Intra-uterine growth restriction (IUGR) is a serious problem in human beings and mammals, which affects approximately 5–10 % of human infants worldwide and 15–20 % of newborn animals(Reference Garite, Clark and Thorp1, Reference Wu, Bazer and Wallace2). It has been observed that at least 13·7 million infants were born with low birth weight each year(Reference De Onis, Blossner and Villar3). IUGR leads to high morbidity and mortality, low feed utilisation and permanent adverse effects on postnatal life. Barker et al. (Reference Barker, Eriksson and Forsen4) originally stated the ‘early’ or ‘fetal’ origins of adult disease hypothesis. The hypothesis described that environmental factors, particularly poor nutrition, act in early life to programme the risks for the early onset of metabolic diseases in adult life and premature death. A significant relationship was observed between children born with IUGR and the development of a variety of adult diseases, including hyperinsulinaemia, dyslipidaemia and non-alcoholic fatty liver disease(Reference Alisi, Panera and Agostoni5, Reference Tarry-Adkins and Ozanne6).
Liver is a major organ for lipid metabolism and insulin target organ, often influenced by IUGR during pregnancy(Reference Liu, Lin and Wang7, Reference Tarryadkins, Fernandeztwinn and Hargreaves8). The liver also plays a critical role in maintaining blood glucose homeostasis by controlling hepatic glucose production. IUGR has been linked to glucose intolerance and insulin resistance (IR)(Reference He, Dong and Xu9). Previous studies suggested that IUGR not only closely linked with lipid dysfunction but also related to fatty liver disease(Reference Magee, Han and Cherian10).
Curcumin is a naturally occurring phenolic compound, which is widely used in food, beverage, medicine and so forth(Reference Aggarwal, Sundaram and Malani11). Curcumin (C21H20O6), first described in 1910 by Lampe and Milobedeska, is the most active ingredient of turmeric and makes up 2–5 % of this spice(Reference Miłobe¸dzka, Kostanecki and Lampe12). The beneficial effects of curcumin on IR have been widely researched in animal models(Reference Jang, Choi and Jung13). Curcumin treatment also has been illustrated to attenuate IR by decreasing insulin receptor substrate 1 (IRS-1) phosphorylation in the muscle of Wistar rats fed with high fructose(Reference Maithilikarpagaselvi, Sridhar and Swaminathan14). In addition, they found that curcumin attenuated hyperinsulinaemia and the homeostasis model assessment for IR (HOMA-IR) index. However, related studies about curcumin on IR in IUGR are very limited.
Accordingly, we hypothesised that dietary supplementation of curcumin has a protective effect on IUGR IR by modulating the insulin signalling pathway. Furthermore, we took advantage of a maternal malnutrition rat model, which is an identified model for human IUGR study, and determined the hepatic lipid content and protein expressions related to fatty acid synthesis and lipid oxidation, to investigate whether these changes of IUGR-induced hyperlipidaemia were alleviated after curcumin administration.
Materials and methods
Curcumin preparation
The curcumin used in the present study was kindly provided by the Kehu Bio-technology Research Center (Guangzhou, People’s Republic of China). The content of curcumin was 98 % measured by using HPLC.
Animal experiment design
The experimental design and procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University following the requirements of the Regulations for the Administration of Affairs Concerning Experimental Animals of China (SYXK(Su)2017-0007). The feed restriction method was used for maternal rats during pregnancy lead to induction of IUGR mode according to a previous study( Reference Desai, Gayle and Babu15 ). First-time-pregnant Sprague–Dawley rats (Nanjing Qinglongshan Experiment Animal Center, Nanjing, People’s Republic of China) were housed in a facility at a constant temperature and humidity. The light regimen was adjusted to a 12 h light–12 h dark cycle. At day 10 of gestation, rats were provided either a diet of standard laboratory diet (LabDiet 5001) (n 12) ad libitum or a 50 % feed-restricted (n 12) diet determined by the quantification of normal feed intake in ad libitum-fed rats. Dams gave birth normally, and birth weights of offspring were recorded on day 1 of postnatal life and divided into normal or IUGR groups. Rats were limited to 10 per litter to normalise rearing (average 11·83 and 12·1 rats per litter in normal and IUGR groups, respectively). During the 21 d of lactation period, each litter of rats from the normal or IUGR group were nursed by their own dams. During the lactation, all dams were free to feed. At 3 weeks of age, offspring in each litter were weaned and housed individually until they were 6 weeks old for observing the early growth of rats. At 6 weeks of age, twenty-four female rats with nearly equal body weights (within each group) were allocated to the NBW (normal birth weight), NBW-C (NBW with curcumin supplementation), IUGR and IUGR-C (IUGR with curcumin supplementation) groups (one rat per litter, (n 6) per group), respectively. The rats were allowed water and a standard granulated diet (American Institute of Nutrition (AIN)-93G diet) ad libitum. During the entire experimental period, rats in the NBW-C and IUGR-C groups were fed a standard diet supplemented with 400 mg curcumin/kg. Curcumin was added to the feed before it was made into pellets. The light regimen was a 12 h light–12 h dark cycle and the temperature was maintained at 20 to 24°C. At 12 weeks of age, all rats were fasted overnight, and blood was collected via cardiac puncture after anaesthesia. Serum was obtained from the blood via centrifugation for 15 min at 3000 g at 4°C. Liver tissue (the same area for each sample) was removed after death and snap-frozen in liquid N2 and then stored at −80°C for further analysis.
Serum biochemistry parameters
Concentrations of total cholesterol (TC) and TAG in serum and liver were measured according to previous studies(Reference Bucolo and David16, Reference Yokode, Hammer and Ishibashi17). Concentrations of VLDL in serum and liver were determined by ELISA kit from Shanghai YILI Biological Technology Co., Ltd. Concentrations of HDL-cholesterol, LDL-cholesterol, glycogen, pyruvate and NEFA and activities of hepatic pyruvate kinase (PK), hepatic lipase (HL) and lipoprotein lipase (LPL) were determined using colorimetric kits (Nanjing Jiancheng Institute of Bioengineering) with a spectrophotometer, according to the manufacturer’s instructions. Total lipase (TL) activity was defined as equal to HL and LPL activities. The concentrations of curcumin in serum and liver of rats were determined by HPLC-MS/MS system according to the previous study(Reference Kunati, Yang and William18).
Gene expression assays
Total RNA from the liver samples stored at –80°C was isolated using the Trizol reagent (Invitrogen). The determination of RNA content, mRNA quantification and real-time PCR (Applied Biosystems) were performed according to previously described methods(Reference He, Dong and Xu9). The primer sequences for the target and housekeeping genes (Cd36, Ppara, sterol regulatory element binding protein 1c (Srebf1), fatty acid synthase (Fasn), hormone-sensitive lipase (HSL) and β-actin (Actb)) used for real-time PCR are listed in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was also used as a control gene to normalise the expression of target genes. Briefly, a reaction system of 20 μl was composed of 0·4 μl of forward primers, 0·4 μl of reverse primers, 0·4 μl of ROX reference dye, 10 μl of SYBR Premix Ex Taq (TaKaRa Biotechnology Co. Ltd), 6·8 μl of double-distilled water and 2 μl of complementary DNA. The 2−ΔΔCt method was used to calculate relative levels of mRNA expression after normalisation with housekeeping genes(Reference Schmittgen and Livak19). The values for the NBW group were used for calibration.
Table 1. Primers
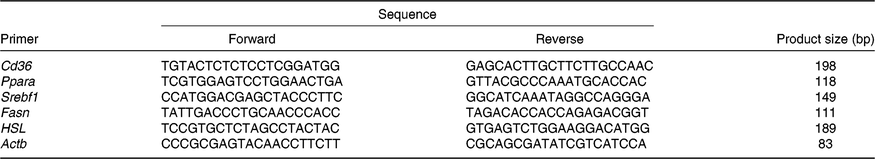
Srebf1, sterol regulatory element binding protein 1c; Fasn, fatty acid synthase; HSL, hormone-sensitive lipase; Actb, β-actin.
Protein analysis
Primary antibodies against IRS-1 (1:1000; Cell Signaling Technology); glycogen synthase kinase-3 α/β (GSK3α/β; 1:1000; Cell Signaling Technology); protein kinase B (Akt; 1:1000; Cell Signaling Technology); phosphoinositide 3-kinase p85 (PI3K; 1:1000; Cell Signaling Technology); sterol regulatory element binding protein 1 (SREBP1; 1:1000; Affinity); FASN (1:1000; Cell Signaling Technology); PPARα (1:500; Affinity); phosphorylated IRS-1Ser302 (pIRS-1Ser302; 1:1000; Cell Signaling Technology); phosphorylated GSK3αSer21/βSer9 (pGSK3αSer21/βSer9; 1:1000; Cell Signaling Technology); phosphorylated AktSer473 (pAktSer473; 1:1000; Cell Signaling Technology); phosphorylated PI3KTyr458 (pPI3KTyr458; 1:1000; Cell Signaling Technology); and antibodies against Actb (1:1000; Cell Signaling Technology) were used in the present study. The total protein and cytomembrane protein of the liver were extracted using assay kits according to the manufacturer’s instructions (Beyotime). The protein content of the sample was measured using the bicinchoninic acid (BCA) Protein Assay Kit (Beyotime). For Western blotting analyses, 40 μg of protein from each sample was subjected to sodium dodecylsulfate–polyacrylamide gel electrophoresis. After electrophoresis, proteins were separated and transferred to polyvinylidene difluoride membranes. The membranes were blocked with blocking buffer (5 % non-fat dry milk) for 12 h at 4°C. Then, the membranes were probed with appropriate primary and secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG; Cell Signaling Technology 1:5000 dilution in 1 × Tris-buffered saline with 0·1 % Tween 20). The blots were detected using enhanced chemiluminescence reagents (ECL-Kit, Beyotime) followed by autoradiography. Photographs of the membranes were taken using the Luminescent Image Analyzer LAS-4000 system (Fujifilm Co.) and quantified by Gel-Pro Analyzer 4.0 software (Media Cybernetics). Results were corrected for total protein.
Statistical analysis
Differences between groups were analysed using a two-way ANOVA. The classification variables were birth weight (NBW + NBW-C × IUGR + IUGR-C), diet (NBW + IUGR × NBW-C + IUGR-C), and the interaction between birth weight and diet (NBW × NBW-C × IUGR × IUGR-C). A Tukey’s post hoc analysis was used to determine the differences between the four groups when a statistically significant birth weight × diet interaction was observed. SPSS 17.0 (SPSS, Inc.) was used for these analyses. A probability level of P < 0·05 was considered statistically significant, and P < 0·01 was considered very significant. Data are presented as mean values and standard deviations.
Results
Serum hormone levels
IUGR rats exhibited higher concentrations of serum insulin (P > 0·05), glucose (P < 0·05) and HOMA-IR (P < 0·05) compared with NBW rats. Curcumin supplementation reduced (P < 0·01) the concentrations of serum insulin, glucose and HOMA-IR in IUGR rats. In addition, a significant effect of birth weight × dietary interaction (P < 0·05) for concentrations of serum insulin, glucose and HOMA-IR were observed. There were no significant differences in the concentrations of serum insulin, glucose and HOMA-IR between rats of the NBW and NBW-C groups (P > 0·05) (Table 2).
Table 2. Effect of curcumin on the serum concentrations of insulin, glucose and homeostasis model of assessment for insulin resistance (HOMA-IR) index of rats with intra-uterine growth restriction (IUGR)Footnote *
(Mean values and standard deviations; n 6 per group)
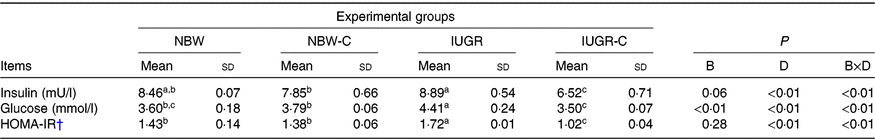
NBW, normal birth weight rats; NBW-C, normal birth weight rats fed diets supplemented with 400 mg/kg curcumin; IUGR-C, IUGR rats fed diets supplemented with 400 mg/kg curcumin; B, birth weight; D, dietary curcumin supplementation; B × D, interaction between the corresponding parameters.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Data were analysed by using two-way ANOVA and Tukey’s post hoc testing, where appropriate.
† HOMA-IR = (fasting glucose (mmol/l) × fasting insulin (μU/ml))/22·5.
Serum biochemistry and hepatic lipid metabolic parameters
The concentrations of TC and TAG in the serum of IUGR rats were significantly lower (P < 0·05) compared with the NBW rats. Dietary curcumin supplementation significantly decreased (P < 0·05) the concentration of HDL-cholesterol and increased (P < 0·05) the concentration of LDL-cholesterol in the serum of NBW and IUGR rats. In addition, a birth weight × dietary interaction effect (P < 0·05) was observed for concentrations of TAG and HDL-cholesterol in the serum. The concentrations of TC, TAG and NEFA in the liver of IUGR rats were significantly higher (P < 0·05) than in the liver of NBW rats. In the IUGR-C group, the concentrations of TC, TAG and NEFA in the liver of rats were lower (P < 0·05) than in the IUGR group. A birth weight × dietary interaction effect was observed in the concentrations of TC and TAG in the liver of rats. The NBW-C group had lower (P < 0·05) concentrations of VLDL and NEFA in the liver than in the NBW group rats (Table 3).
Table 3. Effect of curcumin on serum and liver lipid metabolic measurements of rats with intra-uterine growth restriction (IUGR)Footnote *
(Mean values and standard deviations; n 6 per group)
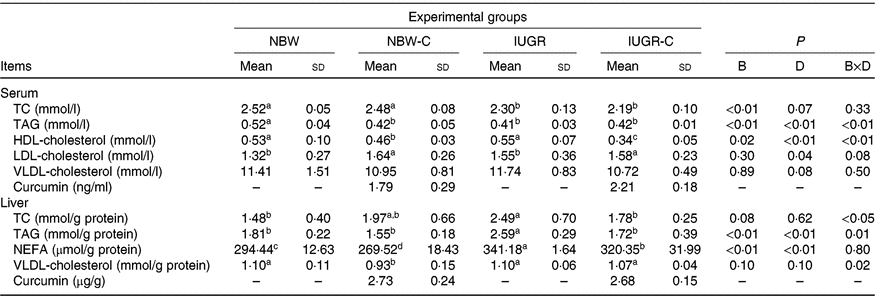
NBW, normal birth weight rats; NBW-C, normal birth weight rats fed diets supplemented with 400 mg/kg curcumin; IUGR-C, IUGR rats fed diets supplemented with 400 mg/kg curcumin; B, birth weight; D, dietary curcumin supplementation; B × D, interaction between the corresponding parameters; TC, total cholesterol; –, means not determined.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Data were analysed by using two-way ANOVA and Tukey’s post hoc testing, where appropriate.
Hepatic glycogen and metabolic parameters
IUGR rats exhibited lower (P < 0·05) concentration of glycogen and higher (P < 0·05) concentration of pyruvate in the liver compared with the NBW rats. Dietary curcumin supplementation significantly increased the concentration of glycogen and decreased the concentration of pyruvate and activity of PK in the liver of IUGR rats (P < 0·05). Dietary curcumin supplementation, the activity of PK and the concentration of glycogen were significantly lower in the liver of the NBW-C group than the NBW group (P < 0·05). In addition, a birth weight × dietary interaction effect (P < 0·05) was observed for concentrations of pyruvate (Table 4).
Table 4. Effect of curcumin on hepatic glycogen and enzymes of rats with intra-uterine growth restriction (IUGR)Footnote *
(Mean values and standard deviations; n 6 per group)
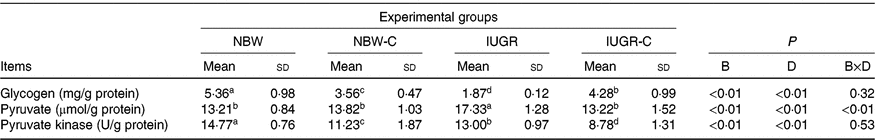
NBW, normal birth weight rats; NBW-C, normal birth weight rats fed diets supplemented with 400 mg/kg curcumin; IUGR-C, IUGR rats fed diets supplemented with 400 mg/kg curcumin; B, birth weight; D, dietary curcumin supplementation; B × D, interaction between the corresponding parameters.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Data were analysed by using two-way ANOVA and Tukey’s post hoc testing, where appropriate.
Hepatic lipolysis enzymes
The IUGR group showed lower activities of LPL (P < 0·01), HL (P < 0·05) and TL (P < 0·05) in the liver of rats compared with the NBW group rats. The activities of hepatic LPL (P < 0·01), HL (P < 0·05) and TL (P < 0·01) were higher (P < 0·05) in the IUGR-C group rats than in the IUGR group rats. Increased (P < 0·05) activities of hepatic LPL, HL and TL were observed in the NBW-C group rats (Table 5).
Table 5. Effect of curcumin on hepatic lipolysis enzymes of rats with intra-uterine growth restriction (IUGR)Footnote *
(Mean values and standard deviations; n 6 per group)
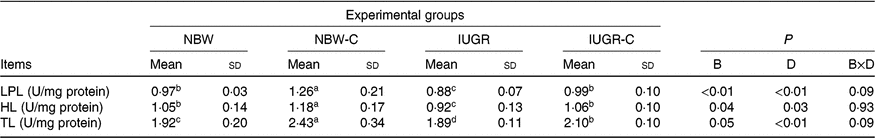
NBW, normal birth weight rats; NBW-C, normal birth weight rats fed diets supplemented with 400 mg/kg curcumin; IUGR-C, IUGR rats fed diets supplemented with 400 mg/kg curcumin; B, birth weight; D, dietary curcumin supplementation; B × D, interaction between the corresponding parameters; I, IUGR; LPL, lipoprotein lipase; HL, hepatic lipase; TL, total lipase, equal to HL and LPL activities.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Data were analysed by using two-way ANOVA and Tukey’s post hoc testing, where appropriate.
Hepatic protein expression
The IUGR rats exhibited higher phosphorylated IRS-1 (P > 0·05) (Fig. 1(A)), Akt (P < 0·05) (Fig. 1(C)) and glycogen synthase kinase 3β (GSK3β) (P < 0·05) (Fig. 2) levels, and lower (P > 0·05) phosphorylated GSK3α and PI3K levels (Fig. 1(B)) in the liver compared with the NBW rats. Dietary curcumin supplementation significantly decreased (P < 0·05) the phosphorylated levels of IRS, Akt and GSK3β, and had a tendency to increase (P > 0·05) the phosphorylated levels of GSK3α and PI3K in the liver of the IUGR-C group. A birth weight × dietary interaction effect (P < 0·05) was observed for the phosphorylated levels of IRS, GSK3β and PI3K.
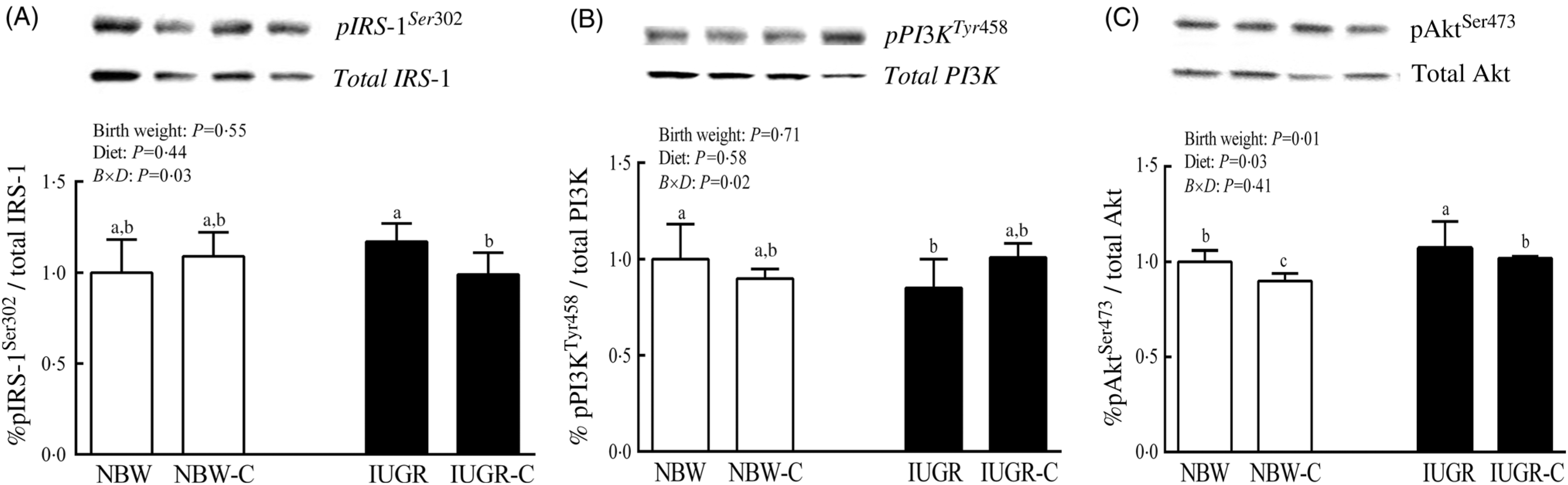
Fig. 1. Abundance of phosphorylated proteins in liver of normal birth weight (NBW) rats, NBW rats supplemented with curcumin (NBW-C), intra-uterine growth restriction (IUGR) rats and IUGR rats supplemented with curcumin (IUGR-C). (A) Insulin receptor substrate-1 (IRS-1); (B) phosphoinositide 3-kinase p85 (PI3K); (C) protein kinase B (Akt). Data are mean values (n 6 per group), with standard deviations represented by vertical bars. Data were analysed by using two-way ANOVA and Tukey’s post hoc testing, where appropriate. a,b,c Mean values with unlike letters were significantly different (P < 0·05). Results were corrected for total protein. B, birth weight; D, diet; B × D, interaction between the corresponding parameters. pIRS-1Ser302, phosphorylated IRS-1Ser302; pPI3KTyr458, phosphorylated phosphoinositide 3-kinase p85Tyr458.
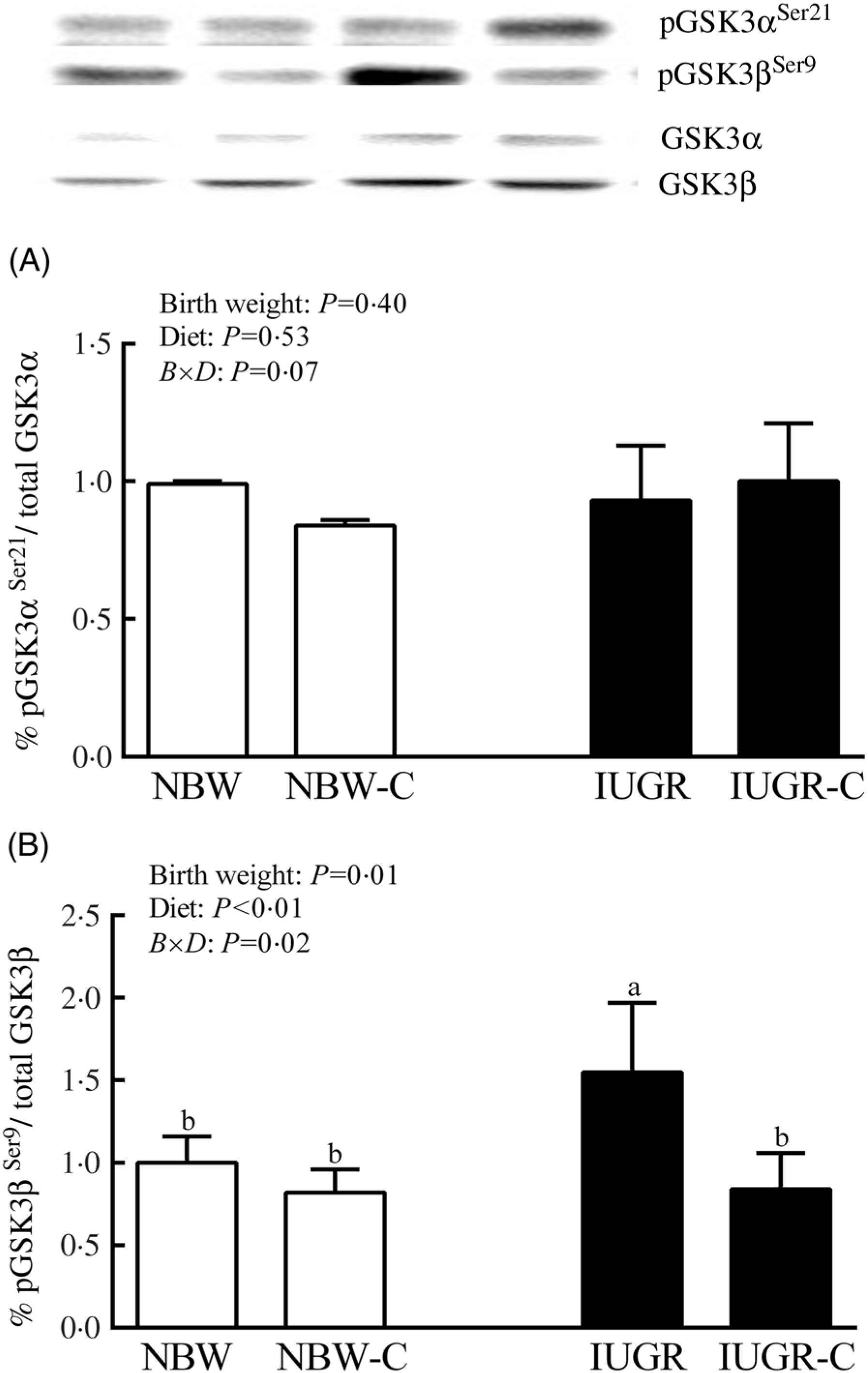
Fig. 2. Abundance of phosphorylated glycogen synthesis kinase-3 α/β (GSK3α/β) in liver of normal birth weight (NBW) rats, NBW rats supplemented with curcumin (NBW-C), intra-uterine growth restriction (IUGR) rats and IUGR rats supplemented with curcumin (IUGR-C). Data are mean values (n 6 per group), with standard deviations represented by vertical bars. Data were analysed by using two-way ANOVA and Tukey’s post hoc testing, where appropriate. a,b Mean values with unlike letters were significantly different (P < 0·05). Results were corrected for total protein. B, birth weight; D, diet; B × D, interaction between the corresponding parameters. pGSK3αSer21, phosphorylated glycogen synthase kinase 3αSer21; pGSK3βSer9, phosphorylated glycogen synthase kinase 3βSer9.
The protein expressions of SREBP1 (Fig. 3(A)) and FASN (Fig. 3(B)) were significantly increased (P < 0·05) in the liver of IUGR rats compared with the NBW rats. The protein expressions of SREBP1 and FASN in the liver of the IUGR-C group were lower (P < 0·05) than in the IUGR rats. The IUGR rats also showed lower (P < 0·05) protein expression of PPARα in the liver compared with the NBW group rats (Fig. 3(C)). Dietary curcumin supplementation significantly increased (P < 0·05) the protein expression of PPARα in the IUGR-C group rats. Dietary curcumin supplementation had no significant effects (P > 0·05) on the protein expressions of SREBP1, FASN and PPARα in the liver of NBW rats. Additionally, a birth weight × dietary interaction effect (P < 0·01) was noted for expressions of SREBP1, FASN and PPARα.

Fig. 3. Abundance of proteins in liver of normal birth weight (NBW) rats, NBW rats supplemented with curcumin (NBW-C), intra-uterine growth restriction (IUGR) rats and IUGR rats supplemented with curcumin (IUGR-C). (A) Sterol regulatory element binding protein 1 (SREBP1); (B) fatty acid synthase (FASN); (C) PPARα. Data are mean values (n 6 per group), with standard deviations represented by vertical bars. Data were analysed by using two-way ANOVA and Tukey’s post hoc testing, where appropriate. a,b,c Mean values with unlike letters were significantly different (P < 0·05). Results were corrected for total protein. B, birth weight; D, diet; B × D, interaction between the corresponding parameters.
Gene expression
In the liver of the IUGR rats, the mRNA expression levels for Cd36, Srebf1 and Fasn were higher (P < 0·05) and the mRNA expression levels for Ppara and HSL were lower (P < 0·05) than in the liver of NBW rats. Dietary curcumin supplementation significantly increased (P < 0·05) the mRNA expression levels for Ppara and HSL, and decreased (P < 0·05) the mRNA expression levels for Cd36, Srebf1 and Fasn in the liver of the IUGR-C group. A birth weight × dietary interaction effect was noted for the mRNA expressions for Cd36, Ppara, Srebf1 and HSL in the liver of rats (P < 0·01) (Fig. 4).

Fig. 4. Effect of curcumin on the hepatic gene expressions of rats with intra-uterine growth restriction (IUGR). Data are mean values (n 6 per group), with standard deviations represented by vertical bars. Data were analysed by using two-way ANOVA and Tukey’s post hoc testing, where appropriate. a,b,c Mean values with unlike letters were significantly different (P < 0·05). Srebf1, sterol regulatory element binding protein 1c; Fasn, fatty acid synthase; HSL, hormone-sensitive lipase; B, birth weight; D, diet; B × D, interaction between the corresponding parameters. ![]() , Normal BW (NBW) rats;
, Normal BW (NBW) rats; ![]() , NBW rats supplemented with curcumin (NBW-C);
, NBW rats supplemented with curcumin (NBW-C);![]() , IUGR rats;
, IUGR rats; ![]() , IUGR rats supplemented with curcumin (IUGR-C).
, IUGR rats supplemented with curcumin (IUGR-C).
Discussion
IUGR impairs liver metabolism during the early period in piglets( Reference He, Dong and Xu9 ) and induces IR in rats( Reference Lim, Armitage and Stefanidis20 ). Previous studies of Magee et al. ( Reference Magee, Han and Cherian10 ) found that the IUGR male rats increased hepatic fatty synthase and TAG contents. Mina et al. ( Reference Desai, Gayle and Babu15 ) found that IUGR female adult had higher per cent of body fat. Our previous study also found that curcumin was beneficial in preventing IUGR-induced inflammation, oxidative damage and injury in the liver of IUGR rats( Reference He, Niu and Wang21 ).Nevertheless, the studies on IR and the relationship between glycogen and lipid metabolism in IUGR female rats are very limited. Therefore, we chose IUGR female rats as our research animal model and investigated the effect of dietary curcumin supplementation on insulin levels, hepatic lipid and glycogen metabolism of female rats.
In the present study, IUGR increased serum HOMA-IR, insulin and glucose levels, which were considered as markers of IR. The results were consistent with the previous study on insulin sensitivity index, which first formally reported the sequence of IR in short children with IUGR( Reference Hofman, Cutfield and Robinson22 ). IR is related to a postreceptor defect in the intracellular insulin signalling pathway, leading to the failure of insulin to reduce the level of glucose and improve hepatic glycogen synthesis( Reference Barthel and Schmoll23 ). Insulin regulation effect on glucose metabolism are mediated by IRS-1, which activates PI3K and Akt. The phosphorylation of Akt leads to inaction of glycogen synthase kinase-3 (GSK3) and thus enhances the glycogen synthesis( Reference Rayasam, Tulasi and Sodhi24 ). Our results showed that IUGR induced rats increased levels of phosphorylated IRS-1 and Akt. It has been demonstrated that the increased serine phosphorylation of IRS-1 plays an important role in the pathogenesis of IR ( Reference Taniguchi, Emanuelli and Kahn25 ). The activation of Akt induced the significant phosphorylation level of GSK3β in IUGR, not GSK3α in our study. GSK3α and GSK3β are two functional isoforms of GSK3, which originate from different genes, but share 97 % amino acid homology. GSK3β is the primary kinase in regulating glycogen synthesis in muscle which has a positive effect on glycogen deposition as compared with GSK3α( Reference Macaulay, Blair and Hajduch26 , Reference Pearce, Arch and Clapham27 ). The different effects of GSK3α and 3β on liver are not well documented. Our research implied that the causes of IR in IUGR might be related to the failure of Akt to suppress GSK3β. Nachimuthu et al. ( Reference Maithilikarpagaselvi, Sridhar and Swaminathan14 ) reported that curcumin treatment attenuated the IR by decreasing IRS-1 phosphorylation in rats and alleviated hyperinsulinaemia and HOMA-IR levels. In the present study, the concentrations of curcumin in serum and liver were accumulated in NBW and IUGR rats after treated with curcumin. We found that dietary curcumin supplementation decreased the levels of serum insulin, glucose and HOMA-IR in IUGR rats. Furthermore, results also showed that the levels of IRS-1 serine phosphorylation, Akt and GSK3β phosphorylation were lower in IUGR rats when supplemented with dietary curcumin. PK acts as a key glycolytic enzyme that catalyses the final step of glycolysis and generates pyruvate in the metabolic process. In our present study, the activity of PK and concentration of pyruvate were decreased and concentration of hepatic glycogen was increased in liver of IUGR rats supplemented with curcumin. These results implied that curcumin could attenuate IR, inhibit hepatic glycolysis and improve hepatic glycogen deposition through regulating IRS-1/Akt/GSK3 pathway and decreasing glycolytic enzyme activity.
TAG, the most common non-toxic form of fatty acid, is the main lipid stored in the liver of patients with non-alcoholic fatty liver disease( Reference Tilg and Moschen28 ). In the present study, IUGR rats significantly increased the liver concentration of TAG and TC and decreased the serum concentration of TAG. These results indicated that more TAG was exported to liver in IUGR rats. Some previous studies revealed that inhibiting TAG synthesis was helpful for the treatment of hepatic steatosis( Reference Yamaguchi, Yang and McCall29 ). In patients with non-alcoholic fatty liver disease, NEFA provide most of lipid content for hepatic TAG synthesis( Reference Musso, Gambino and Cassader30 ). In the present experiment, we observed that hepatic NEFA concentration was increased in IUGR rats. It is well known that accumulation of TAG and increase of NEFA in liver is due to the imbalance between hepatic lipids acquisition and removal( Reference Asai and Miyazawa31 ). Notably, the activities of enzymes related to lipolysis in liver were decreased in IUGR rats. LPL catalyses the hydrolysis of TAG in circulating chylomicrons, thus supplying NEFA for tissue utilisation( Reference Mead, Irvine and Ramji32 ). HL plays a critical role in hydrolysing TAG and phospholipids in the blood. In our experiment, the decreased activities of LPL and HL in IUGR rats might be the main reasons for inefficient NEFA utilisation and excessive TAG accumulation in liver. More importantly, after dietary curcumin supplementation, the activities of LPL, HL and TL in IUGR rats were increased and TAG accumulation was obviously attenuated to a normal level. Moreover, curcumin significantly decreased the concentrations of hepatic NEFA in IUGR rats. The findings were in agreement with the previous results reported by Asai et al. ( Reference Asai and Miyazawa31 ) and Jang et al. ( Reference Jang, Choi and Jung33 ), and reported that curcumin was beneficial to inhibit fatty acid synthases and attenuate hepatic TAG accumulation in high-fat-fed rats. These results suggested that IUGR might have a high risk of hepatic lipid metabolic disorder, and dietary curcumin supplementation could alleviate the accumulation of TAG in liver.
Insulin regulates the lipogenesis through the activation of SREBP-1( Reference Tessari, Coracina and Cosma34 ). Liver plays a vital role in the regulation of gene expression for lipid metabolism, including SREBP-1 c and PPARα( Reference Jump, Botolin and Wang35 ). SREBP-1 c is one of the isoforms of SREBP and first enhances the transcription of genes associated with biosynthesis of fatty acids and TAG. FASN is well known to catalyse the last step in the biosynthesis of fatty acids, directly activated by SREBP and involved in TAG synthesis. Overexpression of SREBP-1 c led to the increase in TAG content by more than 4-fold and expression of FASN to be increased by 3·9-fold in the liver( Reference Shimano, Horton and Shimomura36 ). Emerging evidence has suggested that CD36-mediated fatty acid uptake and increased CD36 expression resulted in the dyslipidemia and hepatic TAG storage( Reference Koonen, Jacobs and Febbraio37 ). We found that the transcription levels of Cd36, Srebf1 and Fasn were increased in IUGR rats. The protein expressions of SREBP1 and FASN in the liver of IUGR rats also were increased. A previous study indicated that IUGR could easily cause hepatic TAG accumulation by increasing the levels of SREBP and FASN proteins during early life( Reference Magee, Han and Cherian10 ), which were similar to our results. PPARα promotes β-oxidation of fatty acids which is mainly expressed in the liver. HSL is believed to be a key enzyme for improving the decomposition of TAG( Reference Langin, Laurell and Holst38 ) and inhibited by insulin( Reference Haemmerle, Zimmermann and Hayn39 ). Our results showed that the transcription levels of HSL and the protein and mRNA expressions of PPARα were decreased in the liver of IUGR rats. The present study suggested that IUGR inhibited the hepatic β-oxidation of fatty acids by regulating HSL and PPARα. In mice administrated with a methionine choline-deficient diet, PPARα-deficiency provoked more severe steatosis, whereas PPARα activation enhanced hepatic lipid turnover( Reference Ip, Farrell and Robertson40 ). Furthermore, activation of PPARα obviously reduced adiposity in mice fed with a high-fat diet( Reference Goto, Lee and Teraminami41 ). These results implied that IUGR could improve lipid accumulation and inhibit lipolysis in the liver which might be related to the alterations of the expression of SREBP1 and target genes. Interestingly, curcumin had a potential ability of attenuating hepatic lipid deposition in IUGR rats. In the present study, dietary curcumin supplementation significantly decreased the protein expressions of SREBP1, FASN and increased the protein expression of PPARα in the liver of IUGR rats. Curcumin also down-regulated the transcription levels of Cd36, Srebf1 and Fasn, and up-regulated the transcription levels of Ppara and HSL in IUGR rats. Previous studies indicated that curcumin could suppress the gene expression of Fasn ( Reference Younesian, Kazerouni and Dehghan-Nayeri42 ) and activate protein expression of PPARα( Reference Kang, Kim and Seo43 ). According to the results of the present study, we could confirm that curcumin could promote lipolysis and fatty acid oxidation in the liver of IUGR rats.
In conclusion, our present data suggested that IUGR rats exhibited a high risk of IR and hepatic lipid accumulation in the liver. Curcumin efficiently attenuated IR of IUGR rats, which might contribute to reduce insulin level in serum and regulate the insulin signalling pathways in liver. Curcumin also alleviated hepatic lipid accumulation of IUGR rats by regulating SREBP target genes and lipid metabolism-related genes in liver, which might contribute to the inhibition of lipogenesis and promotion of lipolysis. Our findings may be helpful in finding a new nutritional strategy for the prevention or treatment of IUGR in humans in future.
Acknowledgements
We are thankful to Kehu Bio-technology Research Center (Guangzhou, People’s Republic of China) for kindly supplying the curcumin.
This work was supported by the National Natural Science Foundation of China (nos. 31572418, 31802101, 31472129 and 31601948); the National key research and development programme of China (2018YFD0501101); and the Fundamental Research Funds for the Central Universities (KJQN201935).
The authors’ responsibilities were as follows: Y. N. designed the research, conducted the research, analysed the data, and wrote and revised the manuscript; H. A. revised the manuscript; J. T. H. and C. W. designed the research; T. C., L. L. Z., X. Z. and J. F. Z. conducted the research; T. W. had primary responsibility for final content. All authors read and approved the final manuscript.
None of the authors declared a conflict of interest.












