Two epidemics have been currently identified that affect industrialized countries: obesity and diabetes(Reference Ogden, Yanovski, Carroll and Flegal1, Reference Dabelea, Bell and D'Agostino2). The forecast for the future is a further progressive increase in the prevalence of these metabolic disorders, also in children. Interestingly, as a consequence of widespread obesity, impaired glucose tolerance and type 2 diabetes, which in the past were almost exclusively limited to adulthood, are becoming more common in children and adolescents(Reference Dabelea, Bell and D'Agostino2). It is likely that the discouraging results of prevention and treatment programmes for childhood obesity available today are the reason behind the rapid rise in the incidence of glucose metabolism disorders in children(Reference Flynn, McNeil, Maloff, Mutasingwa, Wu, Ford and Tough3). Nowadays, although the prevalence of type 2 diabetes is increasing in young individuals, type 1 diabetes is still the most common form of diabetes in children and adolescents(Reference Dabelea, Bell and D'Agostino2).
The management of type 1 diabetes is multifactorial but nutrition plays a key role in both blood glucose control and prevention of micro- and macrovascular complications of the disease(Reference Silverstein, Klingensmith, Copeland, Kaufman, Laffel, Deeb, Grey, Anderson, Holzmeister and Clark4). In this article, we report nutritional recommendations with a special focus on ages at high risk for low compliance: young childhood and adolescence.
Type 1 diabetes
Most cases of type 1 diabetes (type 1A) are due to pancreatic β-cell destruction by an autoimmune attack that leads to absolute insulin deficiency and impaired glucose homeostasis(Reference Eisenbarth5). In a minority of cases (type 1B diabetes) usually occurring in individuals of Asian or African descent, no causes have been identified that underlie the disorder(6). There are two main factors in the pathological process that characterize the immune form of diabetes: susceptibility and environmental triggers(Reference Lambert, Gillespie, Thomson, Cordell, Todd, Gale and Bingley7).
Susceptibility
About 50 % of genetic susceptibility to type 1 diabetes is conferred by the HLA locus. DR3/4, DQA1*0301-DQB1*0302, and DQA1*0501-DQB1*0201 have been identified as high-risk HLA haplotypes, whereas DQA1*0121-DQB1*0602 HLA haplotypes have been associated with diabetes resistance(Reference Eisenbarth8). DR molecules (DRB1*1401) have also been associated with protection from diabetes(Reference Eisenbarth8). Insulin-VNTR (IDDM2) and CTLA-4 (IDDM12) genes cumulatively contribute to explain a further 15 % of the genetic susceptibility to the disease(Reference Anjos and Polychronakos9). Other genes that may be involved in the immune pathogenesis of type 1 diabetes are currently under investigation.
Environmental triggers
Several environmental triggers involved in altering immune function, thereby initiating β-cell destruction, have been suggested: viruses (congenital rubella, enteroviruses, coxsackie, etc), environmental toxins (for example, nitrosamines) or early exposure to some foods such as cows' milk proteins, cereals or gluten(Reference Helgason and Jonasson10–Reference Norris, Barriga, Klingensmith, Hoffman, Eisenbarth, Erlich and Rewers16).
Insulitis and humoral response
In susceptible individuals, the T-cell-mediated immune system is abnormally activated which leads to two main consequences: (a) an inflammatory response within the islets (insulitis) and (b) a humoral (β-cell) response with production of autoantibodies directed toward insulin (IAA), glutamic acid decarboxylase (GADA/GAA) and the protein tyrosine phosphatase IA2 (IA-2AA)(Reference Verge, Gianani, Kawasaki, Yu, Pietropaolo, Jackson, Chase and Eisenbarth17). These autoantibodies are markers of insulitis, but their active role in the pathogenesis of the disease has not yet been demonstrated. One or more of these antoantibodies may be detected years before the clinical onset of diabetes; the presence and persistence of multiple autoantibodies increase the likelihood of progression to clinical disease(Reference Verge, Gianani, Kawasaki, Yu, Pietropaolo, Jackson, Chase and Eisenbarth17, Reference Hagopian, Sanjeevi, Kockum, Landin-Olsson, Karlsen, Sundkvist, Dahlquist, Palmer and Lernmark18). Interestingly, individuals who develop type 1 diabetes are also susceptible to several other autoimmune disorders (Hashimoto's thyroiditis, coeliac disease, Graves' disease, myasthenia gravis, Addison's disease and vitiligo)(Reference Barker, Yu, Yu, Wang, Miao, Bao, Hoffenberg, Nelson, Gottlieb, Rewers and Eisenbarth19).
Incidence
The incidence of type 1 diabetes differs among geographical areas: China has the lowest incidence (about 0·5 cases/100 000 per year) and Finland and Sardinia (Italy) the highest (about 50 cases/100 000 per year), but it is continuously increasing (2–5 %/year) worldwide(Reference Gillespie, Bain, Barnett, Bingley, Christie, Gill and Gale20, Reference Karvonen, Viik-Kajander, Moltchanova, Libman, LaPorte and Tuomilehto21). Moreover, migrating populations bring within a short time a change in incidence that becomes that of the country to which they migrated(Reference Raymond, Jones, Swift, Davies, Lawrence, McNally, Burden, Gregory, Burden and Botha22). This evidence indirectly supports the importance of environmental factors in the development of the disease.
Cumulatively, 5–10 % of the total cases of diabetes in the entire population are due to type 1 diabetes.
The age at onset is progressively decreasing: most cases (40–50 %) are diagnosed before the age of 4 years. Another 20–30 % of cases are diagnosed before the age of 18 years. The remaining cases occur in adulthood(Reference Dabelea, Bell and D'Agostino2).
One of the potential factors involved in the progressive increase in the incidence of type 1 diabetes in young children may be the overload of the β-cell, mediated by a variety of mechanisms, that may sensitize it to become immune to damage and apoptosis, thus accelerating ongoing autoimmune processes leading to its destruction(Reference Dahlquist23). Rapid growth rate, physical stress (infection, inflammation) or psychological stress increase insulin requirement, whereas excess fat cell accumulation promotes insulin resistance. Overfeeding in the intra-uterine life or in early extra-uterine life leads to accelerated growth and overweight(Reference Maffeis, Micciolo, Must, Zaffanello and Pinelli24, Reference Stettler, Kumanyika, Katz, Zemel and Stallings25). Even a moderate excess of child growth, not necessarily associated with obesity, has been associated with the risk of type 1 diabetes as well as with obesity later in life(Reference Patterson, Dahlquist and Soltesz26).
Targets of treatment
The reduced capacity to synthesize and secrete a sufficient quantity of insulin due to the progressive reduction in the number of β-cells leads to hyperglycaemia, glucosuria, ketoacidosis, polyuria, polydipsia, weight loss, etc(Reference Silverstein, Klingensmith, Copeland, Kaufman, Laffel, Deeb, Grey, Anderson, Holzmeister and Clark4). All of these symptoms can be reversed by giving exogenous insulin to the patient. Insulin administration is the cornerstone of treatment for type 1 diabetes. However, insulin requirements depend largely on and change according to time, meals, skeletal muscle activity, stress, infections, etc; therefore, insulin injections should be modified frequently during the day according to blood glucose levels, meal size and composition, as well as the kind, intensity and duration of exercise. Consequently, there are three main targets of the treatment for type 1 diabetes: (a) to obtain good blood glucose control by means of insulin administration, frequent blood glucose monitoring, adequate nutrition and physical activity; (b) to avoid severe hypoglycemia episodes; and (c) to prevent micro- (retinopathy, nephropathy, neuropathy) and macro- (cardio-, cerebro-, peripheral-) vascular complications(Reference Silverstein, Klingensmith, Copeland, Kaufman, Laffel, Deeb, Grey, Anderson, Holzmeister and Clark4).
Nutritional recommendations
Nutrition plays a key role in appropriate blood glucose control as well as in preventing and treating some of the risk factors for diabetes complications, such as obesity, hypertension and hyperlipidaemia. As recently reported in a position statement by the American Diabetes Association, the appropriate goals of medical nutrition therapy are(27):
1. to achieve and maintain:
● blood glucose levels in the normal range;
● optimal lipoprotein profile;
● normal blood pressure;
2. to provide self-management training to guarantee safe exercise sessions;
3. to prevent or slow the development of complications.
These targets should be achieved while at the same time addressing individual nutritional needs, growth and development, as well as maintaining the pleasure of eating. Nutrition counselling should be individualized and sensitive to personal and family needs, willingness to change and ability to make changes. Meal plans should take into account individual preferences but also cultural influences, exercise and physical activity patterns, as well as family eating patterns and schedules.
The main target of nutritional treatment is to educate the patient and the family to follow the RDA and to maintain a reproducible daily meal plan. A free meal plan should be permitted exceptionally. Although insulin injections adequate to carbohydrate intake allow the promotion of glucose disposal and blood glucose control, it is also true that it is very difficult to follow recommended energy and nutrient intake with irregular meal patterns and meal composition, which could easily lead to insulin misuse, weight and fat gain or exposure to hypoglycaemia.
We recommend consulting a qualified dietitian with experience in paediatric nutrition and diabetes, especially if the dietitian is a member of the interdisciplinary team taking care of diabetic children. The main role of the dietitian is to offer nutrition education, in full respect of the RDA, aimed at teaching the child and his family to achieve good reproducibility in their nutritional choices. Once this target has been reached, education on carbohydrate counting should be provided, aimed at managing exceptions in the daily meal plan. Nutrition education should begin at the onset of diabetes and maintained through periodical nutrition counselling sessions with individual families or with patients or groups of patients.
Current nutritional recommendations for children with diabetes suggest to:
(a) limit the intake of foods of animal origin (red meat, cheese, cold cuts);
(b) limit the intake of fats (also by teaching to check food labels);
(c) promote the intake of foods that naturally contain fibre (mainly vegetables, legumes, fruit);
(d) avoid sugar-free foods or ‘special foods’ for diabetics.
Energy
Children and adolescents with type 1 diabetes have the same energy and nutrient requirements as all healthy children and adolescents of the same age, sex and body size. However, in diabetic patients, constant efforts to maintain appropriate body composition also through adequate energy and nutrient intake are crucial to obtaining both insulin sensitivity and blood glucose control. Body-weight control, avoiding overweight and obesity, is important also for reducing micro- and macrovascular complications(Reference Stone, Craig, Chan, Lee, Verge and Donaghue28). Moreover, limiting visceral fat accumulation further reduces the risk of co-morbidities(Reference Sibley, Thomas, de Boer, Brunzell and Steffes29). As for the general population, a healthy lifestyle, regular physical activity and adequate eating habits are necessary to regulate energy balance and to achieve and maintain an appropriate BMI and body fat distribution, also in subjects with type 1 diabetes.
Carbohydrates
Healthy diet recommendations for the population suggest that at least 45 % of the energy intake in a single day should come from carbohydrates(30). ISPAD Consensus Guidelines increases the lower limit of carbohydrate intake to 50 % of total energy(Reference Swift31) (Fig. 1).

Fig. 1 Carbohydrate (CHO) intake recommendation in type 1 diabetes(27, Reference Swift31). FDA, Food and Drug Administration.
Carbohydrates and insulin treatment
Carbohydrate ingestion promotes physiological insulin secretion. Increasing carbohydrate intake increases the insulin secretion rate. However, it is not only the quantity but also the type or source of carbohydrates found in foods, the composition of the meal (i.e. macronutrient and fibre content), digestibility and style of preparation (cooking method and time, etc) that influence postprandial glucose levels(27, Reference Wolever, Yang, Zeng, Atkinson and Brand-Miller32). Therefore, there are two possible strategies to adapt carbohydrate intake and insulin administration in a subject wanting in insulin: (a) to inject a dose of insulin calculated on the basis of the carbohydrate intake with each meal; (b) to inject fixed daily insulin doses, maintaining the energy and composition of daily meals and snacks rather constant. Most children and adolescents with type 1 diabetes use rapid-acting insulin through injections or through an insulin pump so that they have to modify insulin doses and adapt the amount of insulin injected according to the amount of carbohydrates ingested with the meal or snack. A minority of patients use fixed daily insulin doses and they have to maintain their carbohydrate intake constant on a daily basis, regarding time of eating and amount of carbohydrates ingested per eating episode.
Children receiving basal-bolus insulin therapy can use the insulin : carbohydrate ratios to regulate mealtime insulin doses(Reference Silverstein, Klingensmith, Copeland, Kaufman, Laffel, Deeb, Grey, Anderson, Holzmeister and Clark4). Therefore, it is essential that the child/adolescent and his/her parents learn to estimate the nutrient content of a meal. Several methods are available to estimate the nutrient content of a meal: counting carbohydrates, the exchange system and experience-based estimation(Reference Gillespie, Kulkarni and Daly33). Counting carbohydrates must be considered an important way to manage exceptions in food planning. However, the great flexibility offered by one technique, such as counting carbohydrates, is not enough to choose it exclusively and ignore changes in daily meal plan and meal composition. In fact, the line between flexibility and anarchy is very thin, especially in adolescence. The final target of nutrition therapy is to adopt educative interventions that will help diabetics have a healthy, balanced and adequate diet that reflects dietary regularity.
Carbohydrates and exercise
Skeletal muscle activity promotes glucose oxidative and non-oxidative disposal. The kind, intensity and duration of exercise, as well as training and environmental conditions under which exercise is performed, affect glucose metabolism(Reference Steppel and Horton34). Increasing the intensity of exercise increases the proportion of glucose oxidized in muscle compared with fat(Reference Maffeis, Zaffanello, Pellegrino, Banzato, Bogoni, Viviani, Ferrari and Tato35). Moreover, in the post-exercise phase, glycogen synthesis in muscle promotes non-oxidative glucose disposal(Reference McMahon, Ferreira, Ratnam, Davey, Youngs, Davis, Fournier and Jones36). The transfer of glucose from the circulation to muscles may cause hypoglycemia both during and after exercise when glucose output in the liver is unpaired by glycogen depletion. Therefore, a reduced insulin dose injected before planned exercise and the ingestion of carbohydrates before, during and/or after exercise may be necessary to maintain acceptable blood glucose levels. Frequent blood glucose monitoring and insulin adjustments are often necessary to allow the child to participate in school, team and individual sports.
Adults performing moderately intense exercise increase their glucose utilization by 2–3 mg/kg body weight per min above baseline requirements(Reference Wasserman and Zinman37). Assuming that this calculation could also be valid in older children and adolescents, in 1 h of exercise at moderate intensity, a 10-year-old boy with a body weight of 40 kg utilizes about 6 g glucose over baseline requirements. Monitoring blood glucose before and at termination of exercise and at hourly intervals during episodes of prolonged strenuous activity is recommended(Reference Silverstein, Klingensmith, Copeland, Kaufman, Laffel, Deeb, Grey, Anderson, Holzmeister and Clark4). If the blood glucose level should go below 1000 mg/l during the period of exercise, 15 g carbohydrate (10 g for younger children) may be administered as readily absorbed sugar(Reference Silverstein, Klingensmith, Copeland, Kaufman, Laffel, Deeb, Grey, Anderson, Holzmeister and Clark4). For vigorous activity expected to last longer than 30 min, an additional 15 g carbohydrate may be necessary.
Fibre
Adequate nutrition for type 1 diabetes includes a high fibre content(27, Reference Swift31). The intake of legumes, vegetables, fruit, fibre-rich cereals and wholegrain products should be encouraged as for the general population. Fibre-containing foods provide vitamins, minerals and other substances important for good health. Moreover, a high-fibre diet is associated with better glucose control in type 1 diabetes(Reference Cundiff and Nigg38). Optimal fibre intake recommended for the general population is 3·3 g/1000 kJ (14 g/1000 kcal)(27).
Sweeteners
Sugar alcohols (erithritol, isomalt, lactitol, maltitol, mannitol, sorbitol, etc) have lower energy than glucose (8·4 kJ/g; 2 kcal/g) and cause a lower increase in postprandial glucose response than glucose or sucrose(27). Their use appears to be safe, although there is no clear evidence that they may cause reduced glycaemia or energy intake.
Other non-nutritive sweeteners (acesulfame K, aspartame, neotame, saccharin and sucralose) may be used also by children with diabetes(Reference Renwick39).
Fat
A recent study conducted in the USA demonstrated that fat intake is higher in adolescents with type 1 diabetes than in those without diabetes, and fat intake exceeds recommendations (Fig. 2) (Reference Helgeson, Viccaro, Becker, Escobar and Siminerio40). Another study showed that dietary fat intake predicted a 1-year change in body fat in girls with type 1 diabetes (Fig. 3) (Reference Sarnblad, Ekelund and Aman41). Clear evidence is available that individuals with diabetes have a cardiovascular risk equivalent to that of non-diabetic individuals with pre-existing CVD(Reference Barr, Zimmet and Welborn42). Although the most important factor in the development of vascular complications is the glycation process, the role of lipids is also important. High TAG levels are an independent predictive factor of both renal and retinal complications in patients with type 1 diabetes(Reference Hadjadj, Duly-Bouhanick, Bekherra, BrIdoux, Gallois, Mauco, Ebran and Marre43, Reference Chaturvedi, Bandinelli, Mangili, Penno, Rottiers and Fuller44). Moreover, LDL-cholesterol levels are independent risk factors of diabetic nephropathy, whereas total cholesterol is associated with persistent microalbuminuria(Reference Stone, Craig, Chan, Lee, Verge and Donaghue45, Reference Goff, Gerstein and Ginsberg46). Finally, clinical studies have shown that serum levels of advanced glycation endproducts (AGE), for instance, a complex and heterogeneous group of proteins, formed by non-enzymic glycation in a series of reactions, are correlated with clinical stages of diabetes complications such as retinopathy and nephropathy(Reference Singh, Barden, Mori and Beilin47, Reference Vlassara and Palace48). Children and adolescents with diabetes and high serum TAG or LDL-cholesterol had significantly higher serum levels of fluorescent AGE(Reference Galler, Muller, Schinzel, Kratzsch, Kiess and Munch49). The observed effect may be caused by a loss of optimal regulation of lipid metabolism. It could suggest a link between TAG and the formation of AGE.

Fig. 2 Self-reported diet composition in adolescents with and without type 1 diabetes(Reference Helgeson, Viccaro, Becker, Escobar and Siminerio40). CHO, carbohydrate; FA, fatty acids.
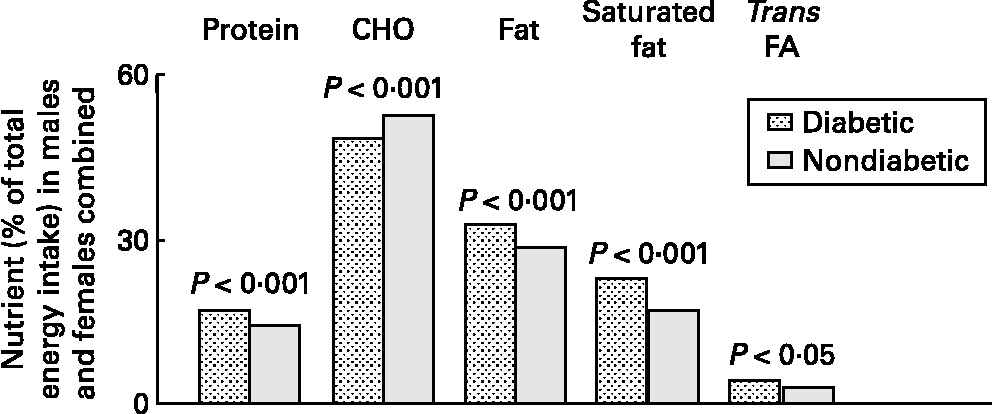
Fig. 3 Fat intake recommendation in type 1 diabetes(27, Reference Swift31).
The relationship between fat intake and fat gain, as well as fat intake and cardiovascular risk factors, emphasizes the need to maintain total fat ≤ 30 % of total enrgy intake in children and adolescents with diabetes, as well as to maintain the composition of fat intake within desirable limits: saturated fat < 7 % of total energy; dietary cholesterol < 24 mg/1000 kJ ( < 100 mg/1000 kcal); trans-fatty acids taken in minimal amounts (Fig. 3)(27, Reference Swift31). Children with diabetes should be encouraged to increase their n-3 PUFA (mainly contained in fish) and MUFA intake (mainly with olive oil), given the demonstrated benefits on lipid metabolism and the lipoprotein profile(27, Reference Swift31, Reference Hilpert, West, Kris-Etherton, Hecker, Simpson and Alaupovic50). Finally, moderate intake of plant sterol and stanol esters may contribute to reduce the absorption of dietary and biliary cholesterol and could contribute to limit cholesterol circulating levels also in children with diabetes(Reference Jarvisalo, Raitakari, Gylling and Miettinen51).
Protein
Also as regards protein, children and adolescents with diabetes should respect dietary recommendations for the population (15–20 % of total energy)(27, Reference Swift31). Good-quality protein (meat, poultry, fish, eggs, milk, cheese and soya) provide all nine indispensable amino acids and are highly digestible. A daily intake of >0·8 g protein/kg body weight is adequate(27). In case of early stages of chronic kidney disease, total protein intake should be reduced, ranging between 0·8 and 1 g protein/kg body weight(27). Intake lower than 0·8 g/kg is recommended in the later stages of kidney disease.
Vegetarian diet
A small fraction of individuals living in industrialized countries have a vegetarian diet, although vegetarianism is becoming more popular. Several studies have shown that a plant-based diet high in fibre-rich foods, such as vegetables, fruits, cereals, whole grains and legumes, is inversely related to BMI, overweight and obesity, blood pressure, blood lipids, cancer, heart disease and all-cause mortality(Reference Appleby, Davey and Key52–Reference Key, Fraser and Thorogood57). Recent data suggest that diabetes care has benefited from a vegetarian diet(Reference de Mello, Zelmanovitz, Perassolo, Azevedo and Gross58, Reference Barnard, Cohen, Jenkins, Turner-McGrievy, Gloede, Jaster, Seidl, Green and Talpers59). In fact, a low-fat plant-based diet influences nutrient intake and body composition in several ways that may, in turn, affect insulin sensitivity. First, dietary energy density and energy intake are reduced in a low-fat, high-fibre diet. The weight-reducing effect of the vegan diet is probably responsible for a substantial proportion of its effect on the reduction of the HbA1C(Reference de Mello, Zelmanovitz, Perassolo, Azevedo and Gross58–Reference Newby, Tucker and Wolk60). Second, reductions in total fat intake and in the proportion of dietary saturated to unsaturated fat as well as the increased intake of low-glycaemic-index and high-fibre foods increase insulin sensitivity(Reference Barnard, Cohen, Jenkins, Turner-McGrievy, Gloede, Jaster, Seidl, Green and Talpers59, Reference Newby, Tucker and Wolk60).
Few data are available in children with type 1 diabetes having a vegetarian diet. Kontessis et al. demonstrated that a vegetarian diet is able to improve microalbuminuria in normotensive, normoproteinuric children with type 1 diabetes(Reference Kontessis, Bossinakou, Sarika, Iliopoulou, Papantoniou, Trevisan, Roussi, Stipsanelli, Grigorakis and Souvatzoglou61). Moreover, the American Dietetic Association and the Dietitians of Canada suggest that well-balanced vegetarian diets are healthy and adequate from a nutritional standpoint and have health benefits for the prevention and treatment of certain pathologies(62). Type 1 diabetes is potentially one of these.
Age at risk for low nutritional compliance
The young child
Controlling blood glucose of preschool children with type 1 diabetes is a big challenge for parents. Young children are very insulin sensitive and they have highly variable and unforeseeable physical activity and nutrition patterns. Considering this, it is very difficult to maintain constant and satisfactory regulation of blood glucose levels. In general, mealtime is usually considered by parents the most difficult part of their child's care(Reference Wysocki, Huxtable, Linscheid and Wayne63). Physiologically the young child has transient food preferences, emotional lability, behavioural resistance, and increasing independence seeking. All these features affect adherence to the diabetes dietary recommendations of the child. A recent study showed a correlation between certain disruptive child mealtime behaviours (children leaving the table at mealtimes, complaining during meals, spitting out their food), children's dietary adherence and average blood glucose control(Reference Patton, Dolan and Power64). Interestingly, some ineffective/coercive parenting strategies (coaxing, interrupted commands, physical prompts and feeding) were identified. Helping parents cope and educating them to adopt successful parenting behaviours could improve mealtimes and allow the child to learn and follow a structured feeding schedule, which is desirable for long-lasting efficacy in diabetes care.
The adolescent
Adolescence is a delicate phase of life. Adherence to metabolic control by adolescents with type 1 diabetes is a big challenge for both physician and parents. The important endocrine changes during puberty, rapid growth, changes in body composition and body fat distribution, as well as the physiological increase in insulin resistance, together with psychological maturation and changes of lifestyle, eating habits and social interactions, all heavily affect glucose metabolism. A combination of factors other than the difficulty in blood glucose control promotes fat gain in some adolescents with diabetes, especially females(Reference Schwab, Doerfer, Hecker, Grulich-Henn, Wiemann, Kordonouri, Beyer and Holl65). Unfortunately, poor eating habits are common in these adolescents and are associated with insulin misuse, poor glycaemic control and the development of microvascular complications(Reference Peveler, Bryden, Neil, Fairburn, Mayou, Dunger and Turner66). Moreover, the cumulative incidence of eating problems continues to increase beyond the teen years and this is strongly associated with poor physical health conditions, which increase the risk of morbidity and mortality(Reference Peveler, Bryden, Neil, Fairburn, Mayou, Dunger and Turner66). There are three main factors associated with unhealthy weight control in adolescents with type 1 diabetes: (a) increased emphasis on food, eating patterns and regulation of dietary intake for diabetes management purposes; (b) increased emphasis on and concern about weight regulation; and (c) quality of family functioning and support(Reference Peveler, Bryden, Neil, Fairburn, Mayou, Dunger and Turner66, Reference Mellin, Neumark-Sztainer, Patterson and Sockalosky67). Maintaining structure and routine of family meals seems to serve as a protective function for adolescent girls with diabetes. Family meals may be helpful for role-modelling healthy eating patterns and for the early detection of emerging eating disorders.
Conclusions
Insulin-dependent diabetes requires maintaining lifelong healthy eating habits. Age- and sex-specific recommendations proposed for the general population are usually valid also for children and adolescents with type 1 diabetes. Attention to diet composition, especially to macronutrient and fibre content, as well as to eating patterns is crucial. The risk for all the complications of diabetes, besides mortality, is increased in obese diabetics. Therefore, it is important that an individual with diabetes maintains normal body weight, much more so than an individual without diabetes. Unfortunately, controlling weight could lead to very dangerous behaviours, especially in adolescent girls, such as insulin misuse or eating disorders. New tools should be available to the physician to efficaciously deal with these emerging problems. Further studies that analyse the relationship between diabetes and appetite regulation, insulin resistance cofactors, postprandial glucose disposal after mixed meals that differ in composition and energy intake and new behaviour-based intervention programmes might contribute to improve the efficacy of intervention and metabolic regulation in these patients.
Conflict of interest statement
None of the authors has any conflicts of interest to report.





