Human salmonellosis is one of the most significant foodborne illnesses worldwide. In Japan, more than 10 000 cases of foodborne salmonellosis cases were reported annually from 1996 to 1999. Salmonella was the predominant aetiological agent in these years, except during 1998 [1]. During this period, Salmonella enterica subsp. enterica serovar Enteritidis (hereafter called S. Enteritidis) was the most frequently isolated strain in the outbreaks and was identified in 10 or more cases in each outbreak. It accounted for 58% of all cases in 1996, 55% in 1997, 62% in 1998, and 46% in 1999. Because S. Enteritidis infections are frequently associated with the consumption of eggs, the Enforcement Regulations of the Food Sanitation Law were partially amended in order to ensure safe distribution of raw shell eggs and liquid egg products in 1998 [2]. Although the number of foodborne salmonellosis cases has significantly decreased since 2000, presumably due to the effect of the Enforcement Regulations, Salmonella remains one of the top two causative agents of bacterial foodborne illness. S. Enteritidis has been the most predominant serovar and contributes to more than 30% of foodborne salmonellosis cases [3]. Almost all (98%) foodborne salmonellosis incidents in 2006 were caused by the consumption of contaminated food in Japan and not travel-related [4]. Japan's laying hen farms produced about 2·5 million tons of eggs in 2006 and the volume accounts for 95% of the total domestic egg consumption [5, 6]. These facts indicate the importance of the reduction of Salmonella contamination in eggs for the prevention or reduction of foodborne salmonellosis. As a basis for the employment of appropriate measures for this objective, the prevalence of Salmonella serovars in eggs needs to be investigated.
Here, we investigated the prevalence of Salmonella in raw shell eggs sold at retail stores in Japan. Eggs may be contaminated with Salmonella on the outer shell surface and/or from within. Internal contamination may be caused because of the penetration of Salmonella through the egg shell or by direct contamination of egg contents before oviposition [Reference Cox, Berrang and Cason7]. Therefore, the shells and egg contents were separately examined in the present study. A total of 2030 raw shell egg samples (10 eggs/sample) were purchased from all eight regions of Japan (Hokkaido, 100; Tohoku, 130; Kanto, 720; Hokuriku, 100; Tokai, 240; Kinki, 330; Chugoku-Shikoku, 190; Kyusyu, 220) in proportion to the size of human population in 2006. Shell eggs with as many different trade names as possible were collected from a number of retail stores across Japan as detailed below. This allowed us to obtain samples representative of the entire population in the absence of data on the market share of shell egg producers at the retail level, because such different trade names represent different feeding types, production areas, producers, breed types and egg colours. Furthermore, in order to obtain the necessary number of samples, eggs bearing the same trade names were accepted, provided that their packing dates were not identical, which led to the inclusion of a larger number of samples from producers holding bigger market shares, who supplied shell eggs to various retail stores on a regular basis. Of 2030 samples, 1128 (55·6%) samples had been maintained at room temperature and 902 (44·4%) had been refrigerated in the retail stores. All the eggs were collected from 220 retail stores located in 49 cities between August 2007 and January 2008 by the Japan Food Research Laboratories (JFRL) staff and transported to their laboratory by express delivery under refrigeration. The eggs were usually collected in boxes of 10 eggs, which is the most common form of egg distribution in Japan. If boxes contained more than 10 eggs, the extra eggs were discarded at the laboratory, and if the boxes contained less than 10 eggs, eggs from other boxes obtained from the same egg grading and packaging (GP) centre that were packed on the same day were purchased and pooled to obtain a sample size of 10 eggs. Eggs bearing 670 different trade names were collected, all originating from domestic producers. At the laboratory, all the boxes were stored in their original boxes in a cold room (at 2–8°C) until further examination. While 1861 (92%) samples were tested within a week after purchase, the range of storage time at the laboratory before testing was from 0 to 15 days. All the eggs were tested before their ‘best-before’ dates.
Raw shell eggs were inspected visually before testing. If the egg shells were cracked in the boxes, new boxes were purchased for the tests. Raw shell eggs were aseptically broken, the shells and egg contents from 10 raw shell eggs were separated and pooled in separate containers. For egg contents, whole egg contents from 10 eggs were placed in a plastic bag and were mixed to homogeneity. Then, 125 ml of the egg contents were mixed with 225 ml buffered peptone water (BPW; Eiken Chemical, Japan: LD1005) maintained at room temperature and cultured at 35°C for 22±2 h. The shells were crushed and mixed with 225 ml BPW. After incubation at 35°C for 22±2 h, 0·1 and 1 ml of each culture was added to 10 ml Rappaport–Vassiliadis broth (bioMérieux Japan Ltd, Japan: 43563) and 10 ml tetrathionate broth (Merck Ltd, Japan: 1.05285.0500), respectively. After incubation at 42°C for 22±2 h, each culture was streaked onto two selective isolation agar plates: one each of xylose-lysine-deoxycholate agar (bioMérieux: 43564) and Brilliant Green agar (bioMérieux: 43588). Candidate colonies were biochemically identified. Salmonella isolates were tested by slide agglutination with O-antisera (Denka Seiken Co., Japan) and tube agglutination with H-antisera (Denka Seiken). Serovars were determined on the basis of reaction with O- and H-group antigen, according to the Kauffmann–White scheme [Reference Poppoff8]. The minimum inhibitory concentration (MIC) of the Salmonella isolates to various antimicrobials was determined using the agar dilution method of the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) [9]. Escherichia coli ATCC 25922 was used as the quality control strain. The resistant breakpoints were adopted from those defined by CLSI [10]. The breakpoints not defined by CLSI were obtained from a previous report [Reference Esaki11]. Antimicrobial susceptibility tests were conducted at the Research Institute for Animal Science in Biochemistry and Toxicology (RIAS). Salmonella species were isolated from five (0·25%, 95% confidence interval 0·03–0·46%) pooled egg shell samples (Table 1). This result shows that Salmonella exists in approximately 1/400 boxes of 10 eggs. S. Enteritidis was isolated from two samples (0·1%). Two S. Derby isolates, one S. Livingstone isolate and one S. Cerro isolate were detected in three other samples. In a Salmonella-positive sample, both S. Derby and S. Cerro isolates were obtained. Previous studies have shown that these four serovars were isolated from the caecal content of laying hens in Japan [Reference Someya, Otsuki and Murase12, Reference Otomo13] and from liquid egg samples [Reference Ohtsuka14, Reference Hara-Kudo and Takatori15]. Although four isolates identified in the present study were resistant to dihydrostreptomycin (⩾32 μg/ml), none of the isolates were resistant to more than two antimicrobials. Asai et al. [Reference Asai16] reported that resistance rates of Salmonella isolates obtained from layer chickens to two or more antimicrobials were the lowest among the Salmonella isolates obtained from cattle, pigs, broilers and layers. Because all the eggs were stored in their original boxes until examination, we believe that the Salmonella contamination of the egg shells occurred before they arrived at the laboratory. The shells may have been contaminated with Salmonella at two probable sources. First, the Salmonella contamination of the egg shells may have occurred at the layer farms and then could not be completely eliminated at the GP centres. Second, the shells may have been cross-contaminated with Salmonella after washing at the GP centres because of inappropriate sanitary conditions at these centres. Davies & Breslin [Reference Davies and Breslin17] reported that Salmonella contamination is observed on floor surfaces, grading tables, conveyer belts or rollers, and candlers present in egg-packaging plants. We contacted the GP centres regarding information on egg treatment strategies. All the GP centres washed their egg shells with water which was at least 5°C warmer than the egg temperature; this practice complied with the guidelines for hygienic practice at GP centres of the Ministry of Health, Labour and Welfare of Japan [2]. Thus, practical control measures need to be reviewed and implemented to mitigate Salmonella contamination of egg shells at GP centres.
Table l. Details of egg supply, Salmonella serovars and MIC (mg/l) values for isolates from shell samples
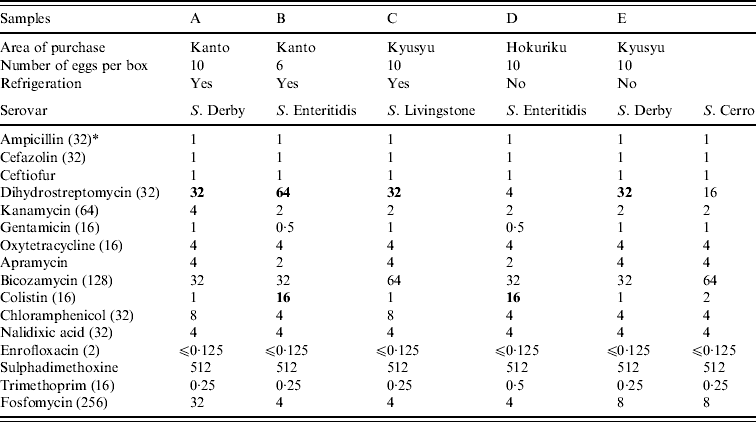
* Breakpoint (mg/l).
Although Salmonella was not isolated from any of the egg-content samples in the present study, it would not be appropriate to conclude that contents of eggs produced in Japan are free from Salmonella. Recently, Lapuz et al. [Reference Lapuz18] reported that three (0·03%) of 9010 pooled egg-content samples of 10 eggs each, produced between 2004 and 2006, were positive for Salmonella, and S. Enteritidis was isolated from two (0·02%) samples. In that study, the number of shell eggs tested was 4·5 times the number of eggs in the present study and 8290 (92%) pooled egg-content samples originated from Salmonella-positive laying hen farms. According to some available reports on the prevalence of Salmonella in laying hen farms in Japan, the range of the positive rates was 25–52% [Reference Otomo13, Reference Sunagawa19, Reference Matsumoto20]. Therefore, less than 50% of shell eggs on the market would have originated from Salmonella-positive laying hen farms. In order to include the same number of eggs originating from Salmonella-positive farms in our sample as in the Lapuz et al. study, approximately 10 times the number analysed in the present study would need to be collected, and even more eggs for estimation of the nationwide prevalence of Salmonella in egg contents with high confidence levels.
The prevalence of Salmonella in egg shells is a potential hazard for foodborne salmonellosis because food handlers directly handle raw shell eggs, and broken pieces of shells may come in contact with the egg content while cooking, which could allow Salmonella to reach the egg contents. It was reported that on average a household with two or more persons purchases 31 kg of shell eggs per year [21]. This shows that a household purchases 52 boxes of 10 eggs if the weight of one shell egg is 60 g and that one in every eight households purchases a box contaminated with Salmonella once a year. Moreover, the frequency might be underestimated due to the pooling method used in this study. Food handlers need to be aware of the possibility of bacterial contamination during handling raw shell eggs and of the means of preservation of raw shell eggs.
ACKNOWLEDGEMENTS
This study was funded by the Ministry of Agriculture, Forestry and Fisheries of Japan. We thank JFRL and RIAS for their cooperation.
DECLARATION OF INTEREST
None.



