Introduction
BirdLife International’s Important Bird and Biodiversity Areas (IBA) programme started in Western Europe in the late 1970s and now has global coverage. Originally, IBAs were called Important Bird Areas. The name change to Important Bird and Biodiversity Areas was approved by BirdLife’s Global Council in June 2014 in order to reflect the wider biodiversity significance of these sites. The programme has identified, documented and mapped over 13,000 sites of international significance for birds, making it the world’s largest network of sites of biodiversity importance. Elsewhere we have reviewed the history of the IBA programme, the criteria and process for site identification, and the characteristics of the resulting network (Reference Donald, Fishpool, Ajagbe, Bennun, Bunting, Burfield, Butchart, Capellan, Crosby, Dias, Diaz, Evans, Grimmett, Heath, Jones, Lascelles, Merriman, O´Brien, Ramirez, Waliczky and WegeDonald et al. in press), while Bennun (Reference Bennun, Brooks and Arico2013) provided a brief overview of IBA policy linkages, local engagement and the relationship between IBAs and wider biodiversity. Here, we review the conservation impacts of the IBA programme, including its influence on international conservation policies, national site protection and land-use planning, and action at individual sites. In doing so we seek to illustrate the conservation gains of assessing and documenting biodiversity importance at the site level, and thereby support and inform the recently launched Key Biodiversity Areas (KBA) Partnership and programme (www.keybiodiversityareas.org), which aims to multiply the successes of the IBA programme by extending a harmonised approach across all taxa.
IBAs in international conservation policy and planning
The following section provides an overview of how IBAs have been used by multilateral environmental agreements (the Convention on Biological Diversity, the Ramsar Convention, the Convention on Migratory Species), the European Union, international financial institutions and the private sector to guide their decisions relating to the conservation or safeguarding of sites of biodiversity importance.
Multilateral Environmental Agreements (MEAs)
MEAs form the cornerstone of coordinated international efforts to tackle environmental problems and degradation. Over 700 MEAs have been signed, covering issues from pollution control to the conservation of individual species (Mitchell Reference Mitchell2003). We briefly review how IBAs have contributed directly to the better implementation of three of these mechanisms.
Convention on Biological Diversity (CBD)
Arguably the largest and most important MEA for the conservation of the planet’s living resources is the CBD, which sets the overarching global framework for biodiversity conservation and sustainable use. In 2010, the Convention adopted a new Strategic Plan for Biodiversity 2011–2020 with 20 global headline targets, known as the Aichi Biodiversity Targets (CBD 2010). These address the underlying causes of biodiversity loss, the responses needed to improve the conservation of biodiversity, the benefits to people provided by biodiversity and its associated ecosystem services, and a set of mechanisms to facilitate implementation. The CBD is implemented primarily through activities at the national and subnational level as set out in each country’s National Biodiversity Strategy and Action Plan (NBSAP), through which global targets are codified into specific national commitments.
IBAs are particularly pertinent to Aichi Target 11, which commits Parties to conserving effectively 17% of the terrestrial surface and 10% of the marine environment, ‘especially areas of particular importance for biodiversity’. IBAs form the most comprehensive network of such sites available worldwide (BirdLife International 2014a). According to a study carried out in 2004 looking at 36 NBSAPs from countries around the world, 28% of the NBSAPs analysed lent strong support to the conservation and sustainable use of IBAs, by developing protected area networks and identifying gaps in protected area coverage (BirdLife International 2004a).
Aichi Target 12 states that “By 2020, the extinction of known threatened species has been prevented and their conservation status, particularly of those most in decline, has been improved and sustained”. IBAs identified for threatened species are also relevant to this Target, given that preventing human-induced extinctions typically requires site-scale interventions (Boyd et al. Reference Boyd, Brooks, Butchart, da Fonseca, Hawkins, Hoffmann, Sechrest, Stuart, van Dijk and Edgar2008), and IBAs represent the most significant sites for conserving threatened bird species. A recent study presented evidence that species occurring in IBAs with greater protected area coverage experienced smaller increases in extinction risk over recent decades (Butchart et al. Reference Butchart, Scharlemann, Evans, Quader, Arinaitwe, Bennun, Besançon, Boucher, Bomhard, Brooks, Burfield, Burgess, Clay, Crosby, Davidson, De Silva, Devenish, Dutson, Díaz Fernández, Fishpool, Foster, Hockings, Hoffmann, Knox, Larsen, Lamoreux, Loucks, May, Millett, Parr, Skolnik, Upgren and Woodley2012).
The CBD Secretariat organized six regional workshops for 124 Parties during 2015 and 2016 in which they helped governments to identify, and commit to, specific actions in order to make greater progress towards achieving Targets 11 and 12. Data on IBAs lacking protected area coverage formed a key input to these workshops, and many countries committed to using them to set priorities for expanding their protected area network. For example, the Lebanese Government outlined as a priority action that all IBAs in the country should be protected. IBAs received equal attention from the Philippines, which has included as part of its priority actions increasing the number of protected IBAs and improving their management effectiveness (CBD 2016). This builds on a long history of national Governments establishing formal protected areas covering IBAs, as a result of advocacy and support from BirdLife Partner organisations (BirdLife International 2004b, 2008a; see below for examples).
IBAs are also particularly relevant to several other Targets of the CBD´s Strategic Plan, including:
• Aichi Target 4, by supporting private and financial sectors to manage their environmental risks related to biodiversity impact (see below, and e.g. International Finance Corporation 2012)
• Aichi Target 14 on restoring and safeguarding ecosystems providing essential services, since IBAs have been shown to be particularly important for also providing ecosystem services to people (e.g. Thapa et al. Reference Thapa, Butchart, Gurung, Stattersfield, Thomas and Birch2014)
• Aichi Target 20, by guiding and catalysing conservation investments by donors; e.g. the Critical Ecosystem Partnership Fund uses KBAs (of which IBAs form the majority of sites) to direct their funding efforts (World Bank IEG 2007).
A recent review (Beresford et al., Reference Beresford, Buchanan, Sanderson, Jefferson and Donald2016) provided evidence that the EU Natura 2000 network of protected areas, of which IBAs form a significant and guiding component (see below), also contributes directly to a number of Aichi Targets, such as 4, 11 and 12 (see below).
This direct contribution of IBAs to targeting protection of sites of high importance within national jurisdictions is mirrored in the parts of the marine environment that fall beyond areas of national jurisdiction. Here, the CBD has led the process of describing Ecologically or Biologically Significant Areas (EBSAs) in Areas Beyond National Jurisdiction, contributing to the efforts of the UN General Assembly to increase the marine protected area network and fill knowledge gaps in the high seas (United Nations General Assembly 2005, CBD 2006). EBSAs are identified using seven scientific criteria (CBD 2008), some of which match closely those used to identify IBAs. Data on IBAs were fed into the series of regional workshops and processes for identifying EBSAs held in 2011–2016, which resulted in the inclusion of 637 marine IBAs within 279 EBSA boundaries (Trathan and Lascelles 2014, BirdLife International 2018a), with the description of some EBSAs drawing almost entirely on IBA data (BirdLife International 2013, CBD 2014). In addition, some countries (e.g. Chile, Japan, South Africa) have opted to identify EBSAs within their territorial waters, using them as target areas for conservation and to promote sustainable activities within their Exclusive Economic Zones.
Additionally, trends in protected area coverage of IBAs provide an indicator that is used to assess progress towards Aichi Target 11 in the scientific literature (Tittensor et al. Reference Tittensor, Walpole, Hill, Boyce, Britten, Burgess, Butchart, Leadley, Regan, Alkemade, Baumung, Bellard, Bouwman, Bowles-Newark, Chenery, Cheung, Christensen, Cooper, Crowther, Dixon, Galli, Gaveau, Gregory, Gutierrez, Hirsch, Höft, Januchowski-Hartley, Karmann, Krug, Leverington, Loh, Lojenga, Malsch, Marques, Morgan, Mumby, Newbold, Noonan-Mooney, Pagad, Parks, Pereira, Robertson, Rondinini, Santini, Scharlemann, Schindler, Sumaila, Teh, van Kolck, Visconti and Ye2014), in CBD reporting processes such as the Global Biodiversity Outlook-4 (Secretariat of the CBD 2014), and in wider processes, such as the global and regional assessments of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (e.g. IPBES 2018a, b, c).
The Convention on Wetlands of International Importance especially as Waterfowl Habitat (the Ramsar Convention)
The Ramsar Convention’s mission is the “Conservation and wise use of all wetlands through local and national actions and international cooperation, as a contribution towards achieving sustainable development throughout the world”. Parties designate wetlands that meet Ramsar criteria for inclusion in the list of “Wetlands of International Importance” and promote the wise use of all wetlands in their territory. Some of the IBA identification criteria (specifically criterion A1 on Globally threatened species and A4 on Congregations – see Reference Donald, Fishpool, Ajagbe, Bennun, Bunting, Burfield, Butchart, Capellan, Crosby, Dias, Diaz, Evans, Grimmett, Heath, Jones, Lascelles, Merriman, O´Brien, Ramirez, Waliczky and WegeDonald et al. in press) were designed to align with particular Ramsar criteria (Criteria 2, 5 and 6), and IBAs have been used to guide the designation of sites under the Ramsar Convention (BirdLife International 2008a, 2014a). For example, the Tana River Delta in Kenya was designated as a Ramsar Site after several years of discussions with local and national stakeholders (http://www.rspb.org.uk/whatwedo/campaigningfornature/casework/details.aspx?id=tcm:9-228564 ).
Nearly a third (29%) of the 3,227 wetland IBAs in Europe, Asia and Africa that qualify under the Ramsar Site criteria have at least some coverage by Ramsar Sites and 15.8% have more than half of their area covered. Trends in Ramsar Site coverage of wetland IBAs that qualify under the Ramsar criteria are used by the convention as a measure of its effectiveness (Gardner et al. Reference Gardner, Finlayson, Davidson, Stroud, Perennou, Barchiesi, Okuno and Dema2017).
Convention on the Conservation of Migratory Species of Wild Animals (CMS)
CMS is a global convention aimed at the conservation of regularly migrating species of animals. Resolution 11.25 on advancing ecological networks to address the needs of migratory species explicitly acknowledges that IBAs comprise the most comprehensive network for such species and that they should be effectively conserved and managed; it also makes specific reference to IBAs in Danger (UNEP CMS 2014). Species-specific and regional initiatives under CMS that encourage the identification and conservation of important sites for birds include the Memorandum of Understanding (MOU) concerning Conservation Measures for the Siberian Crane Grus leucogeranus, the African-Eurasian Migratory Waterbird Agreement (AEWA), the Agreement on the Conservation of Albatrosses and Petrels (ACAP), the MOU on the Conservation of Migratory Birds of Prey in Africa and Eurasia, the MOU on the Conservation of Southern South American Migratory Grassland Bird Species and Their Habitats, and the Central Asian Flyways Initiative (CAF). IBAs have influenced the selection of locations highlighted under these CMS initiatives. For example, the MOU on the Conservation of Migratory Birds of Prey includes a preliminary list of 139 sites that are significant for congregatory bird of prey in Africa and Eurasia, all of which are IBAs (CMS 2008). In the region covered by AEWA, 3,087 Critical Sites were identified as important for 559 populations of 244 waterbird species, all of which are also IBAs (UNEP-GEF African-Eurasian Flyways Project 2011). IBAs with relevance for foraging albatrosses and petrels have been used by ACAP to focus the implementation of by-catch mitigation measures in some oceanic areas, such as in South Atlantic (Dias et al. Reference Dias, Oppel, Bond, Carneiro, Cuthbert, González-Solís, Wanless, Glass, Lascelles, Small, Phillips and Ryan2017).
The European Union’s Birds Directive
In the European Union, the IBA inventory has helped inform the designation of Special Protection Areas (SPAs), which, together with Special Areas of Conservation (SACs), form the Natura 2000 network of sites that provide legal protection to Europe’s most important habitats and species (Council Directive 79/409/EEC, Council Directive 92/43/EEC; European Commission 1979, 1992). For example, the Dutch government used the national IBA directory as the basis for its SPA designation (BirdLife International 2008a) and as a consequence, 91% of the area of IBAs in the Netherlands is now covered by SPAs (Kukkala et al. Reference Kukkala, Santangeli, Butchart, Maiorano, Ramirez, Burfield and Moilanen2016). In Denmark, the information gathered through a network of IBA ‘caretakers’ (volunteers conducting bird surveys and other activities at IBAs) has helped to track populations of qualifying species at IBAs, which has been instrumental in the official designation of 113 of the country’s 128 IBAs as SPAs (BirdLife International 2011d).
The IBA criteria applied in the EU were deliberately aligned with SPA selection criteria (Grimmett and Jones Reference Grimmett and Jones1989, Heath and Evans Reference Heath and Evans2000, Reference Donald, Fishpool, Ajagbe, Bennun, Bunting, Burfield, Butchart, Capellan, Crosby, Dias, Diaz, Evans, Grimmett, Heath, Jones, Lascelles, Merriman, O´Brien, Ramirez, Waliczky and WegeDonald et al. in press). Consequently, the value of the IBA inventory as a ‘shadow list’ of SPAs has repeatedly been recognised by the European Court of Justice and the European Commission in a series of cases brought against Member States for failure to designate sufficient SPAs (European Court of Justice, 1998, 2000, 2002, 2003, 2007a, 2007b, 2007c, 2007d, 2007e, 2016a, 2016b). These cases have helped to catalyse the SPA designation process and as a result, 66% of the terrestrial and 61% of the marine IBA network area in the EU is covered by SPAs (Kukkala et al. Reference Kukkala, Santangeli, Butchart, Maiorano, Ramirez, Burfield and Moilanen2016, Ramirez et al. Reference Ramirez, Tarzia, Dias, Burfield, Ramos, Garthe and Paiva2017). EU Member States with 90% or more of the total terrestrial IBA area covered by SPAs include Estonia, Netherlands, Bulgaria and Latvia (Kukkala et al. Reference Kukkala, Santangeli, Butchart, Maiorano, Ramirez, Burfield and Moilanen2016).
International Financial Institutions
Many international financial institutions (IFIs) have developed social and environmental safeguard mechanisms for their investment projects to minimise or reduce the risk of social or environmental harms caused by their lending activities. Two IFIs make specific reference to IBAs in their safeguards: the European Investment Bank (EIB) and the European Bank of Reconstruction and Development (EBRD). The EIB requires project proposals to specify whether the project will affect and have a negative impact on sites identified as IBAs or that have an equivalent status in detailed scientific inventories endorsed by national authorities (EIB 2013). EBRD considers IBAs as part of “priority biodiversity features”, a subset of biodiversity that is particularly irreplaceable or vulnerable (EBRD 2014).
IBAs are also used to identify sensitive areas that are relevant to the safeguards of other IFIs. In the International Finance Corporation’s (IFC) 2012 Performance Standards on Environmental and Social Sustainability (IFC 2012), Performance Standard 6 on Biodiversity Conservation and Sustainable Management of Living Natural Resources refers to ‘Critical Habitats’ as areas with high biodiversity value. IBAs are relevant to Critical Habitat defined according to particular IFC criteria. The exact equivalence has been mapped spatially for both the marine (Martin et al. Reference Martin, Tolley, Farmer, Mcowen, Geffert, Scharlemann, Thomas, van Bochove, Stanwell-Smith, Hutton, Lascelles, Pilgrim, Ekstrom and Tittensor2015) and terrestrial realms (Reference Brauneder, Montes, Blyth, Bennun, Butchart, Hoffmann, Burgess, Cuttelod, Jones, Kapos, Pilgrim, Tolley, Underwood, Weatherdon and BrooksBrauneder et al. in press). Similarly, the World Bank´s new Environmental and Social Framework (World Bank 2017) refers to Critical Habitats, which includes sites with high biodiversity importance or value (e.g. for Critically Endangered, Endangered, endemic, restricted-range and migratory species). The Equator Principles, a risk management framework adopted by 80 financial institutions in 35 countries for determining, assessing and managing environmental and social risk in projects in developing and emerging economies also adopt and align with the IFC Performance Standards. The IFC, World Bank, Asian Development Bank and 12 of the Equator Principles Financial Institutions and OECD Export Credit Agencies currently access spatial data on IBAs to inform their lending using the Integrated Biodiversity Assessment Tool (IBAT, https://www.ibatforbusiness.org/).
The private sector
Many private sector companies are important stakeholders and decision-makers for IBAs. Their operations within or around IBAs may have a significant negative impact on the bird species for which the site has been identified. Many companies have developed environmental and social policies to avoid or minimise damage to the environment and communities within their range of operations and through their product chains. They are therefore important end-users of IBA data, which can help them to reduce risks related to potential damage to biodiversity and ecosystem services. Forty companies across a broad range of sectors (including automotive, cement and aggregates, chemicals, financial institutions, food processing, insurance, mining and metals, multi-industry companies, oil and gas, and packaging and paper) access data on IBAs through IBAT. This provides a basic risk screening on biodiversity by drawing together spatial datasets from the World Database of Key Biodiversity Areas, the World Database on Protected Areas, and the IUCN Red List of Threatened Species. Through an interactive mapping tool, companies access and use up-to-date information to identify biodiversity risks and opportunities within a specified boundary.
A more targeted platform – the Soaring Bird Sensitivity Mapping Tool (http://migratorysoaringbirds.undp.birdlife.org/en/sensitivity-map) – is used by companies in the wind energy sector, planning authorities and financial institutions to access data on IBAs and other datasets to assess the potential risk of wind energy developments to soaring birds (Allinson Reference Allinson and Köppel2017). The World Bank’s environmental, health, and safety guidelines for wind energy (World Bank, 2015) specifically refer to the tool as a means to avoid and minimise potential adverse impacts of wind energy developments on biodiversity. A sensitivity atlas based on the mapping tool is being developed for Egypt to help raise awareness of decision-makers of particularly sensitive sites, including IBAs, to disturbance by the wind sector.
Donor organizations
Since 2001, CEPF has funded conservation in 24 of the 36 biodiversity hotspots identified globally (Myers et al. Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000). CEPF has used KBAs (including bird-triggered sites, i.e. IBAs) as the lens for identifying geographic priorities since 2003. Before investing in a biodiversity hotspot, CEPF prepares an ecosystem profile: an in-depth analysis of the region that involves hundreds of stakeholders. Part of the process includes identifying KBAs for the region and then prioritising among them for investment (thematic investment priorities are also developed). To date, about one-third of the world’s 15,000 KBAs have been identified through the CEPF ecosystem profiling process under earlier iterations of the KBA criteria, with 53% of these KBAs also qualifying as IBAs.
The Global Environment Facility (GEF) is the biggest donor of biodiversity conservation projects and programmes. During the sixth replenishment period for 2014-18, programme 2 has focused on the expansion of the global protected area estate. This programme requires that that protected areas established with GEF support are globally significant, as defined by a set of criteria on vulnerability and irreplaceability which were modelled on the draft KBA Standard that was under development at the time (GEF 2014). As the large majority of all KBAs are IBAs, this means that GEF funding during this period that was targeted at the establishment of new protected areas was typically directed on the basis of identified IBAs.
National level policy
In this section, we give an overview and examples of how IBAs have been used to inform expansion of the protected area estate.
Protected areas
Protected areas form a central pillar in global efforts to safeguard biological diversity (Bruner et al. Reference Bruner, Gullison, Rice and Fonseca2001). However, although protected areas cover 14.7% of the Earth’s terrestrial surface and 4.12% of the global oceans, including 10.2% of coastal and marine areas under national jurisdiction (UNEP-WCMC and IUCN 2016), the coverage of biodiversity by protected areas remains inadequate and incomplete (Juffe-Bignoli et al. Reference Juffe-Bignoli, Burgess, Bingham, Belle, de Lima, Deguignet, Bertzky, Milam, Martinez-Lopez, Lewis, Eassom, Wicander, Geldmann, van Soesbergen, Arnell, O’Connor, Park, Shi, Danks, MacSharry and Kingston2014, Butchart et al. Reference Butchart, Clarke, Smith, Sykes, Scharlemann, Harfoot, Buchanan, Angulo, Balmford, Bertzky, Brooks, Carpenter, Comeros-Raynal, John Cornell, Ficetola, Fishpool, Fuller, Geldmann, Harwell, Hilton-Taylor, Hoffmann, Joolia, Joppa, Kingston, May, Milam, Polidoro, Ralph, Richman, Rondinini, Segan, Skolnik, Spalding, Stuart, Symes, Taylor, Visconti, Watson, Louisa Wood and Burgess2015). As of February 2018, nearly three quarters of all IBAs (72.7%) are at least partially covered by protected areas, and 22.0% are completely (> 98%) covered; on average, 49.3% of the area of each IBA is covered by protected areas. This incomplete coverage is significant for threatened biodiversity given that greater protected area coverage of IBAs has been shown to be associated with reduced declines in extinction risk of the species for which the sites have been identified (as measured using Red List Indices; Butchart et al. Reference Butchart, Scharlemann, Evans, Quader, Arinaitwe, Bennun, Besançon, Boucher, Bomhard, Brooks, Burfield, Burgess, Clay, Crosby, Davidson, De Silva, Devenish, Dutson, Díaz Fernández, Fishpool, Foster, Hockings, Hoffmann, Knox, Larsen, Lamoreux, Loucks, May, Millett, Parr, Skolnik, Upgren and Woodley2012). This global-scale result mirrors the pattern found by Mwangi et al. (Reference Mwangi, Butchart, Munyekenye, Bennun, Evans, Fishpool, Kanyanya, Madindou, Machekele, Matiku, Mulwa, Ngari, Siele and Stattersfield2010) who reported that IBAs inside protected areas in Kenya were in better condition, with marginally lower pressures but significantly stronger conservation responses, than IBAs outside protected areas. Encouragingly, protected area coverage of IBAs has increased substantially over time (Figure 1), although the proportion of the protected area estate that overlaps IBAs is declining (Butchart et al. Reference Butchart, Scharlemann, Evans, Quader, Arinaitwe, Bennun, Besançon, Boucher, Bomhard, Brooks, Burfield, Burgess, Clay, Crosby, Davidson, De Silva, Devenish, Dutson, Díaz Fernández, Fishpool, Foster, Hockings, Hoffmann, Knox, Larsen, Lamoreux, Loucks, May, Millett, Parr, Skolnik, Upgren and Woodley2012).
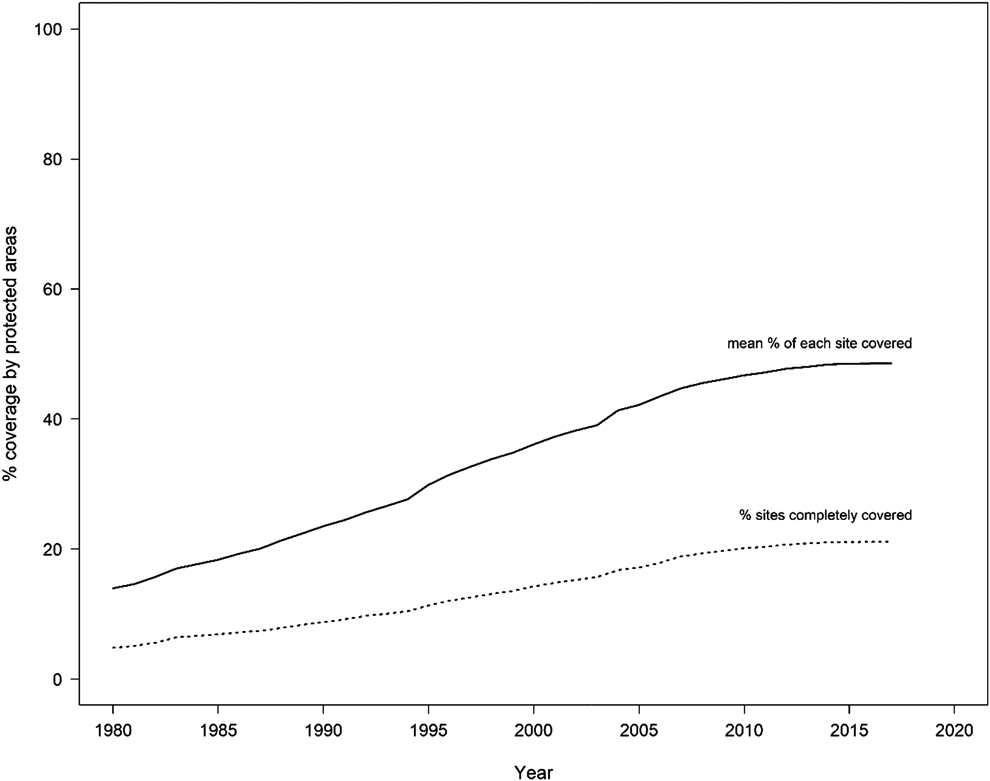
Figure 1. Coverage of the global IBA network by protected areas ( n = 10,708 IBAs). The solid line shows the mean percentage of each IBA covered by protected areas; the dashed line shows the percentage of IBAs that are completely covered (i.e. > 98% of their area) by protected areas.
Under the Sustainable Development Goals (SDG) 14 and 15, protected area coverage of Key Biodiversity Areas, the great majority of which are IBAs, is used for three indicators to assess progress (UN 2016, Sachs et al. Reference Sachs, Schmidt-Traub, Kroll, Durand-Delacre and Teksoz2016):
• Indicator 14.5.1 Coverage of protected areas in relation to marine areas
• Indicator 15.1.2 Proportion of important sites for terrestrial and freshwater biodiversity that are covered by protected areas, by ecosystem type
• Indicator 15.4.1 Coverage by protected areas of important sites for mountain biodiversity.
The identification of IBAs, publication of their details in directories, and lobbying for them by the BirdLife Partnership have also brought about formal protection for some of them. Globally, the mean percentage of each IBA covered by protected areas within Partner countries (53.8%, n = 120) is significantly higher than that within non-Partner countries (39.8%, n = 81) (t-test with unequal variance, P < 0.0001). In countries that have both IBAs and non-IBA KBAs, IBAs have a significantly higher mean percentage coverage by PAs (40.2%) than non-IBA KBAs (28.3%) (P < 0.0001, paired t-test). According to systematic questionnaires completed by 120 BirdLife Partners, 903 IBAs received formal protection during the period 2013–2015 at least partly as a result of BirdLife advocacy (BirdLife International 2016).
There are numerous examples of a direct link between IBA identification and protected area designation. For example, the identification of IBAs in Tunisia prompted the national government to grant reserve status to 29 hitherto unprotected sites (Coulthard Reference Coulthard2002, BirdLife International 2008b). In Timor-Leste, preparation of the IBA directory between 2002 and 2006 represented the first systematic evaluation of the country’s biodiversity sites (Trainor et al. Reference Trainor, Santana, Rudyanto, Pinto and de Oliveira2007); three IBAs identified in the far east of the country were later combined to become the nation’s first national park (BirdLife International 2008c). The identification of Lo Go Xa Mat in Vietnam as an IBA generated considerable interest among local officials, who had previously been unaware of the biodiversity value of the site and who had initiated a programme of wetland drainage and conversion to farmland. Subsequently, provincial leaders sought protected area status for the area and, in July 2002, Lo Go Xa Mat was declared a National Park (BirdLife International 2004b).
Nevertheless, designating formal protected area status may not be the most appropriate option for all IBAs. Indeed, alternative management solutions, such as community-based management for sustainable use, may be more appropriate (BirdLife International 2004c). This is recognised under Aichi target 11, which calls for areas of importance for biodiversity to be conserved through “protected areas and other effective area-based conservation measures”. Preliminary results from a study of over 800 unprotected KBAs (mostly IBAs) in a sample of 10 countries indicates that a high proportion (> 79%) of unprotected KBAs are overlapped by management structures that appear to fit the definition of ‘other effective area-based measures’ such as sacred sites and indigenous reserves. This may partly explain why governments have not designated formal protected areas that overlap these sites. Whatever the governance model for an IBA, community engagement and involvement in its conservation is usually essential (BirdLife International 2010a).
Site-level action
In this section we provide information on how IBAs have been used to champion activities by local volunteers and communities to safeguard and conserve these sites.
Local conservation groups
The task of effectively conserving over 13,000 IBAs worldwide is beyond the power of any centralised agency or even a network of national organisations and is possible only if management and protection have the support and involvement of the local population. Such people make most of the daily land-management decisions, frequently have rights to local land and its resources, and their knowledge of the environment, culture and society mean that they can often play a critical role in conservation. Conservation that involves local people is likely to be more effective, more efficient, more sustainable and more equitable than conservation imposed from outside (e.g. Ancrenaz et al. Reference Ancrenaz, Dabek and O’Neil2007, Vermeulen and Sheil Reference Vermeulen and Sheil2007, Roe et al. 2009, Lambrick et al. Reference Lambrick, Brown, Lawrence and Bebber2014, Shiel et al. 2015).
The IBA concept has focussed efforts to strengthen civil society structures and promote the role of local stakeholders in their management and protection. Collectively, such organisations are referred to as Local Conservation Groups (LCGs; BirdLife International 2014a). Members of these organizations usually live and work within or close to an IBA and their livelihoods therefore often depend on the condition of habitats and the delivery of ecosystem goods and services. While these groups are all based on the same principle of local, civil-society engagement and empowerment, LCGs vary in nature considerably, both between and within countries. In much of Africa for example, they comprise (usually pre-existing) community-based organisations, and an emphasis is put on linking conservation and sustainable management of natural resources to alleviate poverty (Thomas Reference Thomas2011). Thus, LCGs here are often formed around their members’ interest in the management of specific natural resources, such as a fishery (e.g. at Musambwa Islands IBA in Uganda), or tourism (e.g. at Arabuko-Sokoke Forests IBA in Kenya).
In Europe, BirdLife Partner organizations have established national networks of IBA ‘Caretakers’, many of whom are Partner members living locally at an IBA. This approach has its origins in the Czech Republic, where local caretaker groups were established in the early 1990s. In the USA, State Chapters of the National Audubon Society encourage bird clubs and other local community groups to ‘adopt’ one or more IBAs (see http://web4.audubon.org/bird/iba/ibaadopt.html). These ‘IBA Adoption Groups’ provide stewardship of the site, recruit volunteer citizen scientists for monitoring, and offer educational opportunities that help conserve the site. Aves Argentinas (BirdLife in Argentina) has supported a network of ‘Clubes de Observadores de Aves’ (COAS) (See http://www.avesargentinas.org.ar/clubes-de-observadores-de-aves). Under the coordination of Aves Argentinas these independent, voluntary groups of amateur birdwatchers, each led by a local environmentalist, are supporting conservation efforts at 40 of the country’s 268 IBAs. In the Middle East, the Society for the Protection of Nature in Lebanon (BirdLife in Lebanon) is adopting and adapting the hima, a traditional system under which local communities manage natural areas and protect them from over-exploitation (SPNL 2016). By providing support to local institutions they are supporting LCGs to deliver an approach to resource management which is culturally familiar and acceptable, having its roots in traditional practice and Islamic law. In southern Ethiopia, the Ethiopian Wildlife and Natural History Society (EWNHS; BirdLife in Ethiopia) is supporting community groups of local pastoralists at the Liben Plain IBA to strengthen traditional systems of grazing management to benefit their livelihoods and to protect the ‘Critically Endangered’ Liben Lark Heteromirafra archeri.
LCG members support IBA conservation through a whole range of activities, including site monitoring, education and awareness raising, campaigning, and integrating conservation with local development (BirdLife International 2011a, Matiku Reference Matiku, Brooks and Arico2013). For example, in Australia, local communities have worked together to protect beach-nesting Hooded Plover Thinornis rubricollis from disturbance, resulting in a significant increase in the number of fledged chicks of this ‘Vulnerable’ species (BirdLife International 2011b); at Lake Chilwa in Malawi, community-organised hunting clubs have helped bring unregulated hunting under control (BirdLife International 2011c); in Nepal, Forest User Groups at Phulchoki Mountain Forests IBA have helped to reverse forest degradation, ensuring sustainable use of resources and enhanced economic benefits from recreation for local people (see Birch et al. Reference Birch, Thapa, Balmford, Bradbury, Brown, Butchart, Gurung, Hughes, Mulligan, Pandeya, Peh, Stattersfield, Walpole and Thomas2014); and in Denmark data provided by IBA Caretakers have been key to designating IBAs as Special Protection Areas (BirdLife International 2011d).
IBAs in danger
IBAs, particularly unprotected ones, are coming under increasing pressure from a wide range of human activities, leading to the deterioration and conversion of natural habitats, reduction in the population size of trigger species and loss of ecological integrity and ecosystem services of IBAs. In some cases, focused efforts have resulted in mitigating or the elimination of these threats.
In Europe, civil society challenges threats to IBAs through many legal cases every year. Within the EU in 2014 alone, there were 63 cases involving IBAs, all of which have been submitted as complaints to the European Commission as breaches of EU environmental protection laws. For example, the Via Baltica expressway threatened the integrity of several forest and wetland IBAs in north-east Poland at the time when the country was in the process of acceding to the European Union. Thanks to intensive advocacy, which included legal action at the national and EU levels, petitions, letter-writing, demonstrations and a media campaign, the most environmentally damaging section of the road was re-routed to avoid sensitive nature areas (http://www.rspb.org.uk/whatwedo/campaigningfornature/casework/details.aspx?id=tcm:9-228488). In Africa, several IBAs have recently been the focus of intensive, internationally supported campaigns. For example, at Lake Natron, Tanzania, a regional coalition of stakeholders managed to avert the threat of a soda mine to this exceptionally fragile ecosystem holding most of the world´s breeding population of the ‘Near Threatened’ Lesser Flamingo Phoeniconaias minor (BirdLife International 2012, 2018b). In the Americas, the proposed degazettement of the existing protected areas of the Upper Panama Bay, one of the most important wintering and stopover sites of migratory shorebirds in the Western Hemisphere, was averted in February 2015 when the protection of the IBA was reinstated by a Presidential decree, following years of campaigning by a coalition of national NGOs (BirdLife International 2014b). BirdLife’s IBAs in Danger initiative uses systematic site monitoring assessments to identify the most threatened IBAs, in order to direct efforts such as these to the sites that require them most urgently.
Concluding remarks: the past and future impact of IBAs
The global list of IBAs represents the most comprehensive inventory so far of sites identified systematically using standardised criteria. As described above, over the last three decades, data on IBAs have been used by a wide range of stakeholders to maintain and improve the conservation status of these sites through legal protection, sustainable management, safeguarding measures, land use policy and local action. Numerous examples exist to illustrate the contributions that IBAs have made to improving biodiversity outcomes. However, systematic and quantitative data on their global impact is relatively sparse (notwithstanding the exceptions detailed above). This reflects the wider insufficiency in impact assessment and evidence-base across the conservation sector (e.g. Ferraro Reference Ferraro2009) and is stimulating increased efforts to quantify impacts and determine counterfactuals (BirdLife International 2016, Dickson et al. Reference Dickson, Butchart, Dauncey, Hughes, Jefferson, Merriman, Munroe, Pearce-Higgins, Stephenson, Sutherland, Thomas and Trevelyan2017).
Despite the apparent influence of IBAs on policy, from their explicit use in the safeguards of international finance institutions to their role in informing protected area networks and their influence in the implementation of multilateral environmental organisations, many remain highly threatened. For example, Industrial development, including residential and commercial development and transportation corridors are identified as principal threats on 84 out of the 338 IBAs in Danger listed in 2016 (25%). Clearly, the lenders, international credit agencies, and companies that explicitly use IBA data to inform their decisions and operations represent only a subset of those involved in all development globally. Furthermore, the broader context is one of growing pressures on biodiversity driven by growth in human population size and levels of consumption, leading to unsustainable development, environmental degradation, and widespread biodiversity loss (Butchart et al. Reference Butchart, Walpole, Collen, van Strien, Scharlemann, Almond, Baillie, Bomhard, Brown, Bruno, Carpenter, Carr, Chanson, Chenery, Csirke, Davidson, Dentener, Foster, Galli, Galloway, Genovesi, Gregory, Hockings, Kapos, Lamarque, Leverington, Loh, McGeoch, McRae, Minasyan, Morcillo, Oldfield, Pauly, Quader, Revenga, Sauer, Skolnik, Spear, Stanwell-Smith, Stuart, Symes, Tierney, Tyrrell, Vié and Watson2010). In this context, the prevalence of threats to IBAs is not surprising, despite the successes described above.
This context also partly explains why, on average, half of the area of each IBA remains unprotected. In addition, emerging evidence that a large proportion of unprotected IBAs may be covered by ‘other effective area-based conservation measures’ (see above) hints that governments may not have targeted formal protection at these sites partly owing to the presence of alternative management systems. Further research on this issue is needed. The low proportion of IBAs qualifying for Ramsar Site status that have been designated as such is, on the face of it, perhaps surprising. However, it is possible that governments tend to choose just the most outstanding of their wetland areas for nomination as Ramsar Sites, rather than systematically assessing all potentially qualifying areas.
In considering in what ways IBAs have been most successfully used to stem biodiversity loss, it is clear that where governments have adopted tightly defined procedures for determining where to locate protected areas (e.g. through the Birds Directive for determining Special Protection Areas in the EU), backed up by legislative processes (e.g. the European Court of Justice), IBAs have been more influential, compared with countries with more flexibility over land-use planning. Similarly, IBAs have had greater influence with those actors in the private sector that have adopted strict standards and safeguards, while the biodiversity importance of IBAs is regularly ignored by poorly executed environmental impact assessments in countries with weaker governance. Increasing the effectiveness of future efforts to safeguard and conserve IBAs requires greater attention to defining counterfactuals and quantifying impacts (Ferraro Reference Ferraro2009, Dickson et al. Reference Dickson, Butchart, Dauncey, Hughes, Jefferson, Merriman, Munroe, Pearce-Higgins, Stephenson, Sutherland, Thomas and Trevelyan2017), as well as sharing evidence and lessons learned more effectively.
With the publication of the new global Key Biodiversity Areas (KBA) Standard (IUCN 2016), a single, unified set of criteria has emerged which is applicable to all macro-organisms in the terrestrial, freshwater and marine realms. IBAs make up the majority of the KBAs identified to date (BirdLife International 2017). A major challenge now is to apply this Standard to the various other biodiversity elements for which relevant data are available in order to identify a comprehensive set of KBAs around the world. This challenge was taken up by 12 international conservation organisations (Amphibian Survival Alliance, BirdLife International, Critical Ecosystems Partnership Fund, Conservation International, Global Environment Facility, Global Wildlife Conservation, International Union for Conservation of Nature, NatureServe, Rainforest Trust, Royal Society for the Protection of Birds, Wildlife Conservation Society, World Wide Fund for Nature) who established the KBA Partnership and have initiated the KBA programme. The goal of this is: “to develop and maintain an up-to-date, fully documented list of sites identified against the KBA Standard, and to communicate, promote and position this information to enable the achievement of the KBA vision”. It is hoped that decision-makers at all levels will welcome this new, unified list of KBAs (available at www.keybiodiversityareas.org) as the basis for further protected area designations, conservation management and land-use planning.
To ensure this happens, a number of developments need to be undertaken urgently. Information on KBAs will need to be shared with end users, including governments, multi-lateral environmental agreements and international financing institutions to mainstream them into relevant decisions affecting KBAs. The KBA Consultative Forum of end users has been established to ensure that KBA data are presented in a way that is most helpful and relevant. Another structure, the KBA Community serves as a platform to KBA practitioners, including scientific experts and practitioners working at KBAs. Local Conservation Groups established at IBAs can also form part of the Community, which helps the exchange of experiences and best practices of conserving IBAs and KBAs and formation of new groups at an increasing number of sites. Most of the KBA Partners are already working on individual KBAs, many of which are highly threatened by development and land-use changes. Increased collaboration and coordination between these international organisations is expected to lead to more efficient conservation gains both at the individual site and at site network levels.
The degree to which the KBA programme succeeds in scaling up the conservation outcomes and impacts of IBAs, as reviewed here, may play a critical role in shaping the fate of the planet’s most important locations for nature over the coming decades.
Acknowledgements
The authors would like to express their thanks to the thousands of experts, volunteers and other stakeholders who have been actively engaged in the identification, documentation and location action for the conservation of IBAs since the inception of the IBA Programme more than three decades ago. We are particularly grateful to the 120-strong network of BirdLife Partner organizations around the world for adopting the IBA approach and for their tireless work in promoting and conserving IBAs at the national and international level.