2003). In marine organisms, the δ13C values ultimately reflect the composition of the DIC or the particulate organic matter (POM) assimilated by the organisms at the very base of the trophic chain, with metabolic effects superimposed.
Both the δ13CDIC and the δ13CPOM decrease with increasing latitude, distance from the shore, and depth (Fry Reference Fry1988; Hobson Reference Hobson1993; Goericke and Fry Reference Goericke and Fry1994; Hobson et al. Reference Hobson, Piatt and Pitocchelli1994; Marchal et al. Reference Marchal, Stocker and Joos1998; Clementz and Koch Reference Clementz and Koch2001; Hofmann and Heesch Reference Hofmann and Heesch2018), meaning that the δ13C composition of marine organisms strongly depends on their foraging ground. In our context, we would thus expect a given taxon from the high-paleolatitude site (Antarctica, 64°S) to have lower δ13CCO3 values than those from the medium-paleolatitude site (Patagonia, 45°S). Furthermore, anatomic and isotopic evidence suggests that some mosasaur taxa were divers and had deeper foraging grounds (Sheldon Reference Sheldon, Callaway and Nicholls1997; Schulp et al. Reference Schulp, Vonhof, van der Lubbe, Janssen and van Baal2013, Reference Schulp, Janssen, van Baal, Jagt, Mulder and Vonhof2017; Harrell et al. Reference Harrell, Pérez-Huerta and Suarez2016). A deeper and offshore food source would cause their bioapatite to have lower δ13C values than coexisting shallow-water, nearshore organisms. Clementz and Koch (Reference Clementz and Koch2001) compared the carbon isotope composition of the tooth apatite of marine mammals from California with different foraging grounds and reported δ13C values between −14‰ and −12‰ for pinnipeds feeding offshore, while nearshore cetaceans had values between −11‰ and −9‰, showing that differences in the foraging ground can induce an offset of ~5‰. Our results show a very clear difference between marine reptiles and bony fish, the former having significantly lower δ13C values (−11.2‰ and −11.8‰ for mosasaurs and plesiosaurs, respectively, compared with −4.8‰ for bony fish), supporting more offshore and/or deeper food sources for reptiles. We obtained similar δ13C values for all mosasaurs and plesiosaurs (average −11.4‰, n = 20), independent from their latitude of provenance, with the mosasaurs showing a wider scatter, possibly partly explained by a larger and taxonomically more diverse sample set (15 mosasaur samples vs. 5 plesiosaur samples). While the average δ13C composition is similar between mosasaurs from Patagonia (−11.1‰, n = 2) and Antarctica (−11.3‰, n = 13), we observed more variation in the Antarctic material (2.2‰ SD), with some samples clearly standing out (ARG-15 = −15.4‰, ARG-10 = −6.8‰). Again, this is most likely driven by the larger sample size and higher taxonomic variety, and hence potentially different feeding habits and ecological niches. However, a limited contribution of dentine in the enamel samples during sampling may also explain the few higher δ13C values when the dentine values are also especially high, as in sample ARG-10 (Fig. 3). The bony fish teeth have clearly higher δ13C compositions (average −4.8‰), suggesting that these fish had a foraging ground shallower and closer to the shore than the marine reptiles. Literature δ13C data are scarce when it comes to the inorganic fraction of Late Cretaceous marine fish teeth, but a study on Maastrichtian fish otoliths presented isotopic values several per mille higher than those measured in our tooth material (Carpenter et al. Reference Carpenter, Erickson and Holland2003; see Fig. 3A). Otoliths are known to reflect the composition of seawater (i.e., DIC; Degens et al. Reference Degens, Deuser and Haedrich1969; Patterson Reference Patterson1999), which is isotopically heavier than the dietary carbon source from which other biomineralized tissues such as teeth and bones derive. As a further comparison, shelled mollusks from the same formation and site as our Antarctic material (LBF, Isla Marambio) have on average comparatively 13C-enriched average values (−1.9‰ for ammonites and +1.9‰ for benthic bivalves; Tobin and Ward Reference Tobin and Ward2015; see Fig. 3A). Higher values are expected in shells, because their δ13C strongly correlates with that of water DIC (McConnaughey and McRoy Reference McConnaughey and McRoy1979; Poulain et al. Reference Poulain, Lorrain, Mas, Gillikin, Dehairs, Robert and Paulet2010).
Diving Behavior and Isotopic Signature
A deep diving behavior has been proposed for some mosasaurs based on osteological observations (Sheldon Reference Sheldon, Callaway and Nicholls1997; Yamashita et al. Reference Yamashita, Konishi and Sato2012) and their low δ13C values compared with non-diving taxa such as sharks (Schulp et al. Reference Schulp, Vonhof, van der Lubbe, Janssen and van Baal2013). A study on extant sea turtles (leatherback and olive ridley turtles; Biasatti Reference Biasatti2004) suggests that breath holding during diving could deplete 13C in body fluids. This depletion would be reflected in low bioapatite δ13C values—closer to the food source composition—in sea turtles that spend a large part of their life diving (dives up to 80 minutes long for the leatherback turtle [López-Mendilaharsu et al. Reference López-Mendilaharsu, Rocha, Domingo, Wallace and Miller2009] and up to 200 minutes long for the olive ridley turtle [McMahon et al. Reference McMahon, Bradshaw and Hays2007]). Although we do not challenge the hypothesis that some mosasaur species were deep divers and hence held their breath for prolonged periods of time, we are cautious regarding the actual driver of their low δ13C values and question whether these are induced by breath holding itself.
Schulp et al. (Reference Schulp, Vonhof, van der Lubbe, Janssen and van Baal2013) mainly base their conclusions on a large offset (17‰) between the δ13C values of mosasaur enamel and shark enameloid, which they assume to result from contrasting respiratory physiologies. However, we have already mentioned that shark enameloid is known to have especially high δ13C values compared with other taxa, a peculiarity likely linked to biomineralization rather than ecology (see discussion in “δ13C Values of Shark Enameloid: Driven by Biomineralization Rather Than Diet”). Because sharks apparently do not record environmental parameters in their tooth enameloid δ13C values, a comparison with mosasaur enamel to draw ecological conclusions is misleading. We also observe a striking difference between mosasaur and shark enamel(oid) (18.4‰) in our material, but interestingly, this offset diminishes dramatically when mosasaurs are compared with another non-diving taxon, namely bony fish (6.4‰). In that case, because enamel(oid) of both bony fish and mosasaur is thought to reflect environmental parameters, part of this offset can be attributed to different respiratory physiologies: dissolved CO2 in the water respired by gill-breathing vertebrates is 13C-enriched compared with atmospheric CO2 respired by pulmonate vertebrates (Biasatti Reference Biasatti2004), which is reflected in their tissues. However, the largest part of the offset is likely explained by different foraging grounds, as revealed in the previous section; that is, an offshore, deeper food source for mosasaurs than for the bony fish investigated here. Clementz and Koch (Reference Clementz and Koch2001) indeed reported similarly low δ13C values for two pulmonate and diving taxa, both considered offshore dwellers (northern elephant seal and northern fur seal) but strongly differing in the duration of their dives (up to 2 hours for the former, and in the minute range for the latter; Costa Reference Costa2007; Kuhn et al. Reference Kuhn, Tremblay, Ream and Gelatt2010). This suggests that breath holding is not the main driver of low δ13C values, even if it might contribute to them.
To sum up, the food source of diving animals (deeper water, hence isotopically lighter POM and/or DIC reflected in the prey items) is a major driver of their δ13C values, more than the process of breath holding itself, and might well fully explain the low δ13C values measured in plesiosaurs and mosasaurs (Fig. 3B).
δ18OPO4: Water Temperature, Thermoregulation, and Latitudinal Temperature Gradient
The δ18O values of vertebrate bioapatite depend on the isotopic composition of the body water from which it precipitated, as well as on the temperature during mineral formation (i.e., body temperature). Two parameters that have a combined effect on the ambient and/or body temperature are (1) the latitude generally representing the distribution of solar heat on the Earth's surface, which could somewhat be modified by oceanic currents in the marine realm; and (2) whether an animal is thermoconformer (i.e., body temperature is linked to external, ambient conditions) or thermoregulator (i.e., using external or internal heat to increase body temperature; Withers Reference Withers1992). There is strong evidence suggesting that mosasaurs and plesiosaurs could produce metabolic heat (endothermy) and maintain a constant body temperature (homeothermy) independent from the ambient temperature, and thus from the latitude at which they lived (Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010; Harrell et al. Reference Harrell, Pérez-Huerta and Suarez2016). For these taxa, variations in the δ18OPO4 values between samples from different paleolatitudes should thus be limited to—and mainly driven by—the δ18Obody water values, which can vary with the isotopic composition of the ambient water, as well as with physiological processes that can respond to body mass (e.g., Tejada-Lara et al. Reference Tejada-Lara, MacFadden, Bermudez, Rojas, Salas-Gismondi and Flynn2018; Villamarín et al. Reference Villamarín, Jardine, Bunn, Marioni and Magnusson2018). In contrast, both the δ18Obody water values and the body temperature of aquatic, thermoconforming animals (e.g., ectotherms) such as most fish are similar to that of the surrounding water, meaning that their δ18OPO4 values are closely related to latitudinal thermal variations. Fish bioapatite mineralizes in isotopic equilibrium with the ambient water, and according to water–phosphate fractionation equations (Kolodny et al. Reference Kolodny, Luz and Navon1983; Lécuyer et al. Reference Lécuyer, Amiot, Touzeau and Trotter2013), δ18OPO4 values increase with decreasing precipitation temperature. The assumption of high-latitude Antarctic fish δ18OPO4 values being higher than those of Patagonian fish is supported by our data (Δ18OAnt-Pat = 1.1‰; Fig.4).
Meanwhile, the δ18OPO4 values of homeothermic–endothermic marine reptiles are more stable across latitudes, as expected. Additionally, the ambient water temperature at our study sites was most likely lower than mosasaur and plesiosaur body temperatures (e.g., Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010), which is reflected in reptiles’ δ18OPO4 values being overall lower than those of fish (Fig. 4).
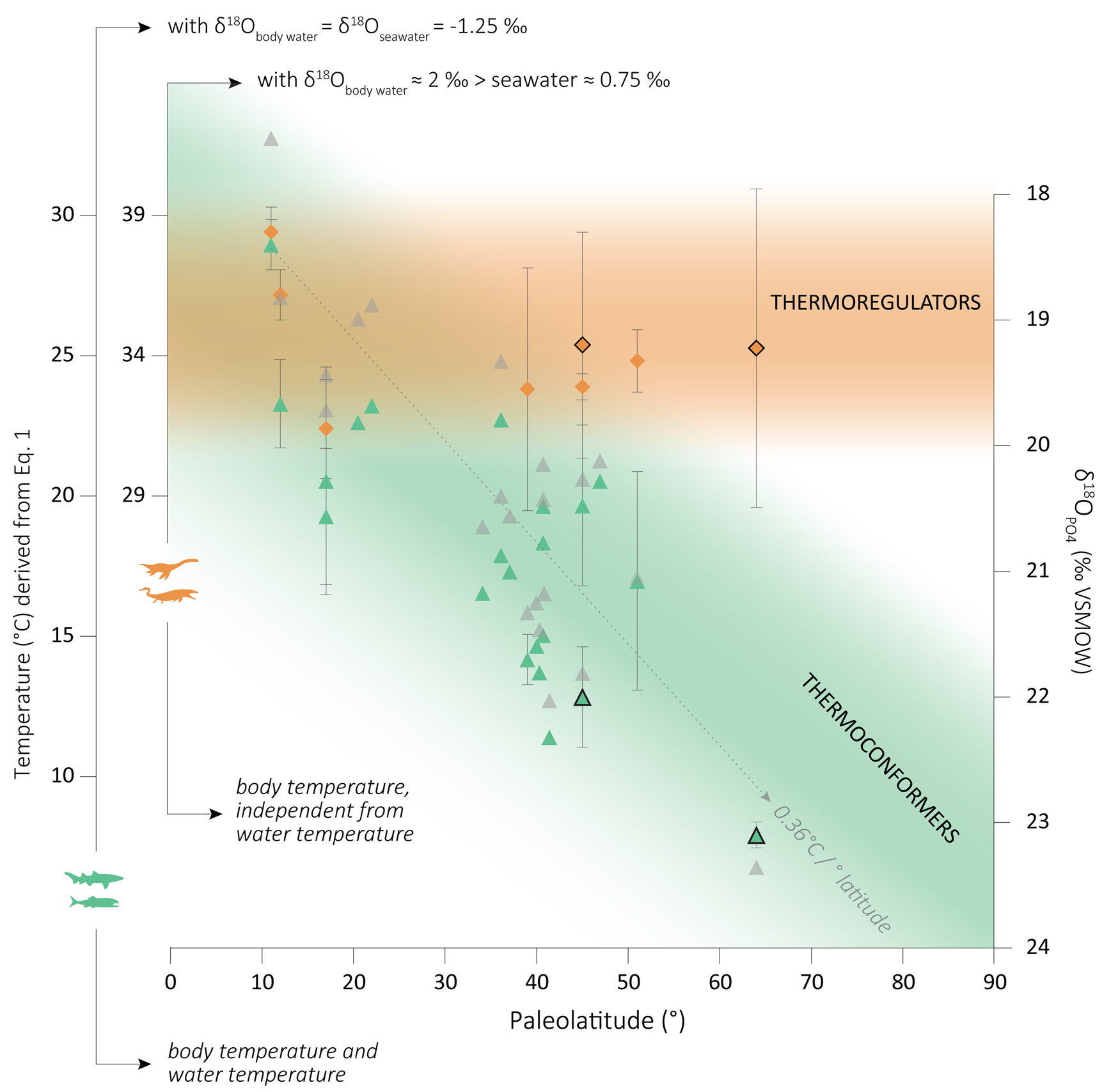
Figure 4. Paleolatitudinal distribution of Campanian–Maastrichtian marine vertebrate δ18OPO4 data from this study (colored closed symbols) and from the literature (colored open symbols), and respective body temperature according to eq. 1, using different δ18Owater values (see main text). Green symbols: thermoconforming bony fish and sharks; orange symbols: thermoregulating mosasaurs and plesiosaurs; gray symbols: water temperatures derived from fish δ18OPO4 values after a latitudinal correction of δ18Oseawater following Zachos et al. (Reference Zachos, Stott and Lohmann1994), and resulting thermal gradient. Note that the gray data points do not correlate with the δ18OPO4 y-axis, because the latitudinal adjustment is nonlinear. Literature data from Pucéat et al. (Reference Pucéat, Lécuyer, Donnadieu, Naveau, Cappetta, Ramstein, Huber and Kriwet2007), Bernard et al. (Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010), and Kocsis et al. (Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Yans and Amaghzaz2014). VSMOW, Vienna Standard Mean Ocean Water.
An estimation of the water temperature can be derived from fish δ18OPO4 values using the Lécuyer et al. (Reference Lécuyer, Amiot, Touzeau and Trotter2013) fractionation equation defined as:
where T is the temperature in degrees Celsius and δ is expressed in ‰ against the VSMOW scale.
Considering a global δ18Oseawater of −1.25‰ (as in Pucéat et al. [2007], for the sake of comparison), that is, lower than today's average due to the absence of a permanent ice cap during the Late Cretaceous (Shackleton and Kennett Reference Shackleton and Kennett1976), our fish enameloid material results in a water temperature of 12.7 ± 1.9°C for the Patagonian site (45°S) and 7.9 ± 0.5°C for the Antarctic (64°S) site. In Figure 4, we have plotted published water temperatures derived from fish δ18OPO4, with a latitudinal correction of the δ18Owater following Zachos et al. (Reference Zachos, Stott and Lohmann1994: Supplementary Table 2). For our material, we obtain slightly different temperatures after that correction: 13.7°C and 6.8°C for our Patagonian and Antarctic sites, respectively (see Fig. 4).
When compared with published values, higher temperatures have been reported by some authors for 40°–50° paleolatitudes based on fish bioapatite δ18OPO4 (e.g., Pucéat et al. Reference Pucéat, Lécuyer, Donnadieu, Naveau, Cappetta, Ramstein, Huber and Kriwet2007) and other proxies such as TEX86 (Woelders et al. Reference Woelders, Vellekoop, Kroon, Smit, Casadío, Prámparo, Dinarès-Turell, Peterse, Sluijs, Lenaerts and Speijer2017). On the other hand, our Patagonian data follow a latitudinal trend when compared with other fish δ18OPO4 data from slightly higher paleolatitudes (Fig. 4). Regarding our Antarctic water temperatures, they are fall within the ~5°C–14°C range calculated based on δ18O values for Maastrichtian cephalopods and shelled mollusks from the same area as our material, adjusted to a δ18Oseawater of −1.25‰ (Ditchfield et al. Reference Ditchfield, Marshall and Pirrie1994; Li and Keller Reference Li and Keller1999; Dutton et al. Reference Dutton, Huber, Lohmann and Zinsmeister2007; Tobin et al. Reference Tobin, Ward, Steig, Olivero, Hilburn, Mitchell, Diamond, Raub and Kirschvink2012; Woelders et al. Reference Woelders, Vellekoop, Kroon, Smit, Casadío, Prámparo, Dinarès-Turell, Peterse, Sluijs, Lenaerts and Speijer2017). To our knowledge, no other water temperatures have been reported for high latitudes based on fish δ18OPO4.
As for the body temperature of marine reptiles, the water–phosphate fractionation equation (eq. 1) cannot be directly transferred to endotherms, because it was defined for thermoconforming organisms, and unknown and/or unquantified vital effects might drive the bioapatite mineralization out of equilibrium. Nevertheless, some authors (e.g., Séon et al. Reference Séon, Amiot, Martin, Young, Middleton, Fourel, Picot, Valentin and Lécuyer2020) have used it as an approximation for marine reptile body temperature, considering an 18O enrichment of about 2‰ of the δ18Obody water relative to ambient water, that is, similar to that observed in extant aquatic pulmonate vertebrates (see Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010). Using the same criteria, we obtain elevated body temperatures (roughly between 30°C and 40°C) for both our middle- and high-latitude reptiles (Fig. 4). We do not aim here to define the absolute body temperatures of mosasaurs and plesiosaurs, but these estimates illustrate well the order of magnitude in terms of body temperature differences between thermoconformers and thermoregulators (here endotherms) with increasing latitude.
These new high-latitude δ18OPO4 values add a missing piece to the latitudinal gradient of fish bioapatite for the Cretaceous, which so far did not exceed 52° paleolatitude (Pucéat et al. Reference Pucéat, Lécuyer, Donnadieu, Naveau, Cappetta, Ramstein, Huber and Kriwet2007; Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010). Zhang et al. (Reference Zhang, Hay, Wang and Gu2019) proposed a global gradient of 0.41°C/1° of latitude for the latest Cretaceous based on δ18OPO4 values of fish remains (otoliths and teeth) and foraminifera, as well as the TEX86 paleotemperature proxy, after correcting for different biases such as latitude and seasonality. These authors highlighted two points of inflection on their latitudinal gradient (at 35° and 52°, respectively), resulting in a steeper middle-latitude gradient and comparatively shallower low- and high-latitude gradients. These changes in the gradient slope are interpreted as reflecting the formation of subtropical and polar fronts and an evolution toward a more stratified ocean. Based on δ18OPO4 fish data, Pucéat et al. (Reference Pucéat, Lécuyer, Donnadieu, Naveau, Cappetta, Ramstein, Huber and Kriwet2007) described the latest Cretaceous marine gradient as globally similar to the present gradient (around 0.4°C/1° of latitude) between 10° and 50° paleolatitude. These authors apply a latitudinal adjustment of the δ18Oseawater following the correction of Zachos et al. (Reference Zachos, Stott and Lohmann1994) established for Cenozoic times between 0° and 70° latitude. This nonlinear correction results in changes in the gradient slope similar to the model of Zhang et al. (Reference Zhang, Hay, Wang and Gu2019), that is, a steeper slope at the middle portion. Applying the same latitudinal correction to our data, we obtain a similar latitudinal gradient of 0.36°C/1° of latitude (Fig. 4).
The combination of our new high-latitude fish δ18OPO4 values with the Campanian–Maastrichtian literature fish data supports the global gradients established to date (around 0.4°C/1° of latitude). This reiterates the potential of fish bioapatite as a paleotemperature proxy as reliable as foraminifers and bivalves. This global gradient possibly reflects the combination of shallower low- and high-latitude gradients with a steeper middle-latitude gradient as proposed by Zhang et al. (Reference Zhang, Hay, Wang and Gu2019) and already suggested by Pucéat et al. (Reference Pucéat, Lécuyer, Donnadieu, Naveau, Cappetta, Ramstein, Huber and Kriwet2007). The water temperatures (around 8°C) calculated from our fish δ18OPO4 values for a 64° paleolatitude are slightly above modern sea-surface temperatures for similar latitude (LEVITUS 1994), even considering the entire range of seasonal variations, which aligns well with the model of a world devoid of long-lasting ice caps on the poles during the latest Maastrichtian. As for marine reptiles, the highest paleolatitude δ18OPO4 data published to date came from 52°S (Australia; Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010). These new mosasaur and plesiosaur high-latitude values further support endothermic and probably also homeothermic thermoregulation and relatively stable δ18OPO4 values across latitudes and allow us to considerably extend the current database for marine reptiles by 12° to the south.
Conclusions
The fossil material analyzed here shows well-preserved δ13C and δ18OPO4 values that reflect differences between taxa in their ecology and/or latitudinal provenance. Marine reptile (mosasaurs and plesiosaurs) δ13C values suggest deeper, more-offshore foraging grounds than associated fish. We suggest that an offshore, deep, 13C-depleted food source is the main driver of their globally low δ13C values and that a major influence of breath holding on isotopic fractionation related to diving, as previously proposed, is secondary. We also emphasize that δ13C values of shark enameloid should not be compared with those of other taxa in ecological studies, as they are most likely unrelated to diet or behavior.
The δ18OPO4 values of marine reptiles do not correlate with paleolatitude, suggesting an elevated body temperature (i.e., thermoregulation), whereas coexisting bony fish and sharks show expected latitudinal variations in their δ18OPO4. Based on fish δ18OPO4, we could calculate a seawater temperature around 7°C at 64°S during the latest Maastrichtian, just before the K/Pg extinction event; that is, slightly above present-day values for similar latitudes worldwide and consistent with a world devoid of permanent polar ice caps. These new high-latitude δ18OPO4 values add an important missing piece to the global database of marine vertebrate bioapatite, so far limited to 52° paleolatitude. The high-latitude fish δ18OPO4 values in particular further support a latitudinal gradient around 0.4°C/1° of latitude for the latest Cretaceous as published elsewhere based on fish bioapatite, bivalves, and foraminifera.
Acknowledgments
Partial financial support for this study was provided by the Agencia Nacional de Investigaciones Científicas y Tecnológicas ANPCyT-PICT-2016-1039. Thank you to M. Reguero (Museo de La Plata, Argentina) and D. Cabaza (Museo de Lamarque, Argentina) for the authorization to use fossil samples, and to the Instituto Antártido Argentino (IAA) for logistic support during field trips to Antarctica. We are grateful for the constructive comments of A. Schulp and an anonymous review that helped improving our work.
Data Availability Statement
Data available from the FigShare Digital Repository: https://doi.org/10.6084/m9.figshare.21340176.








