Introduction
In Europe, Irish agriculture is characterized by an Atlantic-influenced climate, with an average rainfall of 600 to 1,000 mm, annual sunshine of 1,100 to 1,600 h, and a soil quality legacy from historical mixed grassland/spring cereal cropping systems, which results in mean grain yields ranging from 7,200 (winter oats [Avena sativa L.]) to 10,700 (winter wheat [Triticum aestivum L.]) kg ha−1, among the highest globally (CSO 2021; Forristal and Grant Reference Forristal and Grant2011; Met Eireann 2021; Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022). The mild damp climate is, however, conducive to the rapid growth and proliferation of grass weeds, fungal pathogens, and some insect vector–transmitted diseases, requiring the intensive use of crop protection chemicals, especially fungicides (15% of production costs per hectare) and herbicides (8%), to achieve high yield potential for cereal crops (Jess et al. Reference Jess, Kildea, Moody, Rennick, Murchie and Cooke2014; Lynch et al. Reference Lynch, Glynn, Kildea and Spink2017; Teagasc 2022). In Ireland, annual bluegrass (also known as annual meadow grass; Poa annua L.) is abundant on all soils, including arable fields, grasslands, forests, gardens, lawns, roadside verges, wastelands, and amenity areas (Bond et al. Reference Bond, Davies and Turner2007; Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022). It is primarily a self-pollinated, allotetraploid (2n = 28) weed, with high fecundity and germination rates (Bond et al. Reference Bond, Davies and Turner2007; Carroll et al. Reference Carroll, Brosnan, Trigiano, Horvath, Shekoofa and Mueller2021; Gardarin et al. Reference Gardarin, Durr and Colbach2009). In arable fields, P. annua populations tend to build up rapidly in early-sown winter cereals in all crop establishment systems, especially non-inversion tillage (Fortune et al. Reference Fortune, Kennedy, Mitchell, Dunne, Murphy, Connery and Grace2005). A recent grower survey of grass weeds in cereals in Ireland ranked P. annua as the third most problematic species after poverty brome (Bromus sterilis L.) and wild oats (Avena fatua L.) (Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022). Nevertheless, a low incidence of just 9% on 103 farms at preharvest coupled with very low population pressure means that growers consider P. annua relatively easy to control with a variety of herbicide options and timings available (i.e., preplant burndown, residual herbicides applied as preemergence/early postemergence and postemergence).
In Ireland, spring barley (Hordeum vulgare L.) (120,000 ha), winter wheat (60,000 ha), and winter barley (60,000 ha) are the most dominant cereal crops, along with broadleaf crops, including canola (Brassica napus L.) and field beans (Vicia faba L.) both 19,700 ha (CSO 2021). At least 388,509 ha of cereal, canola, and bean crops were herbicide treated in 2016, with 31% of this area treated with burndown glyphosate (Group 9: inhibitor of 5-enolpyruvylshikimate-3-phosphate synthase [EPSPS]), 5% with residual herbicides such as pendimethalin (Group 3: inhibitor of microtubule assembly), flufenacet or prosulfocarb (Group 15: inhibitors of very-long-chain fatty-acid synthesis), 26% with early or autumn postemergence isoproturon (Group 5: photosystem II inhibitor), and 38% with spring postemergence acetyl-CoA carboxylase (ACCase) (Group 1) and acetolactate synthase (ALS) (Group 2) inhibitors (DAFM 2016). The application of glyphosate is primarily targeted at the control of quackgrass [Elymus repens (L.) Gould] and/or B. sterilis, as well as volunteer cereals (Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022). Residual herbicides are used primarily for P. annua and broadleaf weeds or limited control of B. sterilis. ACCase/ALS-inhibiting herbicides are applied in cereals to control B. sterilis and/or A. fatua, and other grass weeds not controlled earlier; in broadleaf crops, they are used to control grass weeds and volunteer cereals (Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022). Among the most commonly used ACCase/ALS-inhibiting herbicides, ALS inhibitor mesosulfuron-methyl + iodosulfuron-methyl, used in winter wheat, and ACCase inhibitor clethodim, used in winter canola, are also highly active on P. annua and are registered in Ireland for controlling this species (Anonymous 2017, 2022c). Another ACCase graminicide, propaquizafop, used in broadleaf crops, may also have limited activity on P. annua only at early growth stages (Anonymous 2020). The herbicides ACCase inhibitor pinoxaden (used in barley and wheat) and cycloxydim (graminicide) as well as ALS inhibitor pyroxsulam (used in winter wheat) are not registered in Ireland for P. annua control (Anonymous 2018, 2022a, 2022d).
In Ireland, frequent use of ACCase/ALS-inhibiting herbicides to control key grass weeds threatens future weed control in these regions, as it risks promoting target-site resistance (TSR) due to mutations in the target genes, and non–target site resistance (NTSR), predominately via enhanced metabolism (e.g., Torra et al. Reference Torra, Montull, Taberner, Onkokesung, Boonham and Edwards2021; Yu and Powles Reference Yu and Powles2014). Since 2019, resistance to ACCase/ALS inhibitors via TSR and/or NTSR has been documented in A. fatua and B. sterilis, as well as unintended targets such as black-grass (Alopecurus myosuroides L.) and Italian ryegrass [Lolium perenne L. ssp. multiflorum (Lam.) Husnot] in cereal fields in Ireland (Alwarnaidu Vijayarajan et al. Reference Alwarnaidu Vijayarajan, Forristal, Cook, Staples, Schilder, Hennessy and Barth2020, Reference Alwarnaidu Vijayarajan, Forristal, Cook, Schilder, Staples, Hennessy and Barth2021; Byrne et al. Reference Byrne, Vijaya Bhaskar, Spink, Freckleton, Neve and Barth2021; Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022). Although the ACCase/ALS-inhibiting herbicides are usually not targeted to control P. annua in Ireland, the wet autumns and fewer preemergence spray opportunities and the loss of isoproturon registration in the European Union (EU) in 2017 resulted in more reliance on postemergence herbicides that also had activity on P. annua, raising the possibility of resistance development in non-target species. To date, no reports of herbicide resistance have been reported for P. annua in Ireland. Internationally, there are 49 reports of P. annua resistance, involving 10 different herbicide modes of action, including both TSR and NTSR mechanisms, resulting in P. annua being ranked the third most problematic weed from a resistance perspective (Heap Reference Heap2023). However, all documented P. annua resistance cases in Europe and globally have occurred in non-arable crop situations (golf courses, orchards, turf, etc.), except for one mesosulfuron-methyl + iodosulfuron-methyl-resistant case from a wheat crop in France, reported in 2015. It is worth noting that P. annua is known to exhibit natural tolerance to some ACCase herbicides, including propaquizafop, cycloxydim, and pinoxaden, due to the natural inheritance of the mutation at Ile-1781 in the ACCase protein (Barua et al. Reference Barua, Boutsalis, Malone, Gill and Preston2020; Clay et al. Reference Clay, Dixon and Willoughby2006; Délye and Michel Reference Délye and Michel2005; Ghanizadeh et al. Reference Ghanizadeh, Mesarich and Harrington2020).
Grass-weed challenges have increased in Ireland due to increased winter cropping, earlier autumn sowing, non-inversion tillage adoption, and new weed introductions from seed imports and spread via machinery moving from farm to farm (Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022). Consequently, Teagasc (the state-funded research and farm advisory organization) has developed a herbicide-resistance monitoring program through which a case of suspected resistance in P. annua (POAAN-R) to mesosulfuron-methyl + iodosulfuron-methyl was received from a crop farm in County Dublin, Ireland; POAAN-R is the subject of this research. The main objectives of this research were to: (1) determine the occurrence and level of resistance exhibited by POAAN-R to mesosulfuron-methyl + iodosulfuron-methyl and cross-resistance, if any, to another ALS chemistry, pyroxsulam; (2) verify the resistance mechanisms to ALS-inhibiting herbicides; and (3) evaluate the efficacy of other herbicide modes of action for controlling POAAN-R. In the context of EU policies (e.g., Directive 2009/128/EC Sustainable Use of Pesticides, Farm to Fork Strategy, 2030 Biodiversity Strategy), which aim to reduce chemical pesticide use and promote integrated pest management approaches (McGinley et al. Reference McGinley, Healy, Ryan, O’Driscoll, Mellander, Morrison and Siggins2023; Silva et al. Reference Silva, Yang, Fleskens, Ritsema and Geissen2022; Tataridas et al. Reference Tataridas, Kanatas, Chatzigeorgiou, Zannopoulos and Travlos2022), this work is an essential component of a research approach needed to support a move to more sustainable integrated grass-weed management (IWM, cultural/nonchemical control, and judicious herbicide use) techniques.
Materials and Methods
Plant Material
Mature seeds of the suspected POAAN-R population that survived field application of 11.3 + 3.9 g ai ha−1 of mesosulfuron-methyl + iodosulfuron-methyl (0.75 of the recommended field rate of product Pacifica Plus, Bayer CropScience, Cambridge, UK) were collected before winter wheat harvest in July 2021 from a crop field (53.867°N, 6.6°W) located near Garristown, in County Dublin, Ireland. The field was managed for at least 11 yr using deep non-inversion tillage (tine and disk implements cultivate the soil and mix crop residue to 100- to 150-mm deep) with winter wheat grown in 4 of 5 yr in a rotation with 1 yr of canola or beans. The herbicide treatments always involved use of mesosulfuron-methyl + iodosulfuron-methyl in winter wheat at lower rates, from 7.5 + 2.5 g ha−1 to 11.3 + 3.9 g ha−1 (0.5 to 0.75 of field recommended rates), and propaquizafop in broadleaf crops, used at 75 g ai ha−1 (0.5 of field recommended rate), primarily to control B. sterilis. Glyphosate was also used pre-sowing for grass-weed control on stubbles, at rates from 270 g ai ha−1 to 405 g ai ha−1 (0.5 to 0.75 of field recommended rates). Residual herbicides, including diflufenican (Group 12: inhibitors of phytoene desaturase) in winter wheat, pendimethalin + imazamox (Group 3 and 2) in beans, or propyzamide (Group 3) in winter canola, were used early postemergence on some occasions.
A herbicide-sensitive P. annua population (POAAN-S) was obtained from a commercial seed supplier (WeberSeeds Botany & Ethnobotany, Vaals, Netherlands) to be used as a sensitive reference. Seeds from both populations were stored in paper bags at 15 C.
Growing Conditions and Herbicide Application
During the winter of 2022 to 2023, seeds of POAAN-R and POAAN-S populations were sown in 230 by 176 by 55 mm plastic trays filled with a standard soil mix containing 70% loam, 20% horticultural grit, 10% medium (Professional) peat, and 2 g l−1 Osmocote Mini™ (National Agrochemical Distributors, Lusk, County Dublin, Ireland). Each replicate had 15 to 20 seeds per tray. Seeds were covered with 1 cm of soil. Trays were watered as required throughout the experiment. Herbicide treatments were applied using a Generation III Research Track Sprayer (DeVries Manufacturing, Hollandale, MN, USA) with a TeeJet® 8002-EVS flat-fan nozzle (Spraying Systems, Wheaton, IL, USA), at a pressure of 250 kPa and a water volume equivalent of 200 L ha−1. The postemergence treated and untreated control plants were grown in a glasshouse compartment with 18/12 C (day/night) temperature regime at a photoperiod of 16 h supplemented with artificial lighting to maintain a minimum light intensity of 250 μ mol m−2 s−1. The preemergence treated and untreated control plants were grown in an unheated glasshouse compartment, mimicking outdoor growing conditions for optimum activity of the residual herbicides.
Single-Dose Herbicide-Resistance Testing
Two experiments were conducted using a variety of herbicides at recommended field rates (Table 1) to determine the sensitivity or resistance of POAAN-R. Experiment 1 evaluated two ALS inhibitors, while Experiment 2 tested three ACCase inhibitors, an EPSPS inhibitor, and the two most commonly used residual herbicides, pendimethalin and flufenacet. In this region, the ALS herbicides for grass-weed control are only available commercially as formulated mixtures to target a broad spectrum of species, including broadleaf weeds (Anonymous 2022a, 2022c). The two ALS herbicides used in this research, contain a number of active ingredients of the same chemical family. Pacifica Plus (Bayer CropScience) contains a formulated mixture of mesosulfuron-methyl + iodosulfuron-methyl + amidosulfuron, and Broadway Star (Corteva Agrisciences, Cambridge, UK) contains a formulated mixture of pyroxsulam + florasulam. The amidosulfuron component in Pacifica Plus and the florasulam component in Broadway Star are included to provide broadleaf weed control (Davies et al. Reference Davies, Onkokesung, Brazier-Hicks, Edwards and Moss2020), as evidenced by the weed control information on the labels of products using amidosulfuron alone (e.g., product Eagle, Bayer CropScience, Cambridge, UK) or florasulam alone (e.g., product Lector, Nufarm, West Yorkshire, UK) (Anonymous 2022b, 2023). Most growers rely on these two broad-spectrum postemergence ALS-inhibiting herbicides for spring-applied grass-weed (especially B. sterilis) control in winter wheat in Ireland (Alwarnaidu Vijayarajan et al. Reference Alwarnaidu Vijayarajan, Forristal, Cook, Schilder, Staples, Hennessy and Barth2021). This research evaluated the efficacy of the active ingredients mesosulfuron-methyl + iodosulfuron-methyl in Pacifica Plus and pyroxsulam in Broadway Star on P. annua. control.
Table 1. Resistance status across different herbicide chemistries for sensitive (POAAN-S) and resistant (POAAN-R) populations of Poa annua.

a ALS, inhibitor of acetolactate synthase; ACCase, inhibitor of acetyl-CoA carboxylase; EPSPS, inhibitor of enolpyruvyl shikimate phosphate synthase; microtubule assembly, inhibitor of microtubule assembly; VLCFA, inhibitors of very-long-chain fatty-acid synthesis.
b Treatments with mesosulfuron-methyl + iodosulfuron-methyl were applied with 1% v/v Biopower (alkylethersulfate sodium salt) adjuvant (6.7% w/w 3, 6-dioxaeicosylsulphate sodium salt [EAC1] and 20.1% w/w 3, 6-dioxaoctadecylsulphate sodium salt, Bayer CropScience Ltd, Cambridge, UK); treatments with pyroxsulam contained 1% v/v Kantor (alkoxylated triglycerides) adjuvant (EC 790 g L−1 alkoxylated triglycerides, Interagro Ltd, Hertfordshire, UK).
c S indicates total susceptibility (i.e., all treated plants died), and R indicates 100% surviving plants (i.e., no mortality) based on visual check conducted at 30 d post-treatment.
Foliar application herbicides were applied postemergence at growth stage (GS) 12–13 (BBCH) (Hess et al. Reference Hess, Barralis, Bleiholder, Buhrs, Eggers, Hack and Strauss1997). Residual herbicides were applied preemergence directly onto the seeds sown on moist soil (for herbicide activation) in trays on the same day of sowing. Immediately after spraying, seeds were covered with the soil to which preemergence herbicide was applied and not watered for 48 h. Each experiment consisted of four replicates of each herbicide treatment and nontreated control for each population and was repeated.
Plant survival posttreatment was assessed visually at 30 d and expressed as a percentage of the total number of treated plants. Based on a visual check, surviving plants that continued to produce new shoots or tillers after treatment were recorded as resistant, and plants with symptoms of severe leaf chlorosis, desiccation, or no new active growth, and ultimately total plant death, or plants that did not have at least one emergent leaf (preemergence only), were recorded as susceptible.
Dose–Response Assays
To quantify the levels of resistance or sensitivity of POAAN-R, four independent dose–response assays with foliar application herbicides were established (i.e., two ALS-inhibiting herbicides to confirm resistance and two alternative herbicides, ACCase inhibitor clethodim and EPSPS inhibitor glyphosate, to confirm sensitivity). Plants of POAAN-R and POAAN-S populations at GS 12-13 were sprayed with mesosulfuron-methyl + iodosulfuron-methyl at 0, 0.94 + 0.31, 1.9 + 0.63, 3.8 + 1.3, 7.5 + 2.5, 15 + 5, 22.5 + 7.5, 30 + 10, 60 + 20, and 120 + 40 g ha−1 with each dose mixed with 1% v/v Biopower adjuvant; with pyroxsulam at 0, 1.2, 2.4, 4.7, 9.4, 18.8, 28.1, 37.5, 75.0, and 150.1 g ai ha−1 with each dose mixed with 1% v/v Kantor adjuvant; with clethodim at 0, 7.5, 15, 30, 60, 120, 180, 240, 480, and 960 g ai ha−1; and with glyphosate at 0, 81, 162, 270, 405, 540, 810, and 1,080 g ha−1. The adjuvant types for the ALS herbicides are described in Table 1. The selected ALS/ACCase dose rates for POAAN-R ranged from 0.25 to 8 times of field recommended rates and for POAAN-S range from 0.0625 to 2 of field recommended rates, while glyphosate dose rates for both R and S range from 0.15 to 2 of field recommended rate. Each dose–response experiment was randomized with three replicates per dose and was repeated. At 30 d after spraying, aboveground plant material was harvested from each tray and fresh shoot weight was recorded.
TSR Analysis
Fresh leaf samples from the youngest fully developed leaves of eight plants, 8 from POAAN-R that had survived the field recommended rate of mesosulfuron-methyl + iodosulfuron-methyl (15 + 5 g ha−1), and 8 from untreated control plants in POAAN-S were collected from the single-dose assays. DNA was extracted from a total of 16 leaf samples with the Macherey-Nagel Plant DNA Kit (Lab Unlimited - Carl Stuart Group, Tallaght, County Dublin, Ireland) using the ThermoScientificTM Pharma KingfisherTM flex 96 Deep-well head magnetic particle processor (Lab Unlimited - Carl Stuart Group). The DNA concentration of each sample was determined using the Quanti-iTTM PicoGreenTM dsDNA Assay kit (InvitrogenTM) (Bio-Sciences Limited, Dun Laoghaire, County Dublin, Ireland) and then each diluted to 20 ng µl−1. A sealed elusion plate containing DNA solutions was sent to a commercial company (IDENTXX, Stuttgart, Germany) for TSR genotyping. The samples were analyzed for the most common mutations in the ALS gene at positions Pro-197 and Trp-574; for the ACCase gene at positions Ile-1781, Trp-2027, lle-2041, Asp-2078, and Gly-2096; and for the EPSPS gene at position Pro-106; all of which were identified mutations associated with herbicide resistance in grass-weed species (e.g., Brunharo et al. Reference Brunharo, Morran, Martin, Moretti and Hanson2019; Tranel and Wright Reference Tranel and Wright2002; Yu et al. Reference Yu, Collavo, Zheng, Owen, Sattin and Powles2007). Briefly, the polymerase chain reaction (PCR) was performed on the genomic DNA (5 to 10 ng µl−1) using specific primers (developed by IDENTXX, Stuttgart, Germany) for amplification of the ALS, ACCase, and EPSPS gene fragments covering the positions of interest. PCR products of respective gene fragments were amplified using initial denaturation for 3 min at 95 C, followed by 40 cycles consisting of 95 C for 10 s, 52 to 62 C (annealing temperature depends on primers used) for 35 s, 72 C for 30 s, and 72 C for 5 min for final extension. Successful amplification was checked via electrophoresis on an agarose gel. PCR product was analyzed for single-nucleotide polymorphism using pyrosequencing (Qiagen, Hilden, Germany) according to the manufacturer’s instructions (Keshtkar et al. Reference Keshtkar, Mathiassen, Moss and Kudsk2015).
Effect of Malathion on Resistance to Mesosulfuron-Methyl + Iodosulfuron-Methyl and Pyroxsulam
To assess the presence of NTSR caused by enhanced metabolism, plants of POAAN-R and POAAN-S populations at GS 12-13 were sprayed with ALS inhibitor mesosulfuron-methyl + iodosulfuron-methyl or pyroxsulam at the recommended field rate (15 + 5 g ha−1 or 18.8 g ha−1) and 1.5 of the recommended field rate (22.5 + 7.5 g ha−1 or 28.1 g ha−1) with or without malathion at 1,000 g ai ha−1. Malathion is an organophosphate insecticide known to inhibit P450-mediated metabolic resistance (e.g., Kaundun Reference Kaundun2014; Yu and Powles Reference Yu and Powles2014). Malathion was applied approximately 3 h before the application of ALS herbicides. Visual assessment for survival was conducted at 30 d after spraying. Each experiment consisted of three replicates, with or without malathion for each dose, and nontreated control for each population and was repeated.
Statistical Analysis
Data analyses were performed in R v. 3.6.3 (R Core Team 2020). As ANOVA revealed no significant (P > 0.05) difference between the two experimental runs, survival data from single-dose assays were pooled for analysis, as were the shoot weight data from dose–response assays. Dose–response models were fit to the shoot fresh weight data using the drc package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015). Lack-of-fit F-tests were performed to assess model fit (P > 0.05). A three-parameter log-logistic model was selected to fit shoot weight data of both ALS-inhibiting herbicides, while a four-parameter Weibull-1 model was used to fit clethodim and glyphosate shoot weight data. As residuals were normally distributed, data were not transformed. Fitted models estimated the growth rate GR50 (i.e., the effective dose rate required to obtain a growth reduction of 50% relative to untreated plants). The resistance index (RI) was calculated as GR50 of POAAN-R divided by GR50 of POAAN-S.
Results and Discussion
Resistance Mechanisms of Poa annua to ALS Herbicides
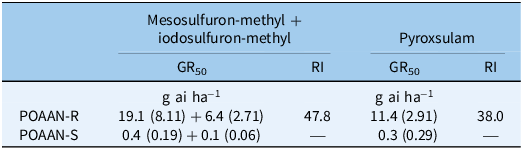
Single-dose assay results indicated that POAAN-R had 100% plants surviving (or no mortality recorded) when treated with field recommended rates of mesosulfuron-methyl + iodosulfuron-methyl (15 + 5 g ha−1) and of pyroxsulam (18.8 g ha−1) (Table 1). In contrast, POAAN-S was totally controlled by both of these herbicides at recommended rates. In the dose–response assays, shoot fresh weights recorded immediately after harvest were used as a metric for measuring resistance levels (Figures 1 and 2). The POAAN-R treated with mesosulfuron-methyl + iodosulfuron-methyl and pyroxsulam had GR50 values of 19.4 + 6.4 or 11.4 (Figure 1A and 1B), giving RIs of 47.8 and 38.0, respectively (Table 2). Despite fresh-weight data indicating that both ALS herbicides affected POAAN-R growth in a dose-dependent manner, survival data (data not shown) indicated high plant survivors at the highest dose rate used (Supplementary Figures S1 and S2). TSR analysis by pyrosequencing confirmed that all plants from POAAN-R had a Trp-574-Leu (heterozygous) mutation of the ALS protein, which corresponded to the high levels of resistance to mesosulfuron-methyl + iodosulfuron-methyl and pyroxsulam. No ALS Pro-197 mutation was detected in POAAN-R. As expected, there were no mutations found either at Pro-197 or Trp-574 in POAAN-S, which corresponded to its high sensitivity to both ALS herbicides. The application of the P450 inhibitor, malathion, followed by mesosulfuron-methyl + iodosulfuron-methyl or pyroxsulam did not reverse resistance in POAAN-R, with 100% plants surviving (or no mortality recorded) at both rates 15 + 5 g ha−1 or 18.8 g ha−1 (recommended field rate) and 22.5 + 7.5 g ha−1 or 28.1 g ha−1 (1.5 of field recommended rate) (Supplementary Figures S3 and S4).
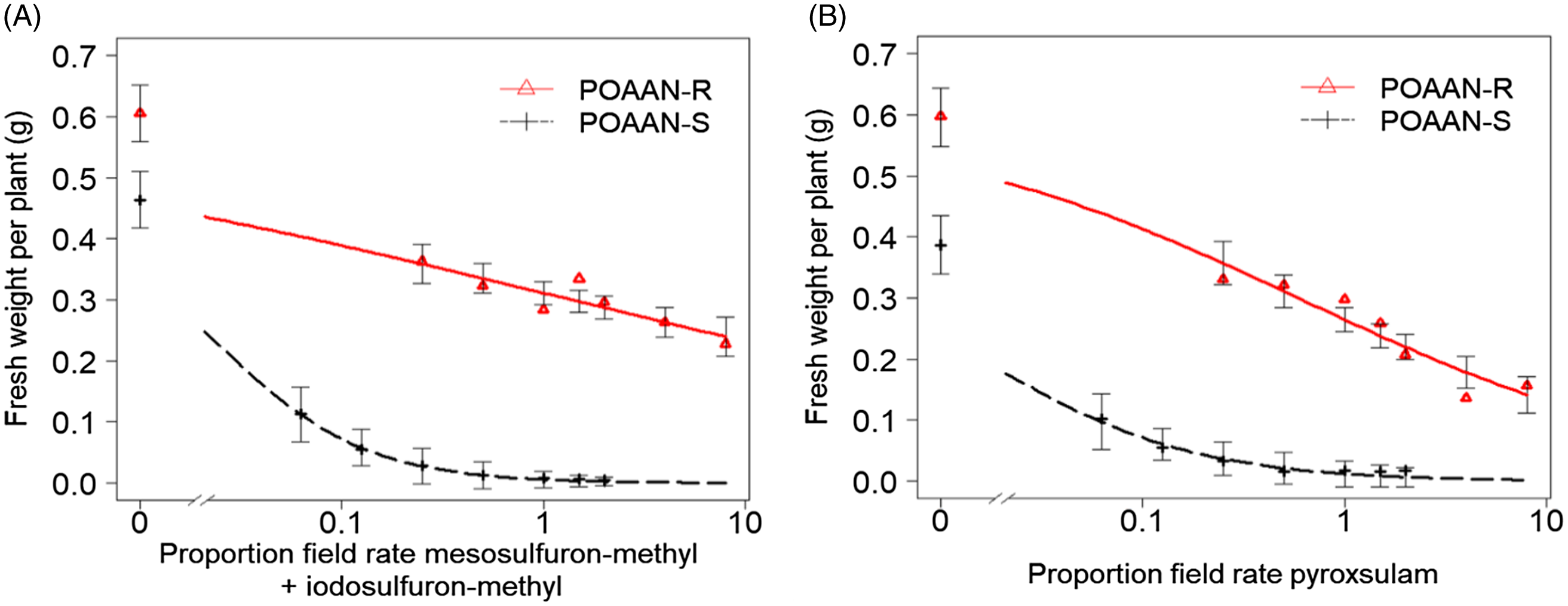
Figure 1. Dose–response curves of sensitive (POAAN-S) and resistant (POAAN-R) populations of Poa annua treated with a range of recommended field rates (±) of (A) acetolactate synthase (ALS) inhibiting herbicides mesosulfuron-methyl + iodosulfuron-methyl (15 + 5 g ai ha−1) and (B) pyroxsulam (18.8 g ai ha−1). Vertical bars represent the SEs.
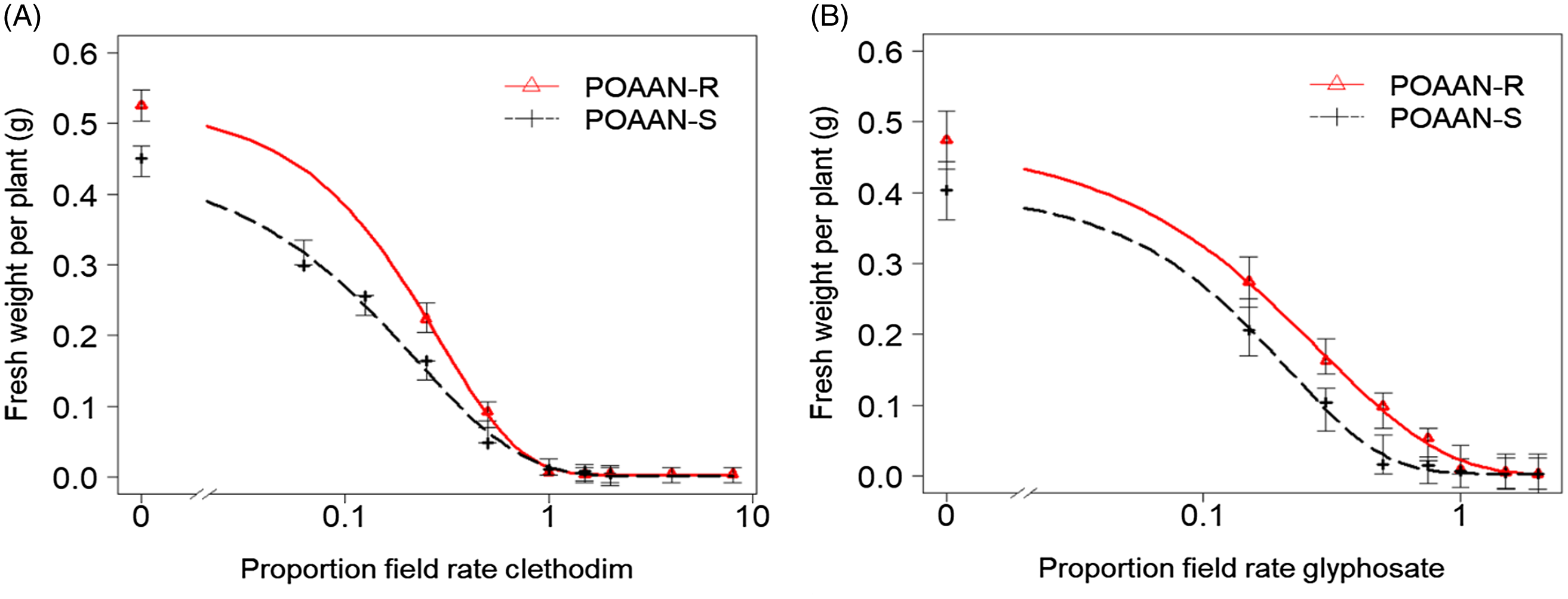
Figure 2. Dose–response curves of sensitive (POAAN-S) and resistant (POAAN-R) populations of Poa annua treated with a range of recommended field rates (±) of (A) acetyl-CoA carboxylase (ACCase)-inhibiting herbicide clethodim (120 g ai ha−1) and (B) 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS)-inhibiting herbicide glyphosate (540 g ai ha−1). Vertical bars represent the standard errors.
Table 2. Shoot fresh weight GR50 values (SEs in parentheses) of sensitive (POAAN-S) and resistant (POAAN-R) populations of Poa annua treated with a range of recommended field rates (±) of mesosulfuron-methyl + iodosulfuron-methyl (15 + 5 g ai ha−1) and pyroxsulam (18.8 g ai ha−1).
Resistance index (RI) was calculated as the ratio of GR50 values of R and S populations.
Repeated use of herbicides with the same mode of action can lead to weed resistance development (Yu and Powles Reference Yu and Powles2014). To date, 171 weed species are reported to have developed resistance to ALS-inhibiting herbicide modes of action, the most for any herbicide group (Heap Reference Heap2023). Resistance to ALS-inhibiting herbicides in different species is mostly caused by at least one of nine mutations at positions associated with ALS (Pro-197, Asp-376, Trp-574, Ala-122, Ala-205, Gly-654, Arg-377, Ser-653, and Phe-206) (Brosnan et al. Reference Brosnan, Vargas, Breeden, Grier, Aponte, Tresch and Laforest2016; Fang et al. Reference Fang, Yang, Zhao, Chen and Dong2022; Tranel et al. Reference Tranel, Wright and Heap2022). In our study, Trp-574-Leu mutations in POAAN-R were associated with resistance to two different chemistries of ALS inhibitors, with stronger resistance to mesosulfuron-methyl + iodosulfuron-methyl than to pyroxsulam (Table 2). In non-agricultural crop situations, where resistant P. annua is prevalent, Trp-574 is the most common mutation conferring resistance to multiple ALS inhibitors (e.g., Brosnan et al. Reference Brosnan, Vargas, Breeden, Grier, Aponte, Tresch and Laforest2016; Cross et al. Reference Cross, McCarty, McElroy, Tharayil and Bridges2015; McElroy et al. Reference McElroy, Flessner, Wang, Fenny, Walker and Wehtje2013). Enhanced metabolism caused by low herbicide dose use can potentially cause NTSR to postemergence herbicides in several grass-weed species (e.g., Kaundun Reference Kaundun2014; Torra et al. Reference Torra, Montull, Taberner, Onkokesung, Boonham and Edwards2021; Yu and Powles Reference Yu and Powles2014). However, similar levels of resistance in POAAN-R with or without the P450 inhibitor malathion treatment in our study suggest that NTSR by enhanced metabolism may be less likely to be the cause of resistance to mesosulfuron-methyl + iodosulfuron-methyl or pyroxsulam. These results reiterate that the effects of low herbicide dose selection could be influenced by modes of action and species biology (mating system, ploidy levels, etc.), as well as standing genetic variation (i.e., the presence of different genetic alleles in a population) (e.g., Busi et al. Reference Busi, Neve and Powles2013; Davies et al. Reference Davies, Hull, Moss and Neve2018; Renton et al. Reference Renton, Diggle, Manalil and Powles2011).
Susceptibility and Resistance of Poa annua to Other Herbicides
Single-dose assay results indicated that both POAAN-R and POAAN-S populations responded similarly, sensitive (i.e., all treated plants died) to clethodim, glyphosate, pendimethalin, and flufenacet herbicides at field recommended rates, but resistant (100% survivors) to propaquizafop and pinoxaden (Table 1). Dose–response assays confirmed that POAAN-R was equally susceptible to POAAN-S to both clethodim and glyphosate (Figure 2A and 2B), with GR50 values of 24.8 g ha−1 and 17.4 g ha−1 or 104.2 g ha−1 and 84.6 g ha−1, respectively, much lower than the recommended rate of 120 g clethodim ha−1 or 540 g glyphosate ha−1, with very low RI (Table 3). Pyrosequencing TSR analysis revealed that the inheritance mutation of Ile-1781-Leu (heterozygous) in ACCase protein has been consistently detected in R and S. On the other hand, the mutations in other positions of ACCase proteins were not detected in both populations. No mutation of the EPSPS gene was detected in R and S, which coincided with their susceptibility to glyphosate at the field recommended rate.
Table 3. Shoot fresh weight GR50 values (SEs in parentheses) of sensitive (POAAN-S) and resistant (POAAN-R) populations of Poa annua treated with a range of recommended field rates (±) of clethodim (120 g ai ha−1) and glyphosate (540 g ai ha−1).

Resistance index (RI) was calculated as the ratio of GR50 values of R and S populations.
The mutant Ile-1781 found in this study, has been frequently identified in several grass weeds with ACCase TSR (Hull et al. Reference Hull, Tatnell, Cook, Beffa and Moss2014; Kaundun Reference Kaundun2014; Yu et al. Reference Yu, Collavo, Zheng, Owen, Sattin and Powles2007) but allotetraploid P. annua exhibits a natural tolerance to ACCase-inhibiting herbicides, due to a fixed Leu residue at Ile-1781 that results in an insensitive form of ACCase (Délye and Michel Reference Délye and Michel2005; Ghanizadeh et al. Reference Ghanizadeh, Mesarich and Harrington2020). Despite this naturally inherited trait, clethodim and haloxyfop are two ACCase herbicides that provide effective control of P. annua (Ghanizadeh et al. Reference Ghanizadeh, Mesarich and Harrington2020). In fact, Délye and Michel (Reference Délye and Michel2005) and Barua et al. (Reference Barua, Boutsalis, Malone, Gill and Preston2020) found P. annua resistance to propaquizafop or was due to mutant Ile-1781. Recently, Ghanizadeh et al. (Reference Ghanizadeh, Mesarich and Harrington2020) found haloxyfop-resistant P. annua caused by an ACCase Ile-2041 mutant. Consistent with this information, both POAAN-R and POAAN-S had a Ile-1781-Leu mutation in the ACCase protein. Furthermore, both R and S were sensitive to clethodim (Figure 2A; Table 3), which has never been used previously in that field, while being resistant to propaquizafop (used previously) and pinoxaden (never been used previously) (Table 1), which is also consistent with the common response to the ACCase-inhibiting herbicides in P. annua. Collectively, our results indicate that predominately heterozygous plants carrying the Ile-1781 mutation are sufficient to affect the sensitivity of specific ACCase-inhibiting herbicides, including propaquizafop and pinoxaden, but not clethodim. It is noteworthy that P. annua exhibits a distinct resistance spectrum, specifically, to ACCase graminicides, despite having the same mutation (Ile-1781) that is known to confer resistance to clethodim in other grass species (Délye et al. Reference Délye, Matéjicek and Michel2008; Yu et al. Reference Yu, Collavo, Zheng, Owen, Sattin and Powles2007). Even though the effectiveness of clethodim in controlling grass weeds with a high degree of ACCase mutation has been well documented (Moss et al. Reference Moss, Riches and Stormonth2012; Yu et al. Reference Yu, Collavo, Zheng, Owen, Sattin and Powles2007), clethodim is not preferentially used by Irish growers (Alwarnaidu Vijayarajan et al. Reference Alwarnaidu Vijayarajan, Forristal, Cook, Schilder, Staples, Hennessy and Barth2021) over the ACCase graminicides propaquizafop and cycloxydim. This is mainly due to the broad range of grass-weed activity of propaquizafop and cycloxydim, their cost-effectiveness, and their possibility to be used in both canola and bean crops.
In this region, P. annua is often considered a lower priority weed in most years and is not thought to have a large economic impact as a grass-weed of cereal crops, as it has been easy to control (Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022), but resistance evolution in P. annua may pose a future threat. In the study field where POAAN-R was sampled, crop production may not be sustainable. The deep non-inversion tillage–based continuous winter cropping and poor management, often associated with: (1) deliberately using glyphosate at suboptimal rates, (2) not selecting suitable residual herbicides or using only narrow-spectrum and suboptimal timings (as early postemergence rather than preemergence), and (3) the selection pressure imposed by the continuous use of the broad-spectrum herbicide mesosulfuron-methyl + iodosulfuron-methyl (frequently at lower rates), could have reduced herbicide efficacy against POAAN-R and unintentionally selected for resistance to ALS inhibitors. Our study also confirmed the susceptibility of ALS-resistant POAAN-R to other herbicides when applied at a field recommended rate, including burndown glyphosate, postemergence clethodim, and the preemergence residual herbicides pendimethalin and flufenacet. Therefore, there is an opportunity to use these options in conjunction with other cultural/nonchemical tools to effectively eliminate or control POAAN-R. For the study field, methods to reduce seed return and deplete the soil weed seedbank should be implemented, such as: multiyear spring cropping or spring-based rotations (as these weeds thrive following autumn germination); inclusion of winter canola allowing the use of preemergence metazachlor (Group 15) to control POAAN-R, or use of early postemergence propyzamide (Group 3) and postemergence clethodim (as a follow-up option) to additionally control B. sterilis; use of glyphosate as a part of a stale seedbed technique; strategic plow-based tillage; spraying-off distinct weed patches; adopting strict machinery hygiene; and finally, establishment of perennial grass–based mixtures [e.g., orchardgrass (Dactylis glomerata L.) and timothy (Phleum pratense L.)] at the field margin as part of wider strategy to prevent the encroachment of these weeds from the headland.
In summary, our study is the first report to characterize the nature of recorded herbicide resistance to inhibitors mesosulfuron-methyl + iodosulfuron-methyl and pyroxsulam in a P. annua, caused by the Trp-574 mutation, from an arable field. It is only the second such mesosulfuron-methyl + iodosulfuron-methyl–resistant case reported, globally, in a cereal crop. Grass-weed challenges are increasing in Ireland (Vijayarajan et al. Reference Vijayarajan, Fealy, Cook, Onkokesung, Barth, Hennessy and Forristal2022), contributed to by use of cereal-dominated rotations, earlier autumn sowing and non-inversion tillage adoption, herbicides used alone without supplementary weed control methods, use of lower than field recommended rates of postemergence ACCase/ALS-inhibiting herbicides, and new weed introductions. Because of these challenges, coupled with potential EU legislation change on plant protection products, more effort needs to be devoted to encourage proactive adoption of a total IWM approach. In this regard, this research conveys a message to growers and industry on the importance of crop and herbicide diversification, selecting suitable herbicides, correct application timing, and correct herbicide rates for maximum efficacy. These strategies in combination with cultural/nonchemical control practices will minimize or delay resistance evolution in target and non-target species as well as promoting reduced reliance on herbicides, or preserving the efficacy of existing effective herbicides, for weed control.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2023.55
Acknowledgments
This research was supported by funding from the projects ECT (Enable Conservation Tillage, grant no. LLOC1079), which is a European Innovation Partnership (EIP), funded by the Department of Agriculture, Food, and the Marine (DAFM) under the Rural Development Programme 2014–2020; and EVOLVE (Evolving Grass-Weed Challenges and Their Impact on the Adoption of Carbon Smart-Tillage Systems, grant no. 2021R528), which is funded by the DAFM under the Research Stimulus Programme. No conflicts of interest have been declared. We thank James Byrne and John Mahon for their assistance with seed collection and Gerry Nolan for glasshouse support. We also thank Joel Torra (Universitat de Lleida, Spain) for providing the commercial malathion product for this research.







