INTRODUCTION
Coralligenous assemblage is a hard bottom habitat characteristic of the Mediterranean Sea and particularly important for its extent, biodiversity and role in carbon dynamics (Ballesteros, Reference Ballesteros2006; Relini & Giaccone, Reference Relini and Giaccone2009; Piazzi et al., Reference Piazzi, Balata, Cecchi, Cinelli and Sartoni2010). It originates from biogenic formations produced by the accumulation of crustose coralline algae, growing at low irradiance and stable environmental conditions, and creating calcareous layers overlapping with other carbonate organisms (True, Reference True1970; Laborel, Reference Laborel1987; Marti et al., Reference Marti, Uriz, Ballesteros and Turon2004). These bioconstructions are structurally complex and permanent, and have great ecological importance in creating heterogeneous habitats and representing an attractive substrate for the settlement of many invertebrate species and sessile organisms (Laubier, Reference Laubier1966; Sartoretto et al., Reference Sartoretto, Verlaque and Laborel1996; Relini, Reference Relini2009).
Coralline algae are among the main bioconstructor organisms of coral reefs (Finckh, Reference Finckh1904; Hillis-Colinvaux, Reference Hillis-Colinvaux1986). While they are usually restricted to littoral or shallow sublittoral environments in tropical areas (Lee, Reference Lee1967; Littler, Reference Littler1973), the coralline algal concretions thrive in deeper waters (20–120 m depth) in the coralligenous frameworks of the Mediterranean Sea (Ballesteros, Reference Ballesteros2006). Two major forms of coralligenous habitats have been described: (i) rims, growing on coastal rocks (e.g. vertical cliffs, overhangs and outer parts of marine caves); (ii) platform outcrops, developing on the horizontal continental shelves over consolidated sediments, coalesced rhodolites or pre-existing rocky substrates (Pérès & Picard, Reference Pérès and Picard1964; Laborel, Reference Laborel1987; Ballesteros, Reference Ballesteros2006).
Coral reefs represent one of the most threatened ecosystems in tropical seas, just as the coralligenous bioconstructions are among the most endangered Mediterranean habitats, as confirmed by international protection agreements (Habitat Directive 92/43/CEE; SPA/BIO Protocol; Barcelona Convention; Berne Convention), since they are characterized by the presence of species highly sensitive to human disturbance, such as the fan corals Corallium rubrum, Eunicella cavolinii and Paramuricea clavata (Brown & Macfadyen, Reference Brown and Macfadyen2007; Relini & Tunesi, Reference Relini and Tunesi2009; Bavestrello et al., Reference Bavestrello, Bo, Canese, Sandulli and Cattaneo-Vietti2014). Many anthropic activities, such as fishing, have negative effects particularly on hard bottom benthic communities (Collie et al., Reference Collie, Hall, Kaiser and Poiner2000). Fishing may lead to modifications in communities structure and functioning, shifting species composition towards opportunistic assemblages composed of rapid growth rates species (Schiaparelli et al., Reference Schiaparelli, Chiantore, Cattaneo-Vietti, Novelli, Drago, Albertelli, Faranda, Guglielmo and Spezie2001; Clark & Koslow, Reference Clark, Koslow, Pitcher, Morato, Hart, Clark, Haggan and Santos2007; Daskalov et al., Reference Daskalov, Grishin, Rodionov and Mihneva2007). Furthermore, the pressure of fishing activities reduces coverage, diversity and abundance of habitat-forming species and associated organisms (Blanchard et al., Reference Blanchard, Leloc'h, Hily and Boucher2004; Althaus et al., Reference Althaus, Williams, Schlacher, Kloser, Green, Barker, Bax, Brodie and Schlacher-Hoenlinger2009; Maynou & Cartes, Reference Maynou and Cartes2012). Within coralligenous assemblages, erect and branched organisms with calcareous skeletons and those that are large, such as bryozoans and fan corals, are the most endangered by fishing gears, since their colonies are often damaged, broken and upturned, and show necrosis due to mechanical friction, leading to their being overgrown by epibionts (Hall-Spencer et al., Reference Hall-Spencer, Allain and Fossä2002; UNEP, 2009; Bo et al., Reference Bo, Bava, Canese, Angiolillo, Cattaneo-Vietti and Bavestrello2014a; Angiolillo et al., Reference Angiolillo, di Lorenzo, Farcomeni and Bo2015).
Over the past decade there has been an increase in the amount, distribution and effects of lost fishing gears, due to the rapid expansion of fishing effort and fishing grounds (Gilman, Reference Gilman2015). Lost fishing gears, such as nets and longlines, from both professional and recreational fishing activities can pose substantial ecological and socioeconomic problems. Indeed, ghost fishing, mainly represented by lost nets and longlines, continues to catch target, non-target and protected species (such as turtles, seabirds and marine mammals), alters the benthic environment, introduce synthetic material into the marine food web, facilitates the introduction of alien species and passively entangles marine organisms (Macfadyen et al., Reference Macfadyen, Huntington and Cappell2009).
Generally, lost gears of artisanal fishing (longlines, hooks, etc.) mainly affect the coralligenous assemblages on rocky cliffs and shoals, where fishes are gathered (Sbrescia et al., Reference Sbrescia, Di Stefano, Russo and Russo2008). Professional fishing such as trawling, practiced on the shelf soft bottoms, may affect the deep coralligenous banks scattered on these bottoms. This kind of pressure is unfortunately very common in different areas of the Tyrrhenian Sea (Colloca et al., Reference Colloca, Cardinale, Belluscio and Ardizzone2003; Mangano et al., Reference Mangano, Kaiser, Porporato and Spanò2013); it is among the most destructive fishing methods causing the degradation of large coralligenous bioconstructions, both by damaging the biological structures mechanically, and by affecting the production of crustose algae with the increase of turbidity and sedimentation rates (Kaiser et al., Reference Kaiser, Collie, Hall, Jennings and Poiner2001; Ballesteros, Reference Ballesteros2006; Clark & Koslow, Reference Clark, Koslow, Pitcher, Morato, Hart, Clark, Haggan and Santos2007; Althaus et al., Reference Althaus, Williams, Schlacher, Kloser, Green, Barker, Bax, Brodie and Schlacher-Hoenlinger2009).
The Bay of Naples has been chosen among the assessment areas for inclusion in the Marine Strategy Framework Directive (MSFD-2008/56/EC) in order to study benthic communities. The seabed fauna of the Bay of Naples has been extensively studied since the 19th century (Colombo, Reference Colombo1887; Mazzarelli & Mazzarelli, Reference Mazzarelli and Mazzarelli1918; Ranzi, Reference Ranzi1930). However, information on the types and distribution of benthic habitats has only recently been acquired; particularly, Russo et al. (Reference Russo, Di Donato and Di Stefano2008) described seascape habitats of some MPAs established longshore of the Gulf of Naples.
Along the Campania's coasts, several mass mortality events of benthic communities, especially fan corals, recorded mostly during the summer period, were detected (Gambi et al., Reference Gambi, Barbieri, Signorelli and Saggiomo2010; Bavestrello et al., Reference Bavestrello, Bo, Canese, Sandulli and Cattaneo-Vietti2014). The density of some characteristic species of coralligenous assemblages, such as Eunicella cavolinii (Koch, 1887), E. singularis (Esper, 1791), Paramuricea clavata (Risso, 1826) and Corallium rubrum (L., 1758), has been drastically reduced (Sbrescia et al., Reference Sbrescia, Di Stefano, Russo and Russo2008). In all these cases the highest mortality rates were recorded around 30–40 m depth during the summer and this is mainly correlated with the increase in Mediterranean surface water temperatures, lowering the thermocline at higher depths, possibly in relation with the global warming (Cinelli et al., Reference Cinelli, Relini, Tunesi and Relini2009). The human stress has played, however, an important role in the degradation of these habitats since the areas have been exploited by fishing activities for centuries (Colombo, Reference Colombo1887; Russo et al., Reference Russo, Ascione and Franzese2004).
In this study, a first analysis of some of the most exploited coralligenous habitats of the Bay of Naples was performed, in order to evaluate the characteristics of the different coralligenous assemblages and to assess the extent of fishing impact on these bioconstructions.
MATERIALS AND METHODS
Study area
Although coralligenous habitats are scattered in the whole study area, they are mainly grouped on the north-west side where the large number of rocky outcrops (mostly remains of submarine volcanic cones) play an important biological role as hotspots of biodiversity in a widely distributed muddy bottom (Russo, Reference Russo1997). The distribution of coralligenous habitat patches in the Bay of Naples is shown in Figure 1.
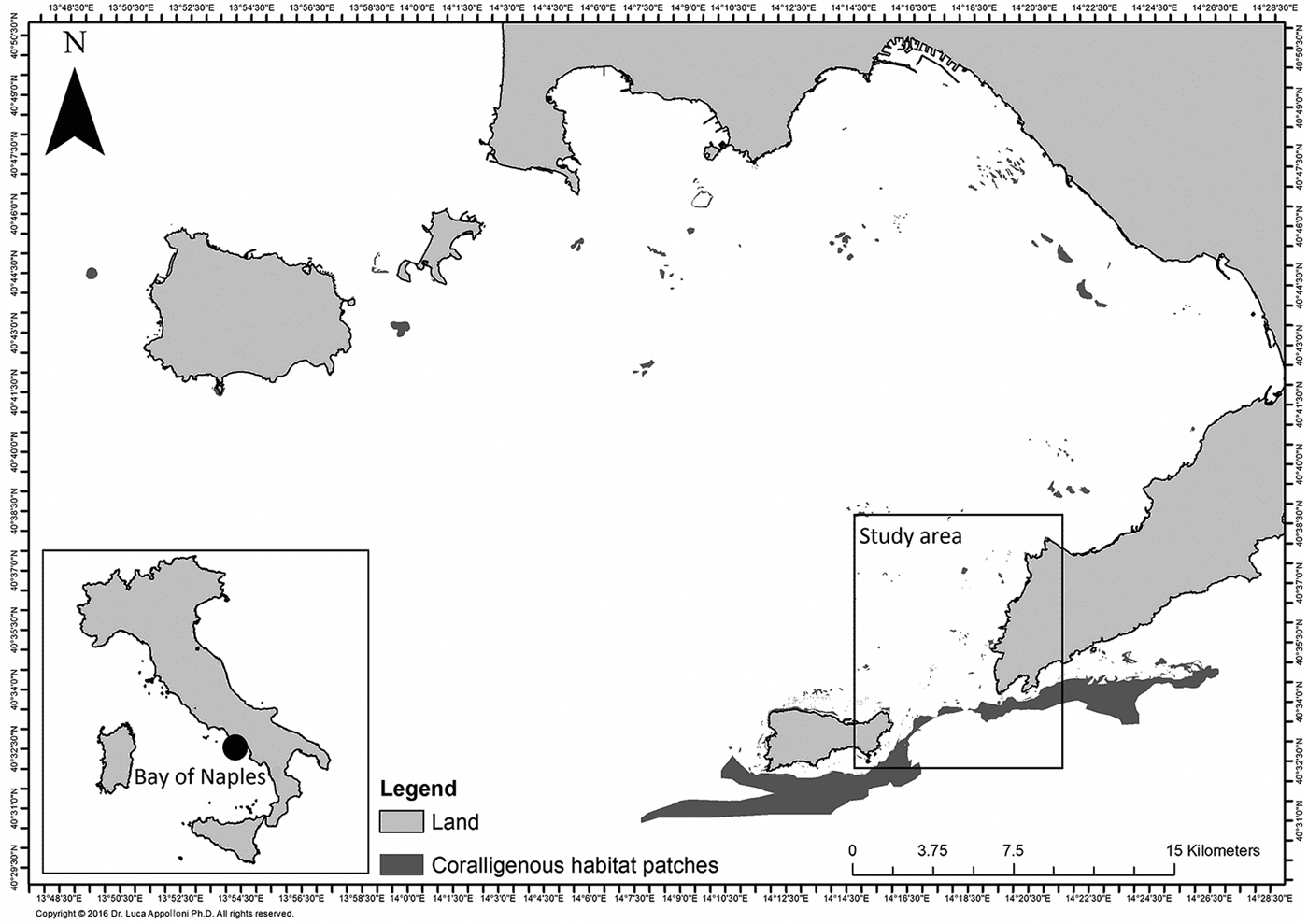
Fig. 1. Coralligenous habitat patches scattered in the whole Bay of Naples.
The south-east side, consisting mostly of a large muddy platform that gently degrades until 200 m depth, is more regular than the north-west side. In this area there are many little outcrops of coralligenous bioconstructions surrounded by soft bottom, principally resulting from accumulation and compaction of biogenic sediment, more or less isolated and variable in size. Their presence, however, is reported mainly from the empirical knowledge of fishermen. At the south-east side, the Sorrento Peninsula separates the Bay of Naples and the Bay of Salerno. Here, the continental slope starts steeply at about 70 m depth and is characterized by a high hydrodynamic regime determining the presence of environmentally and economically important species such as the precious red coral Corallium rubrum (Bavestrello et al., Reference Bavestrello, Bo, Canese, Sandulli and Cattaneo-Vietti2014; Cattaneo et al., Reference Cattaneo, Bo, Cannas, Cau, Follesa, Meliadò, Russo, Sandulli, Santangelo and Bavestrello2016).
Field activities
Following the Marine Strategy protocols (MSFD-2008/56/EC) for coralligenous habitats, surveys with a ROV (Remotely Operated Vehicle) were performed in high natural value areas represented, in this case, by three coralligenous patches. A ROV (Perseus by Ageotec) equipped with a high definition (HD) camera, 2 lights, 2 parallel laser beams for the evaluation of size of the organisms, was used. In addition, a navigation camera with underwater positioning system USBL (Ultra Short Base Line System) interfaced with an on-board navigation system, which allows determination in real time of the geographic location and the exact depth in which the ROV is located, was mounted on the ROV.
The study area was selected on the basis of cartographic and bibliographic information (Russo, Reference Russo and Giammarino1992, Reference Russo, Rosi and Jannuzzi2000) and in relation to the results of some recent video-surveys aimed to assess the presence and the preservation status of some ancient red coral populations (Corallium rubrum) in the Bay of Naples (Bavestrello et al., Reference Bavestrello, Bo, Canese, Sandulli and Cattaneo-Vietti2014).
A preliminary campaign was carried out in May 2014, in order to map and to assess the ecological status of coralligenous habitats. In particular, three sites, located at the SE part of the Bay of Naples were investigated: Bocca Piccola, a platform coralligenous outcrop, located at the north of Sorrentino Peninsula (about 120 m depth); Secchitiello, a small shoal located at SW of Sorrento Peninsula, with a bathymetric range of 55 and 75 m; Ieranto, the shallower part of continental slope, located at about 0.5 miles south off Sorrentino Peninsula, ranging from ~70–150 m depth, with a mean slope of ~25° (Figure 2). These three sites may be considered as representative of the different coralligenous habitats present in the Bay and are exposed to many anthropic impacts affecting deep hard bottoms.
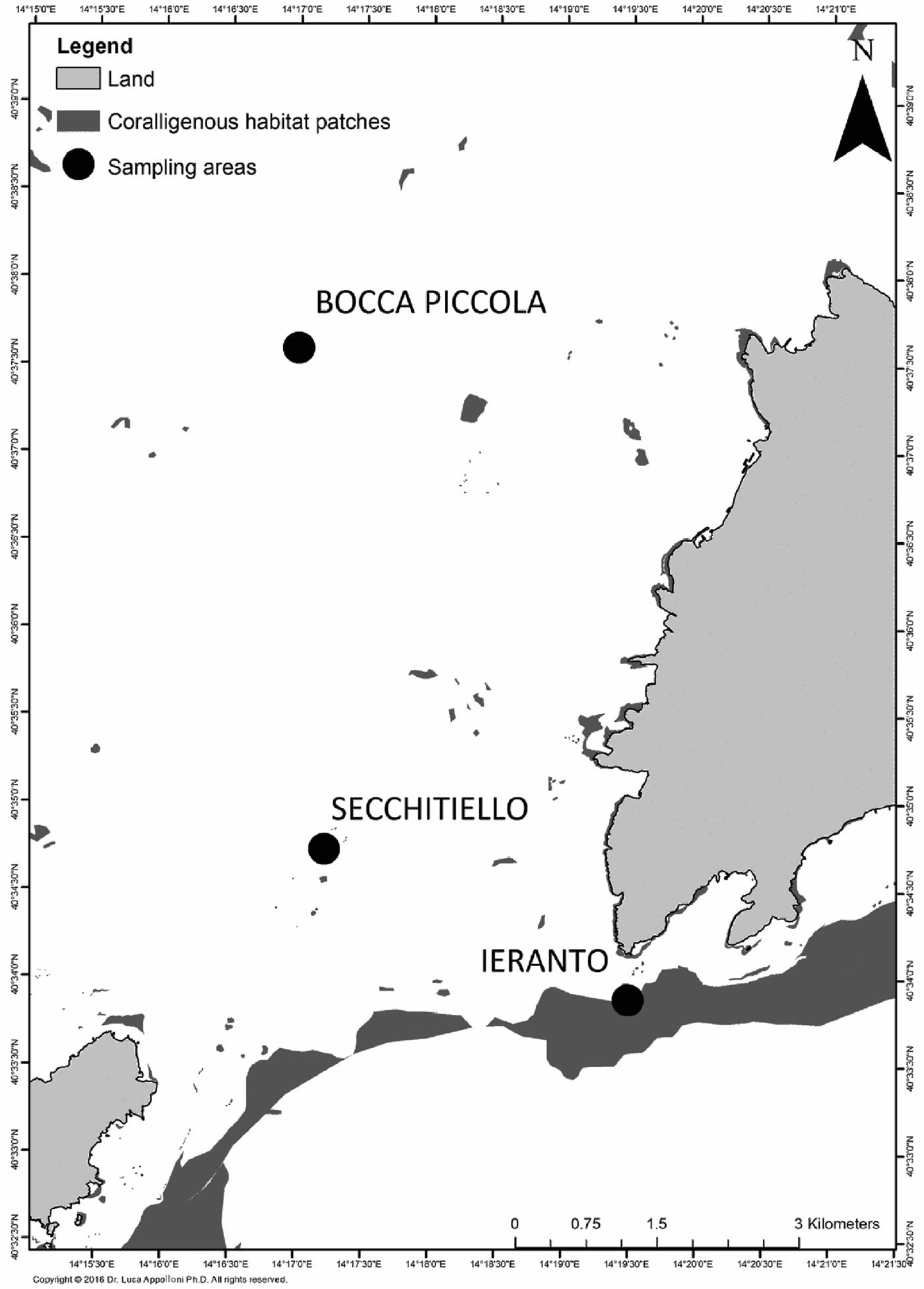
Fig. 2. Sites position within the study area.
Detailed information on type of substrate and structure was acquired for each site through multibeam and side-scan sonar (SSS) surveys. ROV survey routes were then outlined on the basis of the above data, according to the monitoring programme (MFSD).
Data management
Georeferenced videos were defragmented using DVDVideoSoft® software, in order to obtain random frames every 10 s. Of these, only images containing coralligenous bioconstructions were considered.
A random selection of frames featuring coralligenous habitats were analysed and processed in order to assess the total coralligenous cover and abundance of the different morphological groups within macro and mega benthos (from about 2 cm in serpulids and madrepores to several tens of centimetres in sponges and fan corals). Subsequently, the image analysis was carried out using Seascape® software, based on the image segmentation through different tone shades; this software allows the choice of the appropriate level of segmentation and the selection of different segments of the same colour, corresponding to a species or a morphological group; covering surfaces are then calculated. Areas of each frame, referred to the two ROV laser beams, are also calculated using ImageJ® software. Densities on square metres of conspicuous sessile organisms (e.g. fan corals) were finally calculated.
In addition, the total number of frames containing coralligenous bioconstructions was analysed to identify lost fishing gears and different types of damages, especially on fan corals, such as broken or necrotic branches or overgrown with epibionts, or whole ripped colonies, found upturned on the bottom, or, in general, coralligenous bioconstructions entangled or covered by fishing gears, in order to describe and quantify the human pressure and its impact. In particular, fishing pressure was calculated as percentage of frames presenting lost fishing gears, while fishing impact was estimated as percentage of frames presenting damaged coralligenous structures. Longlines and nets (trawl nets, gillnets or trammel nets), the two main lost fishing gears affecting the coralligenous substrate, were considered for fishing pressure, while other lost gears, such as traps, ropes, moorings, anchors, etc., were gathered under the name of ‘other’ (Table 1). Different impacts of fishing activities were classified into three new categories (Figure 3 and Table 1): BUC (Broken/Upturned fan coral Colonies), CNE (Colonies with Necrosis/Epibionts), GCE (Gears Covering/Entangling coralligenous habitat).

Fig. 3. Categories of fishing impact: (A) broken/upturned fan coral colonies (BUC); (B) fan coral colonies with necrosis/epibionts (CNE); (C) gears covering/entangling coralligenous habitats (GCE).
Table 1. New categories for classification of main lost gears and fishing impacts.

A multivariate analysis was performed in order to detect statistical differences among sites. A distance-based permutation multivariate analysis of variance (PERMANOVA; Anderson, Reference Anderson2001), based on Euclidian distance, was carried out on lost gears and fishing impact categories. The statistical analysis design involved the factor Site (fixed, 3 levels), N = 1. Post hoc pair-wise comparisons, using the PERMANOVA t-statistic and 4999 permutations, were also conducted in order to investigate differences among sites. All computations were made with Primer 6 software (Clarke & Gorley, Reference Clarke and Gorley2006).
Finally, additional information regarding sediment cover, type and inclination of substrate, depth, temperature, currents, salinity and turbidity were considered in order to describe the coralligenous habitat in each site and evaluate the anthropic impact.
RESULTS
After defragmentation a total of 782 images containing coralligenous bioconstructions were randomly obtained. A total of 119 best quality images (about 40 for each site) were then selected to perform the image analysis. At the end of this process, each photo appears as a heterogeneous mosaic of different size and colour segments representative of different species or morphological groups, for which it was possible to calculate the percentage of surface occupied.
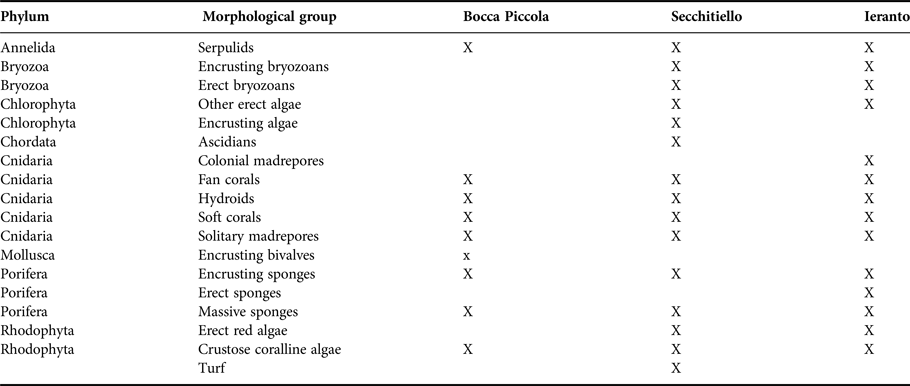
For each analysed photographic area, a list of morphological groups with respective percentage cover or densities (number of individuals/colonies m−2), for the erect organisms with large dimensions and a height up to about 50 cm (e.g. fan corals), was thus obtained. The list of all morphological groups present at each site is shown in Table 2. A total of 10 morphological groups was detected at Bocca Piccola, while 15 and 14 were respectively detected at Secchitiello and Ieranto.
Table 2. List of coralligenous morphological groups, with respective phylum, found in the analysed sites.
Subsequently, the morphological group abundances and the other coralligenous substrate cover (when no morphological groups are evident) were summed in order to evaluate the total coralligenous surface cover, compared with sediment cover or bare substrate, at each site.
The mean value of coralligenous coverage was 23% at Bocca Piccola site (with soft corals, fan corals and crustose coralline algae being dominant), where the lowest number of morphological groups is also recorded. Secchitiello site showed a coralligenous cover of 75% (erect red algae, encrusting sponges, fan corals and crustose coralline algae are the dominant forms), while Ieranto site had 59% cover (fan corals, encrusting and massive sponges, and crustose coralline algae represent the dominant groups) (Figure 4). Generally, fan corals, encrusting sponges and crustose coralline algae are the most abundant morphological groups in the study area.

Fig. 4. Abundances of morphological groups: (A) Bocca Piccola, (B) Secchitiello, (C) Ieranto.
A clear change of species distribution, correlated with bathymetric gradient, was evident at Ieranto site, as also previously shown in a preliminary investigation (Ferrigno et al., Reference Ferrigno, Appolloni, Sandulli, Casoria and Russo2015); in particular, the shallower margin of the cliff is characterized by the dominance of Paramuricea clavata (4.63 ± 1.83 colonies m−2); with the increase of depth Corallium rubrum (43.85 ± 9.01 colonies m−2) and E. cavolinii (13.50 ± 5.85 colonies m−2) become more abundant, and, finally, the deepest part (115–155 m depth) is dominated by sponges mainly of genera Haliclona and Aplysina (Figure 5).
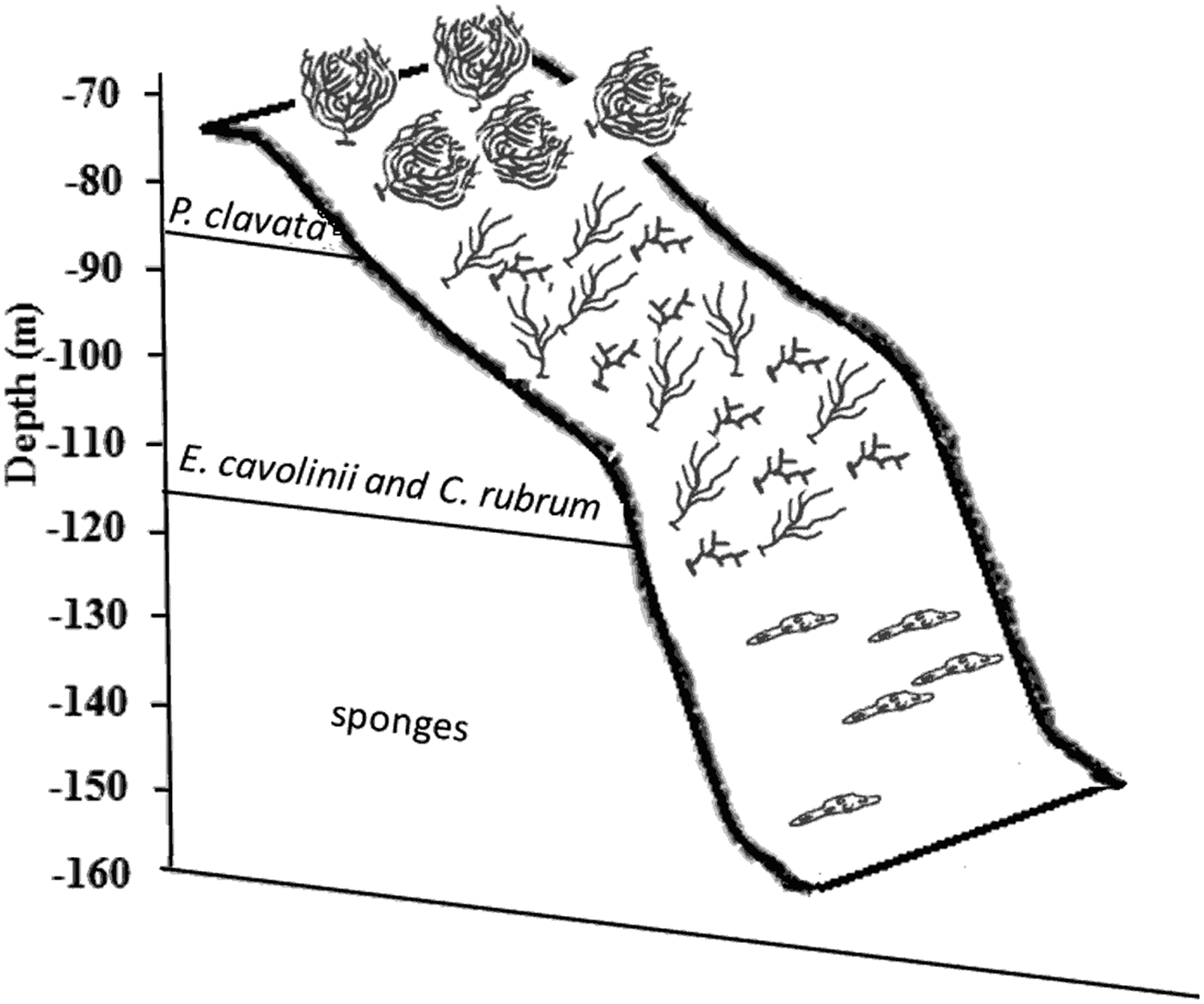
Fig. 5. Schematic representation of dominant species distribution, along bathymetric gradient, at Ieranto site (from Ferrigno et al., Reference Ferrigno, Appolloni, Sandulli, Casoria and Russo2015).
About 53% of frames were impacted by lost gears at Bocca Piccola (46% represented by nets, 38% by longlines, and 16% by other gears); about 31% of frames were impacted by lost gears at Secchitiello (80% represented by longlines, 16% by other gears and 4% by nets); about 27% of frames were impacted by lost gears at Ieranto (78% represented by longlines and 22% by nets) (Figure 6).
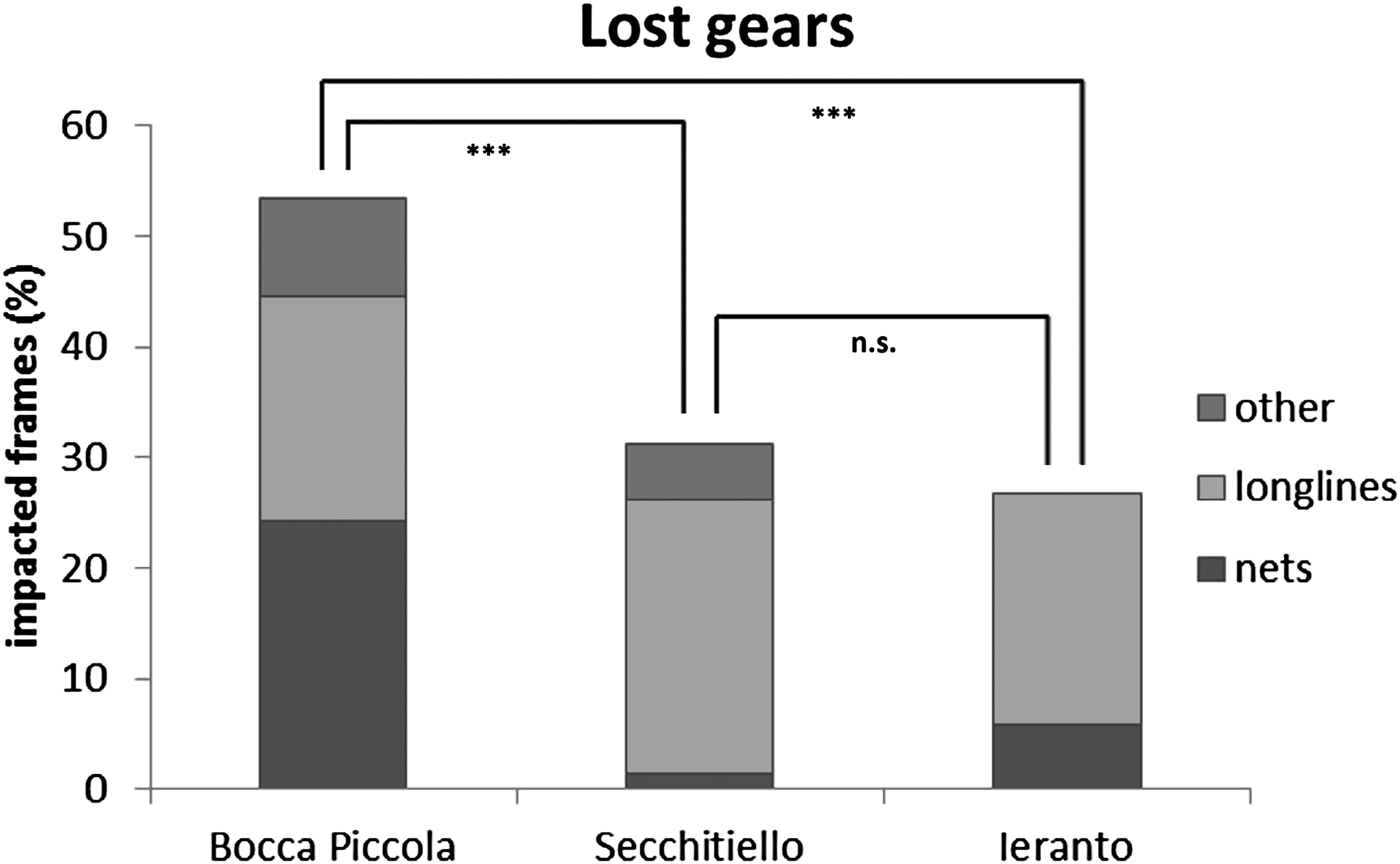
Fig. 6. Abundances of lost fishing gears, in the study area, and differences among sites from PERMANOVA (n.s. = not significant; ***P < 0.001).
GCE (gears covering/entangling coralligenous habitat) is the most frequent category of fishing impact, with the highest values at Bocca Piccola (GCE = 36%), where the highest BUC (broken/upturned fan coral colonies) value was also detected (BUC = 5%); at Secchitiello site the highest values of CNE (fan coral colonies with necrosis/epibionts) was detected (CNE = 10%). The alcionarian Alcyonium coralloides (Pallas, 1766), followed by hydroids, serpulids and encrusting bryozoans were the main epibiontic organisms detected.
Overall, 48% of coralligenous habitat was impacted at Bocca Piccola, 33% at Secchitiello, and 35% at Ieranto (Figure 7).
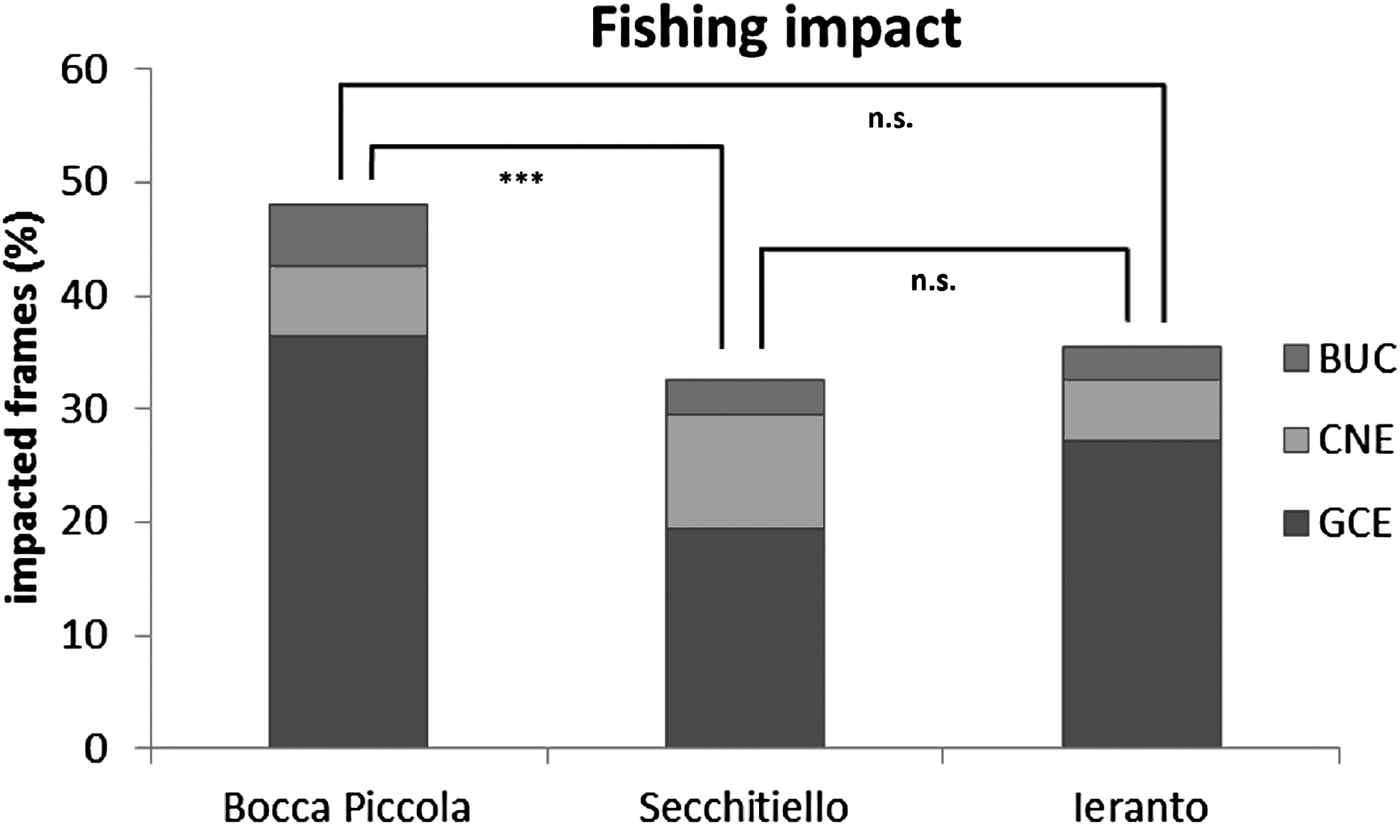
Fig. 7. Abundances of different type of fishing impacts, in the study area, and differences among sites from PERMANOVA (n.s. = not significant; ***P < 0.001).
Multivariate analyses show statistical differences, based on lost gears, between Bocca Piccola and Ieranto sites and between Bocca Piccola and Secchitiello sites; while, based on fishing impact, differences were detected only between Bocca Piccola and Secchitiello (Table 3, Figures 6 & 7).
Table 3. Pair-wise comparisons for differences in site factor for lost gears and fishing impact.

n.s., not significant; ***P < 0.001.
DISCUSSION
Hard bottom assemblages and especially coralligenous habitats are very sensitive to human activities, showing changes in structure and composition (Bavestrello et al., Reference Bavestrello, Bava, Canese, Cattaneo-Vietti, Cerasi, Profeta and Bo2015). Fishing is among the main anthropic activities along coastal areas, affecting several bottom communities (MacDonald et al., Reference MacDonald, Little, Eno and Hiscock1996; Bavestrello et al., Reference Bavestrello, Cerrano, Zanzi and Cattaneo-Vietti1997; Collie et al., Reference Collie, Hall, Kaiser and Poiner2000; Kaiser et al., Reference Kaiser, Collie, Hall, Jennings and Poiner2001; Blanchard et al., Reference Blanchard, Leloc'h, Hily and Boucher2004; Brown & Macfadyen, Reference Brown and Macfadyen2007; Deidun et al., Reference Deidun, Tsounis, Balzan and Micallef2010). Particularly, trawl fishing, due to contact with the seabed, increases turbidity, sedimentation rate and nutrient resuspension, generating plumes which redeposition extends up to about 500 m after 4 h, depending on currents (Durrieu De Madron et al., Reference Durrieu De Madron, Ferré, Le Corre, Grenz, Conan, Pujo-Pay, Buscail and Bodiot2005). Furthermore, trawling may cause severe damage to branched organisms, mostly in the form of broken and smothered colonies (Bavestrello et al., Reference Bavestrello, Bava, Canese, Cattaneo-Vietti, Cerasi, Profeta and Bo2015).
In this study, an assessment of fishing pressure and impact was carried out using ROV-imaging. Particularly, new, representative and synthetic categories for the detection of the main lost fishing gears (longlines, nets and other) and their impact (BUC, CNE and CGE) on sensitive benthic organisms, such as fan corals, were developed. Since the last decade, ROV surveys in the Mediterranean Sea have provided clear evidence of fishing impacts, through the use of footage analysis; this technology is particularly useful in obtaining quantitative data on lost fishing gears and other litter (Hyland et al., Reference Hyland, Cooksey, Bowlby, Brancato and Intelmann2005; Bo et al., Reference Bo, Bava, Canese, Angiolillo, Cattaneo-Vietti and Bavestrello2014a, Reference Bo, Cerrano, Canese, Salvati, Angiolillo, Santangelo and Bavestrellob; Angiolillo et al., Reference Angiolillo, di Lorenzo, Farcomeni and Bo2015; Vieira et al., Reference Vieira, Raposo, Sobral, Gonçalves, Bell and Cunha2015; Cánovas-Molina et al., Reference Cánovas-Molina, Montefalcone, Bavestrello, Cau, Bianchi, Morri, Canese and Bo2016; Clark et al., Reference Clark, Althaus, Schlacher, Williams, Bowden and Rowden2016). ROV surveys have highlighted the widespread presence of fishing impact in the western Mediterranean Sea by reducing the coverage of habitat-forming taxa and lastly the diversity and abundance of associated invertebrates and fish (Orejas et al., Reference Orejas, Gori, Lo Iacono, Puig, Gili and Dale2009; Bo et al., Reference Bo, Bava, Canese, Angiolillo, Cattaneo-Vietti and Bavestrello2014a, Reference Bo, Cerrano, Canese, Salvati, Angiolillo, Santangelo and Bavestrellob). Furthermore, Angiolillo et al. (Reference Angiolillo, di Lorenzo, Farcomeni and Bo2015) showed the positive correlation between the number of dead colonies and the presence of lost gears in the habitat, indicating the destructive effects of fishing activities.
The study area, located in the Bay of Naples, presents several coralligenous bioconstructions (Colombo, Reference Colombo1887; Lo Bianco, Reference Lo Bianco1909; Mazzarelli & Mazzarelli, Reference Mazzarelli and Mazzarelli1918; Ranzi, Reference Ranzi1930; Russo, Reference Russo and Giammarino1992, Reference Russo1997, Reference Russo, Rosi and Jannuzzi2000; Gambi et al., Reference Gambi, Dappiano, Lanera, Iacono, Gambi, De Lauro and Jannuzzi2003), most of which are not yet characterized. From this work it emerges that the analysed sites have specific coralligenous assemblages varying in richness and abundance, probably linked to particular geographic and environmental characteristics, and they significantly differ for both fishing pressure and impact.
Bocca Piccola site is a deep and small coralligenous outcrop created by bioconstructors, located on a wide muddy platform at about 120 m depth and, due to the inner position in the Bay of Naples, it is affected by slow local currents and water turnover, with a resulting higher turbidity (De Ruggiero et al., Reference De Ruggiero, Napolitano, Iacono and Pierini2016). It is probably an important refuge from adverse conditions for marine benthic organisms and demersal fishes, being on a wide and muddy bottom far away from the coast (Bongaerts et al., Reference Bongaerts, Ridgway, Sampayo and Hoegh-Guldberg2010). Here trawling activity was very heavy, as highlighted by a high number of lost nets affecting coralligenous assemblages; trawling may cause several types of damage, particularly on mega-benthic assemblages, due to mechanical impact and increase of sedimentation rate, especially with deeper and finer sediments, also far away from the trawled seabed (Durrieu De Madron et al., Reference Durrieu De Madron, Ferré, Le Corre, Grenz, Conan, Pujo-Pay, Buscail and Bodiot2005; Bavestrello et al., Reference Bavestrello, Bava, Canese, Cattaneo-Vietti, Cerasi, Profeta and Bo2015), leading to loss of richness, diversity and density of communities (Smith et al., Reference Smith, Papadopoulou and Diliberto2000; Althaus et al., Reference Althaus, Williams, Schlacher, Kloser, Green, Barker, Bax, Brodie and Schlacher-Hoenlinger2009; Clark et al., Reference Clark, Althaus, Schlacher, Williams, Bowden and Rowden2016).
Secchitiello was the richest and most diverse site, with the highest number of morphological groups, coralligenous cover and fan coral densities. This site is an offshore rocky shoal, surrounded by sandy and debris bottom, attracting numerous and different organisms and representing a hotspot of biodiversity. The distance from the coast may partially protect this site from pollution and might also allow growth of a high number of species (Devlin & Brodie, Reference Devlin and Brodie2005; Bo et al., Reference Bo, Bertolino, Borghini, Castellano, Covazzi Harriague, Di Camillo, Gasparini, Misic, Povero, Pusceddu, Schroeder and Bavestrello2011). Due to the high biodiversity of this site, artisanal fishing is popular here and, indeed, as demonstrated by high number of longlines entangling the coralligenous bioconstructions, is quite intense. No strongly different hydrology among sites should be observed and the environmental variables are quite homogeneous (Cianelli et al., Reference Cianelli, Uttieri, Buonocore, Falco, Zambardino, Zambianchi and Williams2012); nevertheless, Secchitiello, the shallower site, shows slight differences, with temperature higher by about 0.5°C and salinity lower by about 0.5 PSU (De Ruggiero et al., Reference De Ruggiero, Napolitano, Iacono and Pierini2016).
Ieranto site is a vertical underwater cliff close to the southern coast of the Sorrento Peninsula, and it is located at the edge of continental shelf. Here, strong upwelling currents (De Ruggiero et al., Reference De Ruggiero, Napolitano, Iacono and Pierini2016) generating water turnover and promoting nutrients dispersion allowed the establishment of a deep well-structured fan corals facies down to about 115 m depth. Beyond this depth the coralligenous richness and abundance decreased, probably due to an effect of the observed increase of sediment accumulation. The presence of several morphological groups and, overall, a high coralligenous cover, characterizes the Secchitiello and Ieranto sites, where sediment cover is lower compared with Bocca Piccola, which is surrounded by muddy bottoms. They host a very rich and diversified coralligenous assemblage mainly dominated by big and erect suspension feeders, such as the fan corals Eunicella cavolinii, Paramuricea clavata and Corallium rubrum, characteristic species of highly hydrodynamic (Bo et al., Reference Bo, Bavestrello, Canese, Giusti, Salvati, Angiolillo and Greco2009) and quite eutrophic (Ballesteros, Reference Ballesteros2006) environments. However, the results showed that at depths greater than ~110 m the coralligenous habitat extension decreases and sediment cover increases. This was observed also with slope decrease; indeed, Bocca Piccola site, where coralligenous assemblage develops on horizontal substrate, seems to be less rich and diverse than the other sites.
The surveys documented an important presence of coralligenous assemblages impacted by lost fishing gears at all sites, confirming the heavy fishing pressure of the area, as previously reported (Sbrescia et al., Reference Sbrescia, Di Stefano, Russo and Russo2008). Bocca Piccola was significantly different from the other sites, with the highest anthropic pressure and impact. Despite its offshore location, here the highest number of lost nets entangling and covering coralligenous assemblages was detected. On the contrary, in the other two sites, longlines used by artisanal and recreational fishing were mainly detected. Usually a higher abundance of marine litter is observed closer to the coastline and urban centres (Mordecai et al., Reference Mordecai, Tyler, Masson and Huvenne2011). However, Watters et al. (Reference Watters, Yoklavich, Love and Schroeder2010) observed this pattern does not always occur and there may be a positive relationship between density of lost gears and distance from the coast. This applies to Bocca Piccola site, and it is probably due to the presence of commercially relevant fishing stocks inhabiting off-shore deep rocky banks, attracting local recreational and professional fishermen (Bo et al., Reference Bo, Cerrano, Canese, Salvati, Angiolillo, Santangelo and Bavestrello2014b). Indeed, occurrence of commercial target species, along with physical characteristics of an area, such as depth and topography, influence the fishing effort and consequently the impact on benthic communities (Bo et al., Reference Bo, Bava, Canese, Angiolillo, Cattaneo-Vietti and Bavestrello2014a).
Between 2003 and 2014 the fishing fleet has constantly contracted up to 20% and, in particular, trawling activity is the most affected by the downsizing process, followed by small-scale fishing (MIPAAF, 2014). Nevertheless, trawling and small-scale fishing are among the major fishing methods, representing respectively 10 and 81% of the number of vessels, with 118 and 957 units, and 30 and 17% of GT (gross tonnage), operating during 2010 in the Bay of Naples (IREPA, 2011), highlighting the strong fishing effort in the study area.
Strong human pressure may endanger high biodiversity areas (Sandulli et al., Reference Sandulli, Carriglio, Deastis, Marzano, Gerardi, Gallo D’Addabbo and de Zio Grimaldi2004), affecting the benthic communities by breaking and upturning large and branched colonies and inducing diseases due to mechanical friction (Asoh et al., Reference Asoh, Yoshikawa, Kosaki and Marschall2004; Bo et al., Reference Bo, Bava, Canese, Angiolillo, Cattaneo-Vietti and Bavestrello2014a; Ferrigno et al., Reference Ferrigno, Bianchi, Lasagna, Morri, Russo and Sandulli2016). Several sensitive organisms of coralligenous habitats, such as C. rubrum, already seriously damaged as a result of a long history of commercial exploitation (Gallmetzer et al., Reference Gallmetzer, Haselmair and Velimirov2010; Tsounis et al., Reference Tsounis, Rossi, Grigg, Santangelo, Bramanti, Gili, Gibson, Atkinson and Gordon2010; Bavestrello et al., Reference Bavestrello, Bo, Canese, Sandulli and Cattaneo-Vietti2014), and, for this reason, added to the IUCN Red List of Threatened Species, are present in this area. Some coral mortality events were observed particularly in branched colonies of C. rubrum, E. cavolinii and P. clavata (Bavestrello et al., Reference Bavestrello, Bertone, Cattaneo-Vietti, Cerrano, Gaino and Zanzi1994; Cerrano et al., Reference Cerrano, Bavestrello, Bianchi, Cattaneo-Vietti, Bava, Morganti, Morri, Picco, Sara, Schiaparelli, Siccardi and Sponga2000, Reference Cerrano, Arillo, Azzini, Calcinai, Castellano, Muti, Valisano, Zega and Bavestrello2005; Garrabou et al., Reference Garrabou, Perez, Sartoretto and Harmelin2001; Gambi et al., Reference Gambi, Barbieri, Signorelli and Saggiomo2010; Gambi & Barbieri, Reference Gambi and Barbieri2012), probably due to thermal crisis, hydrodynamic exchanges and water pollution. Nevertheless, fishing activities might also induce stressing conditions leading to the proliferation and infection of pathogenic microorganisms on fan coral colonies (Bally & Garrabou, Reference Bally and Garrabou2007; Vezzulli et al., Reference Vezzulli, Previati, Pruzzo, Marchese, Bourne, Cerrano and Consortium2010).
In this study, the impact of fishing activities on biocoenoses was also emphasized by different types of damage caused by several lost gears covering or entangling coralligenous habitat. Furthermore, several fan coral colonies were broken or upturned on the bottom and damaged by necrosis or overgrown with epibionts, particularly at Secchitiello site, confirming the high sensitivity to fishing of fan corals. Differently from C. rubrum, the elastic skeletons of E. cavolinii and P. clavata usually do not break, but, if abraded by longlines friction became necrotic and may be colonized by epibionts, which cover and smother the colonies (Bo et al., Reference Bo, Cerrano, Canese, Salvati, Angiolillo, Santangelo and Bavestrello2014a, Reference Bo, Bava, Canese, Angiolillo, Cattaneo-Vietti and Bavestrellob; Angiolillo et al., Reference Angiolillo, di Lorenzo, Farcomeni and Bo2015). Among the most common epibionts is the parasitic Alcyonium coralloides; other opportunistic fast growing organisms, such as hydroids, polychaetes and bryozoans have been widely found on dead coral branches.
In areas exploited by trawl fishing, a detailed assessment of communities distribution and fishing routes may provide useful management information, allowing the design and management of Marine Protected Areas with different protection levels (Bavestrello et al., Reference Bavestrello, Bava, Canese, Cattaneo-Vietti, Cerasi, Profeta and Bo2015). Particularly, the abundance and the health status of the most structuring species, such as fan corals, may be considered in order to evaluate the fishing impact (Bo et al., Reference Bo, Bava, Canese, Angiolillo, Cattaneo-Vietti and Bavestrello2014a). Management tools, extended to coralligenous habitats, should propose guidelines for a more sustainable fishing, also including fishing ban periods or allocating a budget for removal of lost fishing gears found on the bottom. Moreover, several technical measures could significantly reduce the fishing impact on these benthic ecosystems. For example, the contact zone of trawling nets with bottom can be reduced by decreasing the net length or by using composite and soft material; for gillnets and trammel nets, adjusting the mesh size, and choosing the size of the targeted species, may significantly reduce the capture of unwanted organisms (Sacchi, Reference Sacchi and Wurtz2012). Past assessments of regional fisheries management organizations concluded that there are substantial deficits in monitoring, surveillance and enforcement (Gilman, Reference Gilman2015). Thus, monitoring high biodiversity areas and drawing due attention to identify needed improvements in local and international management is urgently required.
CONCLUSIONS
ROV-imaging detected a general decrease of coralligenous per cent cover and morphological groups number and abundances with increasing depth or decreasing slope, probably due to sedimentation rate increase. In particular, the studied sites are characterized by rich and diversified coralligenous assemblages, with a high presence of fan corals; particularly Eunicella cavolinii and Paramuricea clavata were abundant at Secchitiello site, while several colonies of the sensitive and quite rare Corallium rubrum were present at Ieranto site.
Nevertheless, different fan corals colonies were clearly damaged by fishing gears, entangling these branched organisms and leading to overgrowth of epibiontic and parasitic species, such as the alcionarian Alcyonium coralloides. The pressure of fishing activities was particularly demonstrated at Bocca Piccola by the high presence of lost gears on coralligenous habitats. Overall, a moderate presence of coralligenous assemblages covered or entangled by gears was detected at all sites (on average 28% of analysed frames). The impact of fishing activities was, instead, established by damaged coralligenous assemblages, shown by high values of gears covering or entangling coralligenous habitat at Bocca Piccola and by fan coral colonies with necrosis or epibionts at Secchitiello.
The continuous exploitation of these rich and diverse coralligenous habitats may lead to endangerment of the whole ecosystems, these being highly sensitive to human activities and particularly to fishing, as confirmed by this study. Nevertheless, still too little is known about fishing impact on benthic and deep habitats of hard bottoms. More sustainable fishing techniques, protection measures and monitoring programmes are urgently required, in order to improve the ecosystem management.
ACKNOWLEDGEMENTS
Thanks are due to the crew members and captain of RV ‘Cormorano V’, the ROV pilots of Subonica, and informatics system technicians of Install, for field activities and data acquisition.
FINANCIAL SUPPORT
This work was financed and supported by University of Naples ‘Parthenope’, CoNISMa, within the Marine Strategy Framework Directive (MSFD-2008/56/EC) for coralligenous habitats monitoring.












