Mycotoxins are some of the most frequent natural contaminants of cereals. A list of mycotoxins which are of concern for the safety of animal feed in the European Union (EU32/2003) has been already published. For instance, Fusarium culmorum and Fusarium graminearum are ubiquitous species of the Fusarium genus that can release a large array of mycotoxins: fumonisins, toxin T-2, toxin HT-2, ochratoxin A, deoxynivalenol (DON), zearalenone (ZEA), etc.(Reference Smith, McMillan and Castillo1). DON and ZEA have a particular importance with their common co-occurrence, high frequency (particularly in the temperate climate) and very high toxic effect. In feed, high doses of DON and ZEA (higher than 3 and 1 parts per million (ppm), respectively) provoked effects such as diarrhoea, vomiting, feed refusal, reduced weight, reproductive disorders and ultimately death. Chronic low-dose toxicity is characterised by anorexia, reduced weight gain, nutrient malabsorption, neuroendocrine changes and immunological effects(2, Reference Pestka and Smolinski3).
Despite the intense efforts of prevention, mycotoxin contamination of feed occurs in the field(Reference Lillehoj, Diener, Asquith and Dickens4). Therefore, various detoxification strategies have been developed over time. These procedures/methods can be grouped into three categories: physical, chemical and biological(Reference Bhatnagar, Lillehoj and Bennett5). Dietary strategies, including biological mitigating agents, are some of the most recent approaches in reducing the adverse effects of mycotoxins. The extraordinary development in this area has been lately helped by recent advances in molecular biology, genetic engineering and microbial genomics(Reference Kabak, Dobson and Işl6–Reference Karlovsky8). A large number of studies have investigated the ability of several binding dietary agents (activated carbons, hydrated sodium calcium aluminosilicate, bentonite and zeolites) in reducing the negative effects of mycotoxins in feed(Reference Avantaggiato, Havenaar and Visconti9–Reference Döll, Gericke and Dänicke11). While this research demonstrated good results against aflatoxins (especially aluminosilicate), their efficacy to bind other toxins, or more than one mycotoxins in case of co-contamination, must be confirmed. Some of the available mycotoxin binders failed in sequestering the in vitro/in vivo Fusarium mycotoxins in pigs, for example(12). Recently, there has been an increasing interest in the use of bacteria, yeast and fungi as binders to successfully reduce the toxic effect of mycotoxins(13). It has also been shown that substances which do not interact with mycotoxins, i.e. several plant extracts and spice oils, antioxidant compounds (Se, vitamins, provitamins) and feed/food components (phenolic compounds, coumarin, chlorophyll and its derivatives, fructose, aspartame), are efficient in counteracting the toxic effect of contaminants, but, again, available data are provided from the in vitro studies, and especially focus on aflatoxin B1(Reference Galvano, Piva and Ritieni14). It is clear that much more research must be conducted to identify, especially in vivo, the detoxification ability of the aforementioned control agents, their mode of action, and their economical and technical feasibility.
Therefore, the objectives of the present study were to evaluate in vivo the ability of a nutritional complex compound (a protein concentrate additive, enriched in calcium fructoborate (CFrB), a natural ester of boric acid with fructose) in counteracting the toxic effect of feeding a diet containing naturally contaminated maize with low levels of Fusarium toxins (DON as the major contaminant and ZEA, fumonisin and HT-2 toxin as the minor components) in starter pigs. As a great consumer of cereals, the pig is one of a broad range of farm animals frequently exposed to mycotoxins. It is also considered one of the most susceptible mammalian species to mycotoxins, and other contaminants, especially during the weaning period, in which changes in nutrition and regrouping are significant and stressing(Reference Pié, Lallès and Blazy15). Intake of mycotoxin-contaminated feed in this period could lead to decreases in animal growth performance and reproduction, and an increase in susceptibility to infectious diseases. In the present study, the effect of daily low doses of Fusarium mycotoxins and of an organic additive on production parameters, organ weights, plasma biochemistry and the immune response of peripheral blood cells was investigated. We hypothesise that the use of nutritional complex compounds with polyvalent properties might be promising alternatives in reducing mycotoxin effects.
Materials and methods
Experimental animals and diets
A total of sixteen cross-bred starter piglets, with an average body weight of 11·00 (sem 0·58) kg, were identified by ear tags and housed in floored indoor pens. Piglets were divided into four experimental groups (four piglets/group), and were randomly assigned to one of the four treatments: M − CFrB − (diet without mycotoxin and without CFrB additive, control); M − CFrB+ (diet without mycotoxin, with CFrB additive); M+CFrB − (mycotoxin-contaminated diet without CFrB additive); M+CFrB+ (mycotoxin-contaminated diet with CFrB additive) for 24 d. The control diet was formulated to meet all nutritional requirements of 8–30 kg starter pigs (National Research Council, 1988), with a total maize content of 53·31 g/kg (Table 1). The mycotoxin-contaminated diet was prepared by replacing ‘uncontaminated’ control maize with Fusarium mycotoxin-contaminated maize. The contaminated diet contained 1·79 (sem 0·56) mg Fusarium toxins/kg compound feed, whereas the control diet contained 0·427 (sem 0·15) mg Fusarium toxins/kg compound feed, which was considered negligible taking into consideration the literature data and EC recommendation 576/2006(16).
Table 1 Composition of experimental diets (%)

ME, metabolisable energy; DON, deoxynivalenol; ZEA, zearalenone; FB, fumonisin.
* Vitamin–mineral premix/kg diet: 0–24 d: 10 000 IU vitamin A; 2000 IU vitamin D; 30 IU vitamin E; 2 mg vitamin K; 1·96 mg vitamin B1; 3·84 mg vitamin B2; 14·85 mg pantothenic acid; 19·2 mg nicotinic acid; 2·94 mg vitamin B6; 0·98 mg folic acid; 0·03 mg vitamin B12; 0·06 mg biotin; 24·5 mg vitamin C; 40·3 mg Mn; 100 mg Fe; 100 mg Cu; 100 mg Zn; 0·38 mg I; 0·23 mg Se.
† Calculated.
In order to test the capacity of a mitigating additive in reducing the possible negative effects of a mycotoxin-contaminated diet, a protein concentrate additive enriched in CFrB was added daily in the contaminated diet of animals according to the manufacturer's instructions (1·65 g/pig). CFrB (FruiteX B®) was purchased from the FutureCeuticals Company (San Diego, CA, USA) – synthetic product (Miljkovic, US Patent 5,962,049 (1998)), and was identified as Ca [(C6H10O6)2B]2.4H2O, an ester of boric acid with fructose similar to the natural forms of boron found in edible plants(Reference Rotaru, Scorei and Harabor17). Feed samples were taken at the beginning of the experiment, and were analysed for Fusarium mycotoxins and nutrient content; DM, crude protein, fat, crude fibre and ash were estimated according to the International Standards Organisation methods(18–22) (Table 1). The respective treatments were administered for 24 d. At the end of the experiment, pigs were weighed individually, and cumulative feed consumption was measured for each pen. In order to study the effect of various treatments on plasma biochemistry, and the innate and acquired immune response, blood samples were aseptically collected by jugular venepuncture from all animals (heparinised Vacutainer tubes; Vacutest®, Arzergrande, Italy). Liver samples were taken after animals were slaughtered, and stored at − 80°C until analysed.
Animals were cared for in accordance with the Romanian Law 206/2004 for handling and protection of animals used for experimental purposes. The study protocol was approved by the Ethical Committee of the National Research-Development Institute for Animal Nutrition and Biology, Balotesti, Romania.
Analysis of mycotoxins
The content of DON in the feed was analysed by HPLC, with UV detection after clean-up with an immune-affinity column, and a detection limit of 0·03 mg/kg (Table 1). ZEA, fumonisin and T-2/HT-2 toxins were analysed by ELISA using ELISA kits Veratox (Neogen, Lansing, MI, USA) with detection limits of 10, 50 and 25 ppb, respectively (Table 1). Ochratoxin A toxin, also analysed by ELISA, was detected in negligible concentration.
Plasma biochemical parameters
Plasma concentrations of Na, K, Cl, Ca, P, Mg, total protein, urea, glucose, bilirubin, and concentrations of alkaline phosphatase, glutamate pyruvate transaminase, glutamate oxaloacetate transaminase were determined on a BS-130 Chemistry analyser (Bio-Medical Electronics Company Limited, Shenzhen, China).
Plasma total Ig subsets (IgG, IgA, IgM)
Total concentration of Ig subsets was measured by ELISA (Bethyl, Medist, Montgomery, TX, USA) after plasma dilution: 1:4000 (IgA), 1:60 000 (IgG) and 1:6000 (IgM), as reported previously(Reference Marin, Taranu and Pascale23), and according to the manufacturer's instructions. Absorbance was read at 450 nm using a microplate reader (Tecan Sunrise, Salzburg, Austria).
Isolation of pig porcine peripheral blood mononuclear cells
The effect of Fusarium toxins on cellular viability, cell proliferation and T-lymphocyte phenotypes was studied on porcine peripheral blood mononuclear cells (PBMC). Blood collected from all animals was mixed with an equal volume of Dulbecco's PBS (Sigma-Aldrich Chemical Company, Steinheim, Germany), then laid over Ficoll-Hypaque (1.077; Sigma-Aldrich Chemical Company) and centrifuged at 2700 pm, 20 min at room temperature. PBMC were collected, washed twice in PBS and resuspended in RPMI-1640 (Sigma-Aldrich Chemical Company), supplemented with 2 mm-glutamine, penicillin (100 U/ml), streptomycin (50 μg/ml) and 10 % fetal calf serum (Sigma-Aldrich Chemical Company). Cells were counted and viability was assessed using trypan blue (Sigma-Aldrich Chemical Company).
Porcine peripheral blood mononuclear cell proliferation
The ability of isolated and mitogen-activated PBMC to proliferate ex vivo was measured by the [methyl-3H]thymidine proliferation assay. PBMC (1 × 106 cells/ml), stimulated or unstimulated with concanavalin A (10 μg/ml) (Type IV; Sigma-Aldrich Chemical Company), were cultured for 72 h at 37°C and 5 % CO2 in ninety-six-well flat-bottomed tissue culture plates (NUNC, Langelnselbold, Germany). For the last 18 h of cultivation, cells were labelled with 1 μCi/well of [methyl-3H]thymidine, and then harvested through glass-fibre filters (Skatron, Sterling, London, UK). Incorporation of [methyl-3H]thymidine was measured with a Canberra-Packard Beta Counter (PerkinElmer Life and Analytical Science, Downers Grove, IL, USA), and the results were expressed as counts/min.
Immune phenotyping of lymphocytes
The percentage of peripheral T-lymphocyte subsets CD3+CD4+ and CD3+CD8+ was assessed by flow cytometry. Antibodies were purchased from Becton Dickinson Pharmingen (San Diego, CA, USA). Briefly, 100 μl of blood harvested in EDTA-coated vacutainers (Vacutest®) were incubated for 20 min at room temperature, in the dark, with 0·6 μg (1·2 μl) of fluorescein isothiocyanate-conjugated mouse anti-pig CD3ɛ monoclonal antibody (clone BB23-8E6-8C9), and 0·5 μg (2·5 μl) of R-phycoerythrin-conjugated mouse anti-pig CD4a monoclonal antibody (clone 74-12-4) or 0·5 μg (2·5 μl) of R-PE-conjugated mouse anti-pig CD8a monoclonal antibody (clone 76-2-11). Fluorescein isothiocyanate-conjugated mouse IgG2aκ and R-PE-conjugated mouse IgG2bκ were used as isotype controls (clones G155-178 and 27-35). Thereafter, samples were treated for 15 min with Cell Lyses (BD Biosciences, Heidelberg, Germany) for the removal of erythrocytes. After two washes with 2 ml Cell Wash (Becton Dickinson), cells were finally fixed with 400 μl Cell Fix (Becton Dickinson), and analysed by flow cytometry using a FACSCanto flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and CellQuest software (Becton Dickinson). At least 10 000 events were analysed.
Respiratory burst of granulocytes
The intracellular production of H2O2 by peripheral granulocytes was performed by flow cytometry in whole blood using the fluorogenic substrate dihydrorhodamine 123 (BurstTest kit; ORPEGEN Pharma, Heidelberg, Germany). Briefly, ice-cold heparinised blood (100 μl) was activated with unlabelled opsonised Escherichia coli for 10 min at 37°C. Dihydrorhodamine 123 was then added, and incubation was continued for another 10 min at 37°C. Erythrocytes were lysed and fixed for 20 min at room temperature. Samples were washed twice with washing solution by centrifugation (5 min, 1200 rpm, 4°C), and the supernatant was discarded. Finally, 200 μl of propidium iodide (DNA-staining solution) were added while samples were kept on ice, in a dark place. Cell analysis was done by flow cytometry using a FACSCanto flow cytometer (Becton Dickinson) and CellQuest software (Becton Dickinson). At least 10 000 events were analysed. By manual gating, we first selected the population of granulocytes from a forward-scatter v. side-scatter plot. Then, we selected single cells and excluded aggregation artifacts, as defined by propidium iodide incorporation. Finally, flow-cytometry data were expressed as the percentage of responsive cells under basal conditions or to a particular ex vivo stimulus, meaning the percentage of cells with fluorescence intensity above a defined threshold (M2). The mean intensity of the cellular response was calculated as the geometrical mean of the fluorescence channel.
Measurement of cytokine production
Samples of liver were weighed and homogenised in phosphate buffer containing 1 % igepal, 0·5 % sodium deoxycholate, 0·1 % SDS, and complete (EDTA-free) protease inhibitor cocktail tablets. The homogenates were kept 30 min on ice, and then centrifuged at 10 000 g at 4°C for 10 min. TNF-α, IL-1β and IL-8 concentrations in the supernatants were determined by ELISA, using commercially available kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. Optical densities were measured on an ELISA reader (Tecan Sun Rise) at a wavelength of 450 nm. Dilutions of recombinant swine TNF-α, IL-1β and IL-8 were used as standards, and data were analysed against the linear portion of the generated standard curve. Results are expressed as pg cytokine/ml supernatant.
Statistical analyses
ANOVA tests were used to analyse the differences in terms of weight, plasma biochemistry parameters, cell proliferation, Ig synthesis and so on, between the M − CFrB − , M − CFrB+, M+CFrB − and M+CFrB+ groups. P values of 0·05 were considered significant.
Results
Animal performance
We first investigated the effect of dietary treatment on clinical signs and animal performance. Piglets were fed with control or Fusarium mycotoxin-contaminated diets (1·79 ppm) and a mitigating additive, CFrB, for a period of 24 d. Control animals as well as piglets fed with the mycotoxin-contaminated diet appeared clinically normal during the whole experiment, and no deaths resulted from the intoxication; but at the end of the feeding period, the performance of the contaminated piglets was lower than those in the control and CFrB groups (Fig. 1). Indeed, Fusarium mycotoxin significantly retarded average daily weight gain, from 0·458 (sem 0·12) kg for the control animals to 0·292 (sem 0·17) kg for the Fusarium mycotoxin-treated animals. The gain:feed ratio was also reduced in pigs fed with contaminated diets (2·312 (sem 0·67) for the control v. 3·029 (sem 1·43) for the contaminated pigs). However, this reduction was not significant, and the effect was alleviated by the supplementation of feed with the mitigating additive (0·431 (sem 0·11) and 2·912 (sem 1·01) kg, respectively).

Fig. 1 Influence of dietary mycotoxins and calcium fructoborate (CFrB) additive on body weight. Pigs received the control diet (□), calcium fructoborate (CFrB) additive diet (![]() ) or diet contaminated with Fusarium mycotoxins (■) or Fusarium mycotoxins and CFrB additive (
) or diet contaminated with Fusarium mycotoxins (■) or Fusarium mycotoxins and CFrB additive (![]() ). Animals were weighed at the end of the feeding trial (24 d). Values are means of body weight (n 4), with standard errors represented by vertical bars. An ANOVA test was performed to compare the different groups. The table presents the performance (average daily weight gain (AWG) and feed:gain) obtained from pigs under the action of the four treatments.
). Animals were weighed at the end of the feeding trial (24 d). Values are means of body weight (n 4), with standard errors represented by vertical bars. An ANOVA test was performed to compare the different groups. The table presents the performance (average daily weight gain (AWG) and feed:gain) obtained from pigs under the action of the four treatments.
Organ weights
The weight of the internal organs was also measured in animals from the different diet groups. The absolute weights of liver, kidney, spleen and lung were not influenced by the diet supplemented with CFrB alone, but were lower in pigs that received the mycotoxin-contaminated diet compared with control, without significant differences between mean values (Table 2). However, the supplementation of the contaminated diets with the CFrB additive restored the decreased organ weights caused by mycotoxins, with a significant difference for lung weight (M+CFrB+ v. M+CFrB − , P < 0·05). The expression of the internal organ weight assessed as the ratio of organ weight:metabolic body weight (BW0·75)was still reduced in pigs fed with the M+CFrB − diet and ameliorated in pigs fed with the M+CFrB+ diet (data not shown).
Table 2 Effect of Fusarium mycotoxins (M) and calcium fructoborate (CFrB) additive on organ weight*
(Mean values with their standard errors, n 4)
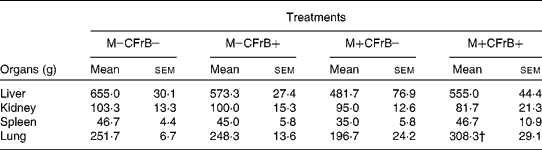
* The weight of the internal organs was measured at the end of the experiment in animals from the different diet treatments.
† Mean value was significantly different from the M+CFrB − treatment (P < 0·05).
Plasma biochemistry
Biochemistry analysis was also performed in the plasma of animals receiving the different diet treatments. Supplementation of the diet with CFrB additive resulted in a significant increase in Na (P < 0·05) concentration, the other plasma biochemistry parameters being less influenced, and registered either a non-significant increase (P, aspartate aminotransferase and alanine aminotransferase enzymes) or decrease (Cl, Ca, urea and glucose) in concentration with respect to the control diet. Compared with the control, the ingestion of the mycotoxin-contaminated feed had no effect on the total glucose, urea, P and alkaline phosphatase; however, it significantly increased the plasma concentration of total protein, Na and Ca (P < 0·05). Aspartate aminotransferase and alanine aminotransferase enzymes also increased, not significantly, from 37·9 (sem 4·8) and 47·8 (sem 9·8) U/l in control groups to 41·8 (sem 1·7) and 55·2 (sem 1·9) U/l, respectively, in piglets feeding the contaminated diet, while bilirubin and chloride concentrations decreased (Table 3). The affected biochemistry parameters were partially attenuated by the addition of CFrB to the mycotoxin-contaminated diet.
Table 3 Effect of dietary Fusarium mycotoxins (M) and calcium fructoborate (CFrB) additive administration on selected blood biochemical parameters in piglets*
(Mean values with their standard errors, n 4)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* At the end of the experiment, plasma from the piglets was used to measure the blood biochemical parameters. ANOVA tests were performed to analyse the effect of different treatments on biochemical parameters.
Plasma Ig concentration
Table 4 shows that the concentration of the different plasma Ig subsets (IgA, IgG and IgM) ranged within the normal values for starter pigs, but the comparison between the control and Fusarium mycotoxin-contaminated diets resulted in 25·9 % increase in IgG, and 29·8 % in IgM level in the mycotoxin group at day 24 of the experiment. Feeding the diet supplemented with CFrB increased the concentration of IgM and IgG by 29·8 and 25·9 %, suggesting that the fructoborate compound per se stimulates the Ig synthesis, though it was not able to prevent the increased plasma Ig level in pigs that consumed the diet containing the contaminated maize which remained higher (P < 0·05) than the control (3·23 (sem 0·6) and 10·17 (sem 3·2) v. 2·05 (sem 0·4) and 6·36 (sem 2·5)). A slight decrease in plasma IgA level induced by DON, and reversing by the mitigating additive, was observed.
Table 4 Immunological values in pigs fed diets contaminated with Fusarium mycotoxins (M) and calcium fructoborate (CFrB) additive*
(Mean values with their standard errors, n 4)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* At the end of the experiment, blood samples were collected, and the total concentration of Ig subsets from plasma was measured by ELISA after plasma dilution: 1:4000 (IgA), 1:6000 (IgM) and 1:60 000 (IgG). Results are expressed as IgA, IgM or IgG content in the plasma of piglets. ANOVA tests were performed to analyse the effect of treatment on Ig levels.
Proliferation of peripheral blood mononuclear cells
The [methyl-3H]thymidine proliferation assay used to determine the ex vivo proliferation of the PBMC derived from pigs fed with experimental treatments for 24 d, and cultured for 72 h at 37°C and 5 % CO2, showed a significant effect of the Fusarium-contaminated diet on cell proliferation, which dramatically increased by 111·7 % in pigs fed this treatment in comparison with control pigs (P < 0·05). By contrast, the proliferation of the PBMC derived from pigs receiving the contaminated diet and supplemented with mitigating CFrB compound did not exceed the control level (P < 0·05). The effect of treatments on PBMC proliferation was similar when the cells were stimulated in vitro with concanavalin A (Fig. 2). In order to verify that the increased PBMC proliferation was associated with an increased cell number, the trypan blue exclusion experiment was performed. We observed that the cell number increased from 7·9 (sem 1·2) × 106 cells/ml in control PBMC to 15·9 (sem 3·9) × 106 cells/ml in cells derived from mycotoxin-treated pigs (P < 0·05), and to 14·5 (sem 1·0) × 106 cells/ml in cells of pigs fed with the contaminated diet and the CFrB supplement (P < 0·05). Interestingly, even the number of these cells is higher than that of the control, and they are less reactive, with the [methyl-3H]thymidine incorporation assay showing a decreased capacity to proliferate.
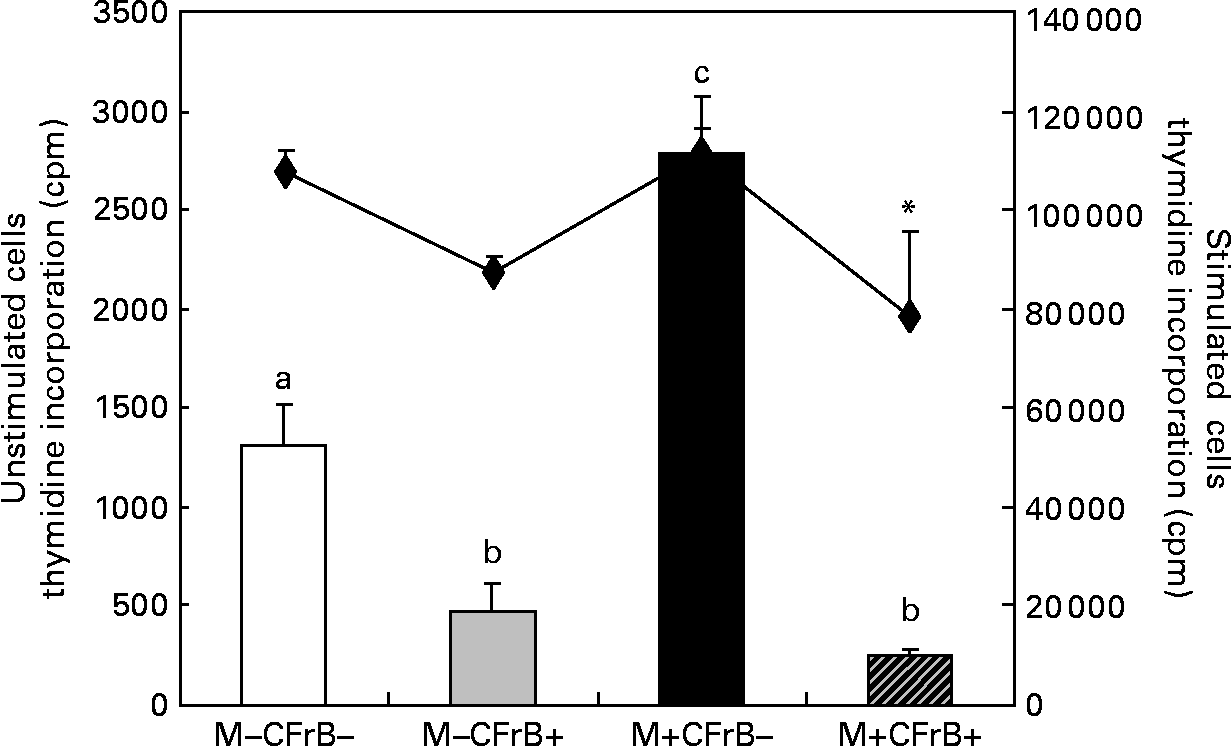
Fig. 2 Effect of dietary mycotoxins (M) and calcium fructoborate (CFrB) additive on ex vivo porcine peripheral blood mononuclear cell (PBMC) proliferation. PBMC derived from pigs receiving the control diet (□), CFrB-additive diet (■) or diet contaminated with Fusarium mycotoxins (![]() ) or Fusarium mycotoxins plus CFrB additive (
) or Fusarium mycotoxins plus CFrB additive (![]() ) were stimulated or unstimulated with concanavalin A (ConA, 10 μg/ml) and cultured (1 × 106 cells/ml) for 72 h at 37°C and 5 % CO2. The ex vivo proliferation of PBMC was measured by the [methyl-3H]thymidine incorporation assay, and the results are expressed as counts/min (cpm). Values are means, with standard errors represented by vertical bars (n 4). ANOVA tests were performed to compare PBMC proliferation in control and treated animals; a,b,c Mean values with unlike letters were significantly different between the control and treated animals for proliferation under the basal condition (P < 0·05). * Mean values were significantly different between the contaminated diet and contaminated diet plus CFrB additive under the ConA-stimulated condition (P < 0·05).
) were stimulated or unstimulated with concanavalin A (ConA, 10 μg/ml) and cultured (1 × 106 cells/ml) for 72 h at 37°C and 5 % CO2. The ex vivo proliferation of PBMC was measured by the [methyl-3H]thymidine incorporation assay, and the results are expressed as counts/min (cpm). Values are means, with standard errors represented by vertical bars (n 4). ANOVA tests were performed to compare PBMC proliferation in control and treated animals; a,b,c Mean values with unlike letters were significantly different between the control and treated animals for proliferation under the basal condition (P < 0·05). * Mean values were significantly different between the contaminated diet and contaminated diet plus CFrB additive under the ConA-stimulated condition (P < 0·05).
Immune phenotyping of peripheral T-lymphocytes
The effect of Fusarium toxins on T-lymphocyte subsets defined by the expression of CD3+, CD3+CD4+ and CD3+CD8+ was measured by flow cytometry with fluorescently labelled anti-CD4 and anti-CD8 antibodies (Fig. 3). The results indicated the capacity of the diet containing the contaminated maize, and especially of that containing the mitigating additive, to increase the percentage of peripheral T, CD3+, CD3+CD4+ and CD3+CD8+ subsets of treated pigs in comparison with the control; while the increase induced by the mycotoxin diet was not significant, that produced by the fructoborate additive was significant (P < 0·05). The addition of the organic additive to the contaminated feed determined a cumulative effect on the percentage of CD3+ (P < 0·05) and CD3+CD8+ (P < 0·05) lymphocyte subsets, which increased to 133·25 and 142·70 % in comparison with the control.

Fig. 3 Flow cytometric identification of CD3+ (□), CD3+CD4+ (![]() ) and CD3+CD8+ (
) and CD3+CD8+ (![]() ) blood T-lymphocytes following treatments with Fusarium mycotoxins (M) and calcium fructoborate (CFrB) additive. Blood samples taken after 24 d from pigs fed the M − CFrB − diet, M − CFrB+ diet or M+CFrB − diet and M+CFrB+ diet were stained for CD3ɛ, CD4a and CD8a subsets, and analysed by flow cytometry. Values are means, representing the average of the percentage of CD3+, CD3+CD4+ and CD3+CD8+ blood T-lymphocytes at 24 d following treatments with Fusarium mycotoxins and CFrB additive, with their standard errors represented by vertical bars (n 4). ANOVA test was performed to compare the percentage of T-cell subsets between the control and treated animals. a,b Mean values with unlike letters were significantly different between the treatments (P < 0·05).
) blood T-lymphocytes following treatments with Fusarium mycotoxins (M) and calcium fructoborate (CFrB) additive. Blood samples taken after 24 d from pigs fed the M − CFrB − diet, M − CFrB+ diet or M+CFrB − diet and M+CFrB+ diet were stained for CD3ɛ, CD4a and CD8a subsets, and analysed by flow cytometry. Values are means, representing the average of the percentage of CD3+, CD3+CD4+ and CD3+CD8+ blood T-lymphocytes at 24 d following treatments with Fusarium mycotoxins and CFrB additive, with their standard errors represented by vertical bars (n 4). ANOVA test was performed to compare the percentage of T-cell subsets between the control and treated animals. a,b Mean values with unlike letters were significantly different between the treatments (P < 0·05).
Respiratory burst of granulocytes
To assess the effect of mycotoxins on the innate immune response, the intracellular production of H2O2, which was induced ex vivo by opsonised E. coli, was evaluated by flow cytometry in peripheral granulocytes derived from different diet groups (Fig. 4). Mycotoxin-contaminated feed increased the percentage of responsive cells in both unstimulated (15·4 v. 5·1 % in control), and, especially, under stimulated conditions (70·95 v. 22·65 % for the control). The diet containing the CFrB additive seemed to normalise the exacerbated cellular immune response induced by Fusarium toxins (decreased by 42·6 %, P < 0·05).
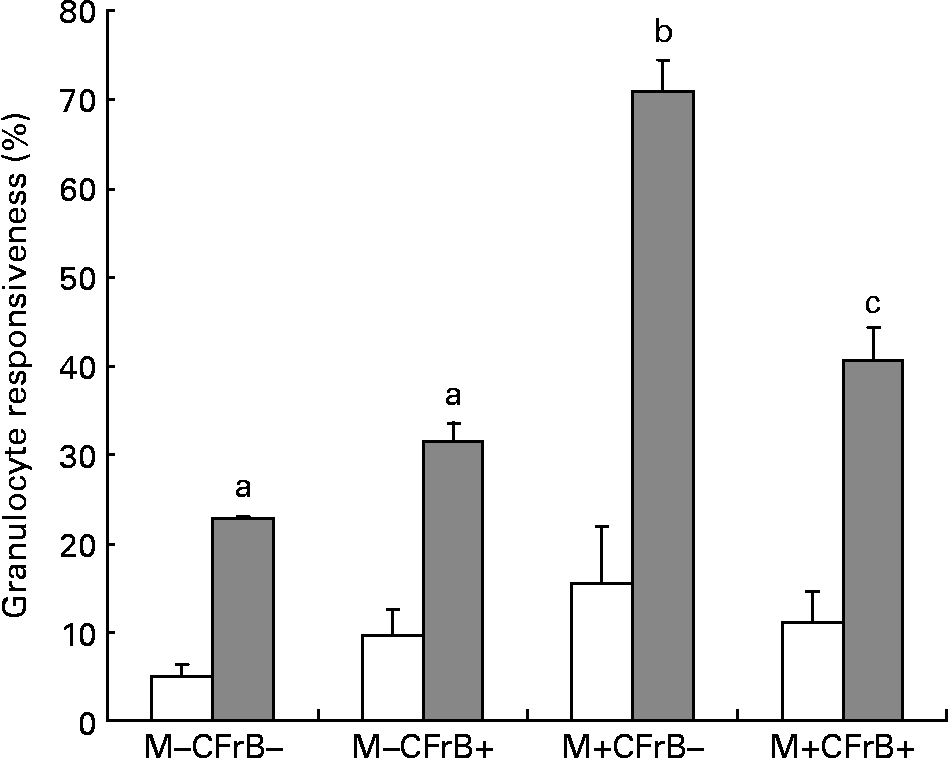
Fig. 4 Effect of dietary mycotoxins (M) and calcium fructoborate (CFrB) additive on the respiratory burst of circulating granulocytes. Ice-cold heparinised blood (100 μl) was activated with unlabelled opsonised Escherichia coli for 10 min at 37°C. The intracellular production of H2O2 by peripheral granulocytes was performed by flow cytometry in the whole blood using the fluorogenic substrate dihydrorhodamine 123 and propidium iodide as the DNA-staining solution. Cell analysis was done by flow cytometry using CellQuest software (Becton Dickinson, San Diego, CA, USA). At least 10 000 events were analysed. Data were expressed as the percentage of responsive cells under basal conditions (no stimulus, □) or in response to a particular ex vivo stimulus (E. coli, ![]() ), meaning the percentage of cells with fluorescence intensity above a defined threshold (M2). Values are means, with their standard errors represented by vertical bars (n 4). ANOVA tests were performed to analyse the effect of different treatments on cytokine production. a,b,c Mean values with unlike letters were significantly different between the treatments (P < 0·05).
), meaning the percentage of cells with fluorescence intensity above a defined threshold (M2). Values are means, with their standard errors represented by vertical bars (n 4). ANOVA tests were performed to analyse the effect of different treatments on cytokine production. a,b,c Mean values with unlike letters were significantly different between the treatments (P < 0·05).
Liver cytokine synthesis
As expected, the synthesis of IL-1β, TNF-α and IL-8 was revealed by ELISA measurement in the liver of pigs, but this cytokine production was altered in samples from animals which ingested the mycotoxins. As already observed for other immune parameters, the exposure to Fusarium mycotoxins for 24 d increased the synthesis of IL-1β, TNF-α and IL-8 by 123·8, 217·1 and 255·1 %, respectively; the increase was significant for IL-8 and TNF-α in comparison with the control group (M − CFrB − ) and the mitigating additive group (M − CFrB+) (P < 0·05). In contrast, the ingestion of the toxin associated with the fructoborate supplement significantly decreased (P < 0·05) the synthesis of the investigated cytokines (Fig. 5).

Fig. 5 Effect of dietary mycotoxins (M) and calcium fructoborate (CFrB) additive on the synthesis of cytokines, IL-8, TNF-α and IL-1β, in the liver of pigs. Samples of the liver were weighed and homogenised in phosphate buffer containing 1 % igepal, 0·5 % sodium deoxycholate, 0·1 % SDS and complete (EDTA-free) protease inhibitor cocktail tablets. The homogenates were kept for 30 min on ice and then centrifuged at 10 000 g at 4°C for 10 min. TNF-α, IL-1β and IL-8 concentrations in the supernatants were determined by ELISA using R&D Systems kits (according to the manufacturer's instructions; R&D Systems, Minneapolis, MN, USA). Optical densities were measured on an ELISA reader (Tecan, Sun Rise, Austria) at a wavelength of 450 nm. Values are means, with their standard errors represented by vertical bars (n 4). ANOVA tests were performed to analyse the effect of different treatments on cytokine production. a,b Mean values with unlike letters were significantly different between the treatments (P < 0·05).
Discussion
Feed consumption is the first zootechnical parameter affected by Fusarium mycotoxins, which decrease, for example for DON, from a level >1 ppm in the feed of pigs(Reference Etienne24, Reference Grosjean, Callu and Pinton25). A direct consequence of the reduced feed intake is the alteration of pig growth rate(Reference Swamy, Smith and MacDonald26–Reference Friend, Trenholm and Elliot28). Indeed, in the present study, we demonstrated that the growth rate of pigs fed with a diet contaminated with Fusarium mycotoxin, mostly DON, decreased by 36·2 % compared with the controls over 24 d, and feed efficiency increased by 30·98 %, confirming that the alteration of feed consumption represents the main effect of fusariotoxins on pigs. The lowered performance induced by Fusarium toxins was alleviated in pigs of the M+CFrB+ group following the supplementation with the fructose and the boron compound. Our results are in agreement with those of Armstrong et al. (Reference Armstrong, Flowers and Spears29) and Armstrong & Spears(Reference Armstrong and Spears30), who also observed an increase in average daily weight gain and feed intake in pigs that consumed boron-supplemented diets following an intramuscular injection of lipopolysaccharide. Other studies testing the efficacy of polysaccharide products have shown either a beneficial or no effect for counteracting Fusarium toxins. Dried apple pomace (a by-product of apple juice production rich in pectin and other NSP) incorporated at the level of 8 % in a Fusarium mycotoxin-contaminated feed (3100 ppb DON and 65 ppb ZEA) was able to restore the growth rate of weaner piglets(Reference Gutzwiller, Czegledi and Stoll31), while polymeric glucomannan (0·05, 0·1, 0·2 and 1 % in feed) had no beneficial effect on the zootechnical performance of growing pigs fed with a Fusarium mycotoxin-contaminated diet (mainly DON)(Reference Swamy, Smith and MacDonald26, Reference Wetscherek, Huber and Lew32). However, in these studies, the level of mycotoxin was higher than that reported in the present study (5·5 and 2–2·5 ppm, respectively).
The supplementation of the CFrB compound to the diet containing contaminated maize counteracted organ-weight alterations in the present study, but it had no beneficial effect on the biochemistry parameters modified by mycotoxin. The inability of 0·1 and 0·2 % of dietary glucomannan polymer to prevent some of the Fusarium toxin-induced alterations in serum chemistry, but to restore the significant reduction of liver weight in pigs, was also reported by Swamy et al. (Reference Swamy, Smith and MacDonald26). Nevertheless, these authors observed that the same concentrations of glucomannan polymer were more effective in reversing the alterations in haematology, serum chemistry and biliary IgA concentrations in broiler chickens(Reference Swamy, Smith and Cotter33).
Similar to other mycotoxins, the presence of DON and other Fusarium toxins in animal feed could have serious consequences on their immune functions(Reference Oswald, Marin and Bouhet34, Reference Pestka, Zhou and Moon35). Many in vivo and in vitro studies have described the effects of trichothecenes on the cellular and humoral response, leading to the alteration of Ig and cytokine synthesis, decreased lymphocyte proliferation, etc.(Reference Pestka, Zhou and Moon35, Reference Meky, Hardie and Evans36). Of particular interest is the effect of DON on antibody synthesis, which seems to be related to the induction of certain cytokines such as IL-2, IL-5 and IL-6 at Payer's patch level(Reference Pestka and Smolinski3). In pigs, the results are contradictory, showing either an increase in serum Ig(Reference Grosjean, Pinton and Callu37–Reference Rotter, Prelusky and Pestka40) or no effect in Ig levels(Reference Accensi, Pinton and Callu41–Reference Swamy, Smith and MacDonald44). In our experiment, the feeding of contaminated diet to pigs significantly increased (P < 0·05) the plasma IgG and IgM concentrations, and did not influence the plasma IgA level (Table 4). The elevated IgM and IgG concentrations induced by Fusarium toxins in the present investigation are in accordance with the experiments conducted by Goyarts et al. (Reference Goyarts, Danicke and Tiemann38), Swamy et al. (Reference Swamy, Smith and MacDonald26) and Pinton et al. (Reference Pinton, Accensi and Beauchamp39) with growing pigs fed with a higher concentration of DON (5·7, 5·5 and 2·5 ppm, respectively). A stimulative effect on the humoral and cellular immune response was also determined by CFrB alone (increased plasma IgG and IgM concentrations and increased percentage of CD4+ and CD8+ lymphocyte subsets), but the additive was not effective in preventing these increased parameters in pigs that consumed the Fusarium toxin-contaminated diet (Table 4; Fig. 3). In contrast to the present results, the supplementation of Fusarium-contaminated feed with glucomannan was able to counteract the increased IgM and IgG concentrations in the experiment conducted by Swamy et al. (Reference Swamy, Smith and MacDonald26) and, partially, the increased percentage of CD4+ and CD8+ lymphocyte subsets in the trial conducted by Girish et al. (Reference Girish, Smith and Boermans45).
It has been shown that DON, a marker of Fusarium infestation, is able to increase the synthesis of IL-2, a cytokine with a key role in cell proliferation(Reference Swamy, Smith and MacDonald26, Reference Swamy, Smith and MacDonald44); this fact along with the stimulation of ribotoxic stress and the activation of mitogen-activated protein kinases, enzymes which catalyse reactions in signal transduction related to proliferation, differentiation and apoptosis(Reference Pestka and Smolinski3), could explain the effect of Fusarium mycotoxins on lymphocyte proliferation observed in the present study and in other similar studies. Thus, a series of in vivo studies have already shown that the non-specific capacity of lymphocytes to proliferate is dramatically increased in the growing pigs receiving 1·6 and 3·6 ppm DON(Reference Pinton, Royer and Accensi46, Reference Grosjean, Taranu and Skiba47). In poultry(Reference Girish, Smith and Boermans45), increased proliferation was observed in the crypt and the villi of the small intestine when birds ingested diets containing Fusarium toxins (diacetoxyscirpenol or T-2 toxins). Data from the present trial showed that the oral administration of 1790 ppb DON and less amount of ZEA, fumonisin and HT-2 toxins for 24 d resulted in a significantly higher ex vivo proliferation response of peripheral blood lymphocytes to a mitogen stimulation or under basal conditions (P < 0·05).
Dietary fructoborate supplementation was very effective in reversing the alteration of PBMC proliferation, as well as in the respiratory burst of circulating granulocytes, and in the production of liver pro-inflammatory cytokines, which are important aspects of the immune response modified under the mycotoxin action in the present trial. In vitro studies of Scorei et al. (Reference Scorei, Ciofrangeanu and Ion48, Reference Scorei, Cimpoiasu and Iordachescu49) have highlighted the anti-inflammatory and antioxidant properties of fructoborate, showing that the addition of 0·2–1 mm-CFrB to cultured RAW 264.7 macrophages reduced the secretion of IL-6, IL-1β and NO by lipopolysaccharide-stimulated cells in a dose-dependent manner, and markedly diminished the intracellular reactive oxygen species in keratinocytes. These authors have supported the hypothesis that the soluble carbohydrate compounds of boron formed by the combination of boric acid with free glucides, glycolipids and glycoproteins buffer the reactive species of oxygen by developing organic peroxyborates. Other authors(Reference Luo and Eckhert50–Reference Nielsen52) have also suggested that boron decreased the production of H2O2 generated during the respiratory burst by the up-regulating activity of some enzymes (superoxide dismutase and glutathione peroxidase) involved in the respiratory burst cascade. In our feeding experiment, dietary boron compound supplementation significantly decreased the synthesis of liver TNF-α and IL-8, and the exacerbated respiratory burst developed in the peripheral granulocytes.
To our knowledge, boron esters (fructoborate) or other feeds containing fructose have never been tested as possible antidotes to mycotoxins in pigs. We consider that its efficacy to alleviate the performance and some aspects of the immune response of pigs altered by Fusarium mycotoxins may derive from the specific and various properties of its components (proteins, boron and fructose), and from the mechanism that such a complex compound could start. It has been hypothesised that carbohydrates might trap the mycotoxin molecule, preventing toxin absorption, or might contribute to the biodegradation of mycotoxins into non-toxic metabolites by the stimulation of intestinal microflora activity; at the intestine level, carbohydrates increase the amount of water in the gut lumen, protein synthesis in small-intestinal mucosal cells and stimulate cell division(Reference Gutzwiller, Czegledi and Stoll31). Boron provided support (as a cofactor) to important enzymes involved in the antioxidant processes, and demonstrated immunomodulatory function (anti-inflammatory and Ig-stimulant), which enhanced the resistance of animals to mycotoxin. Boron is also implicated in Ca metabolism, and these two elements affect similar systems including cell membrane characteristics, trans-membrane signalling, etc.(Reference Nielsen53). Although we can conclude that the addition of complex compounds to animal feed provides versatile tools to prevent mycotoxicosis, the present study has to be completed with further research aiming to establish whether fructoborate additive remains efficient against a higher dose of mycotoxin under mycotoxin co-contamination conditions. Different dietary rates of fructoborate additive are required in order to study in more detail the health-promoting effects.
Acknowledgements
The present study was supported by funds from the National Research Project ‘CEEX 25’ 2005–08 and ‘PNII’ 2008–11 granted by the Romanian Ministry of Research and Technology. The authors would like to thank Dr Romulus Scorei for providing the CFrB additive and technical information. Thanks are also due to Dr Olga Starodub for her help with the English text. I. T. conducted and designed the research and wrote the manuscript. D. E. M. contributed to the manuscript preparation and, along with M. M. performed biochemistry, Ig and cytokine determinations and analysed the data. G. M. and I. N. performed thymidine proliferation assay, flow-cytometry experiments and analysed the data. C. T. and M. S. contributed to the progress of the in vivo experiment (animal performance, sample collection and preparation). M. O. analysed the chemical composition of the feed used in the trial. All authors read and approved the final manuscript.











