Human subjects are colonised, at birth, by bacteria, archaea, fungi and viruses, which are collectively called microbiota. Distinct microbiota inhabit all epithelial surfaces of the body: skin, oral cavity, respiratory, gastrointestinal and reproductive tracts(Reference Reid, Younes and Van der Mei1); with the largest and most diverse microbiota residing in the colon. The intestinal microbiota is composed of 100 trillions of bacteria which represent about 25 times as many genes as our own Homo sapiens genome. The diversity and complexity of the microbiota is influenced by the host genetic background, the diet and the environment. Reciprocally, this microbiota encodes thousands of genes absent in human genome that exert diverse functions often associated with beneficial physiological effects for its host(Reference Lepage, Leclerc and Joossens2–Reference Rakoff-Nahoum, Kong and Kleinstein4). From this close symbiotic relationship emerged the notion that human subjects and their microbiota form a composite organism, namely a holobiont(Reference van de Guchte, Blottiere and Dore5). Advances in next-generation sequencing and bioinformatics tools have shown that this relationship is far more complex than anticipated. Indeed, over the past decade, studies highlighted that perturbation of the microbiota, referred to as dysbiosis, and loss of bacterial diversity affect different host systems, particularly metabolic and immuneo processes, that participate in host physiology and pathophysiologic conditions(Reference Lepage, Leclerc and Joossens2). Moreover, growing lines of evidence suggest that the dialogue between microbiota and the host systems has a homoeostatic role beyond the gut, and contributes directly to the global wellbeing of the host. In agreement with this, animal studies have demonstrated that microbiota is implicated in liver diseases, allergy, diabetes, airway hypersensitivity, autoimmune arthritis and even neurological disorders(Reference Atarashi and Honda6–Reference Benakis, Brea and Caballero8).
The human body has evolved to functionally interact with thousands of naturally occurring or microbiota-derived metabolites. Thus, the intestinal microbiome provides an extended repertoire of molecules and metabolites that influence the host health. Amongst those molecules, SCFA, derived from bacteria-dependent hydrolysis of fibres, have attracted considerable attention because of their role in host health (Fig. 1a). Indeed, decreased abundance of SCFA-producing bacteria or decreased genomic potential for SCFA-production have been identified in many studies such as type-1 diabetes, type-2 diabetes, liver cirrhosis, inflammatory bowel diseases and atherosclerosis(Reference Qin, Li and Raes9–Reference Vatanen, Franzosa and Schwager14). Here, we aim to provide an overview of bacterial SCFA production in the gut, their impact on intestinal cells and host functions, and their different mechanisms of action.
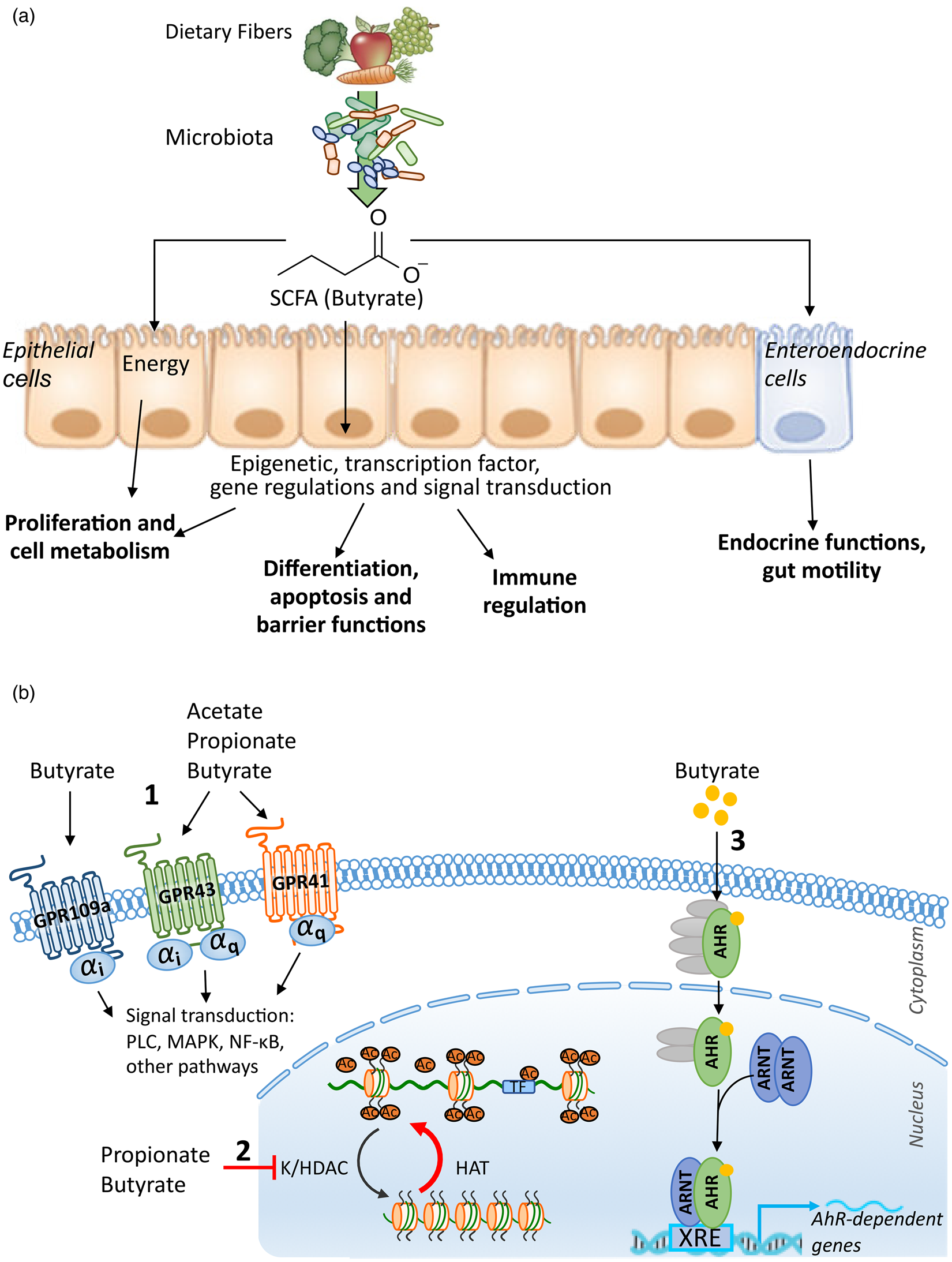
Fig. 1. (a) Functional impact of SCFA on the host. (b) Mechanisms: (1) G protein-coupled receptor (GPCR)-dependent signalling, (2) histone and transcription factor acetylation by SCFA and (3) role of butyrate as a ligand of transcription factors. AhR, aryl hydrocarbon receptor; ARNT, aryl hydrocarbon receptor nuclear translocator; HAT, histone acetyltransferase; K/HDAC, lysine/histone deacetylase; MAPK, mitogen-activated protein kinase; PLC, phospholipase C; TF, transcription factor; XRE, xenobiotic response element.
SCFA production and transport
Production of SCFA
Complex dietary carbohydrates are metabolised by intestinal microbiota through an extensive set of enzymes, mostly absent in mammals and belonging to the large family of carbohydrate-active enzymes (reviewed in(Reference Garron and Henrissat15)). The degradation of dietary fibres by gut microbiota produces organic acids, gases and a large amount of SCFA. Acetate (C2), propionate (C3) and butyrate (C4) are the main SCFA produced (60:20:20 mm/kg in human colon). SCFA can reach a combined concentration of 50–150 mm mainly in the colon where the microbial biomass is the highest(Reference Duncan, Holtrop and Lobley16–Reference Macfarlane and Macfarlane19). Substrates for bacterial fermentation include non-digestible carbohydrates derived from dietary fibres such as polysaccharide plant cell walls, resistant starch, soluble oligosaccharide and endogenous products, such as mucin(Reference Dalile, Van Oudenhove and Vervliet20). Besides bacterial fermentation, SCFA can also be found in plant oil and animal fats. Butter contains 3–4 % of butyrate in the form of tributyrin(Reference Bourassa, Alim and Bultman21). However, when fermentable fibre supply decreases, some bacterial species can switch to amino acids and protein fermentation as an alternative energy source, also contributing to SCFA and branched chain fatty acid production(Reference Smith and Macfarlane22,Reference Davila, Blachier and Gotteland23) . The branched chain fatty acids, i.e. isovalerate, 2-methylbutyrate and isobutyrate, are present at lower concentrations compared to SCFA and originate only from protein breakdown. Acetate is a net fermentation product for most gut bacteria while butyrate and propionate are produced by more specific bacterial species. Butyrate is produced from acetate, lactate, amino acids and various carbohydrates via glycolysis from two different pathways, the butyryl-CoA:acetate CoA-transferase or the phosphotransbutyrylase and butyrate kinase pathway. Using Fluorescence In Situ Hybridization probes and PCR, Flint and colleagues have shown that specific families belonging to the Clostridiales order (Firmicutes) have the capabilities to produce butyrate: Lachnospiraceae (Coprococcus, Eubacterium, Anaerostipes and Roseburia), Ruminococcaceae (Faecalibacterium and Subdoligranulum) and Erysipelotrichaceae (Holdemanella)(Reference Flint24–Reference Louis and Flint26). The butyrate-producing capability of Clostridiales has been confirmed using in vitro culture in other genera such as Clostridium, Butyrivibrio, Lachnoclostridium, Marvinbryantia, Oscillibacter, Flavonifractor, Erysipelatoclostridium, Anaerotruncus, Dorea, Blautia and Ruminiclostridium (Reference Martin-Gallausiaux, Larraufie and Jarry27,Reference Martin-Gallausiaux, Beguet-Crespel and Marinelli28) . Propionate is produced in the gut from various substrates, including amino acids, carbohydrates, lactate and 1,2-propanediol. Hence, most hexoses and pentoses enter the succinate pathway and result in succinate production, a precursor of propionate. The succinate pathway is present in Bacteroidetes and some Firmicutes, such as the Negativicutes (Veillonella and Phascolarctobacterium). Some other Firmicutes, belonging to Negativicutes (Megasphaera), Lachnospiraceae (Coprococcus) and Ruminococcaceae, use the acrylate pathway, in which lactate is the substrate to produce propionate. The propanediol pathway is present in Proteobacteria and Lachnospiraceae species and use deoxyhexose sugars (e.g. fucose) as substrates. The commensal bacterium Akkermansia muciniphila, member of the Verrucomicrobia phylum also produces propionate from this latter pathway(Reference Belzer, Chia and Aalvink29). Some bacteria, notably in the Lachnospiraceae family, can produce both propionate and butyrate but from different substrates, e.g. Roseburia inulivorans (Reference Reichardt, Duncan and Young30).
In vitro experiments have shown that Bacteroides growth is reduced relative to Firmicutes and Actinobacteria because SCFA negatively impact Bacteroides at mild acid pH(Reference Duncan, Louis and Thomson31). Thus, SCFA production by Firmicutes and Bacteroides may to be regulated by pH variations, with more Firmicutes fermentation in the proximal colon (pH about 5⋅6) and conditions favouring Bacteroides fermentation in the distal colon with a more neutral pH (pH about 6⋅3)(Reference Hamer, Jonkers and Venema32). This selective gradient is limiting the propionate production and promoting butyrate formation in the more proximal part of the colon(Reference Flint24). Intestinal pH is not the only factor that impact microbiota composition and consequently SCFA production. Indeed, intestinal gas production (e.g. oxygen and hydrogen) and diet composition and intake (e.g. types of fibres and iron) have been reported to influence the microbiota composition and the gut SCFA concentration(Reference Dostal, Lacroix and Bircher33,Reference Dostal, Lacroix and Pham34) .
Transport of SCFA
In the host, SCFA have distinct roles depending of their absorption and local physiologic concentrations(Reference Conn, Fell and Steele35,Reference Frost, Sleeth and Sahuri-Arisoylu36) . Acetate, propionate and butyrate are weak acids with pK a 4⋅8 for butyrate. Under physiological conditions the colonic pH range from 5⋅5 to 6⋅7, thus most SCFA are in the ionised form and require transporters for absorption(Reference Cummings, Pomare and Branch37,Reference Bergman38) . SCFA transporters are expressed at different levels: in the small intestine: monocarboxylate transporter (MCT)1 (SLC16A1), sodium-coupled MCT(SMCT)2 (SLC5A12) and SLC16A7 and in the colon: MCT1 (SLC16A1), SMCT2 (SLC5A12), SMCT1 (SL5CA8) and SLC26A3(Reference Dalile, Van Oudenhove and Vervliet20,Reference Sivaprakasam, Bhutia and Yang39) . The transporters MCT1, SMCT1 and SLC26A3 show affinities for all three major SCFA while the other ones are more selective, e.g. SMCT2 only transports butyrate. Butyrate is mainly absorbed via MCT1 that is expressed both at apical and basolateral membrane of colonic epithelial cells(Reference Sivaprakasam, Bhutia and Yang39,Reference Sepponen, Ruusunen and Pakkanen40) . From approximately 20 mm in gut lumen, butyrate concentration on portal vein reaches a range of 5–10 μm. The liver significantly uptakes butyrate as there is almost no splanchnic release(Reference Bloemen, Venema and van de Poll41,Reference van der Beek, Bloemen and van den Broek42) . Butyrate venous concentration ranges from 0⋅5 to 3⋅3 μm(Reference Hamer, Jonkers and Venema32). Similarly, a larger amount of propionate is found in portal vein, about 32 μm, but there is only a very small release from the liver. Venous concentration of propionate ranges from 3⋅8 to 5⋅4 μm. In contrast, acetate is weakly absorbed by epithelial cells and the liver. The portal vein concentration of acetate is 98–143 μm. Hence, the liver efficiently metabolises the butyrate and propionate released by the gut epithelium and avoids any acute increase even in the case of artificial enema(Reference Hamer, Jonkers and Venema32,Reference Bloemen, Venema and van de Poll41) .
Cellular uptake of SCFA in their anionic form is through H+- or Na+-coupled transporters. Thus, butyrate transport directly participates in electrolyte absorption with increases of Na+ and Cl− absorption and release of bicarbonate (HCO3−) in the lumen(Reference Sivaprakasam, Bhutia and Yang39,Reference McNeil, Cummings and James43,Reference Binder and Mehta44) . Interestingly, electrolyte absorption is region specific due to different transporter expression levels in each gut region(Reference Lutz and Scharrer45). Transport of butyrate is electroneutral through SMCT2 (Na+), resulting in the transport of one Na+ for each butyrate anion absorbed(Reference Srinivas, Gopal and Zhuang46). On the contrary, SMCT1 transport is electrogenic as two Na+ are transported with one butyrate anion. This results in electrolytes and water absorption(Reference Coady, Chang and Charron47,Reference Gupta, Martin and Prasad48) . MCT1 is a proton-coupled transporter and has no direct role in ion transport. However, MCT1 indirectly regulates bicarbonate secretion through Na+/H+ and Cl−/HCO3− exchangers. Interestingly, butyrate modulates the expression of many transporters including MCT1 and SMCT1, therefore potentially increasing electrolyte exchanges as well as its own transport. Butyrate blocks Cl− secretion by inhibiting Na-K-2Cl cotransporter expression and increases expression of the Na+/H+ transporter NHE3 through histone deacetylase (HDAC) inhibition and a specificity protein dependent pathway(Reference Matthews, Hassan and Meng49–Reference Subramanya, Rajendran and Srinivasan52).
Mechanisms
SCFA receptors
The human genome encodes for six potential G protein-coupled receptors (GPCR) sensitive to SCFA: GPR41 (FFAR3), GPR42, GPR43 (FFAR2), GPR109a (HCAR2), GPR164 (OR51E1) and OR51E2. GPR41 and GPR109a are exclusively Gαi/o-coupled receptors whereas GPR43 can be coupled to either Gαβγq and Gαi/o and OR51E2 is αs coupled(Reference Gelis, Jovancevic and Veitinger53). GPR42 has recently been identified as a functional GPCR-modulating Ca2+ channel flux, but only the Gβγ pathway downstream this receptor was explored(Reference Puhl, Won and Lu54). GPR41, GPR43 and GPR109a are expressed in numerous organs including the small and large intestine by various cell types: immune cells, adipose tissues, heart, skeletal muscle or neurons(Reference Dalile, Van Oudenhove and Vervliet20). GPR43 (FFAR2) and GPR41 (FFAR3) recognise acetate, butyrate and propionate with affinities that differ between species, whereas only butyrate activates GPR109a (Fig. 1b)(Reference Le Poul, Loison and Struyf55–Reference Hudson, Tikhonova and Pandey58). Schematically, GPR41 activation by propionate and butyrate and GPR109a activation by butyrate lead to the inhibition of cyclic adenosine monophosphate (cAMP) accumulation and protein kinase A and mitogen-activated protein kinases (ERK and p38) activation. Conversely, GPR43 is activated by the three main SCFA with approximately the same affinities. GPR43 engagement stimulates the phospholipase-Cβ, which releases intracellular Ca2+ and activates protein kinase C in addition to cAMP accumulation inhibition and protein kinase A and ERK activation(Reference Dorsam and Gutkind59). The highly polymorphic GPR42 is activated by propionate and modulates Ca2+ by a yet unknown mechanism that could be similar to GPR43 due to the very high homology between these two receptors. In human subjects, GPR42 is expressed in the colon and in sympathetic ganglia(Reference Puhl, Won and Lu54). Butyrate is the ligand of GPR164 (OR51E1) expressed all along the gastrointestinal tract and specifically by enteroendocrine cells (EEC)(Reference Priori, Colombo and Clavenzani60,Reference Han, Kang and Oh61) . The olfactory receptor OR51E2 (Olfr78 in mouse) is activated by propionate and acetate and result in cAMP and Ca2+ increase. Olfr78 is expressed at the gut mucosal level by peptide YY (PYY)-positive colonic EEC(Reference Fleischer, Bumbalo and Bautze62). It is also detected in various tissues, including kidney, blood vessels, lung and specific nerves in the heart and gut(Reference Pluznick63).
Transcriptional regulations and post-translational modifications
SCFA have a broad impact on the host: metabolism, differentiation, proliferation mainly due to their impact on gene regulation. Indeed, several studies revealed that butyrate regulates the expression of 5–20 % of human genes(Reference Basson, Liu and Hanly64–Reference Donohoe, Collins and Wali66). Within the cells, butyrate and propionate exhibit strong inhibition capacity of lysine and histone deacetylase (K/HDAC) activity, with butyrate being more potent than propionate(Reference Candido, Reeves and Davie67,Reference Sealy and Chalkley68) . Moreover, butyrate is metabolised into acetyl-CoA which stimulates histone acetyltransferase by further enhancing histone acetylation (Fig. 1b)(Reference Donohoe, Collins and Wali66,Reference Donohoe and Bultman69) . By their HDAC inhibitor and histone acetyltransferase stimulatory properties, SCFA promote post-translational modification of histones through increasing their acetylation. Histone hyperacetylation leads to an increased accessibility of transcription factors to the promoter regions of targeted genes owing to the modulation of their transcription. HDAC inhibition by butyrate does not only up-regulate gene transcription, repression of several genes such as LHR, XIAP or IDO-1 has been reported(Reference Martin-Gallausiaux, Larraufie and Jarry27,Reference Bose, Dai and Grant70) . In a colonic cell line, 75 % of the upregulated genes are dependent of the ATP citrate lyase activity and 25 % are independent at 0⋅5 mm concentration but the proportion is reversed at high concentration (5 mm). This suggests that the gene regulation mechanisms are different, depending on the butyrate concentration. It has been shown that butyrate does not only tune the histone acetylation level but also acetylation of other proteins, including transcription factors such as SP1 and Foxp3(Reference Arpaia, Campbell and Fan71,Reference Thakur, Dasgupta and Ta72) . SCFA derived from the gut microbiota also promote crotonylation through their histone acetylase properties(Reference Fellows, Denizot and Stellato73). Histone crotonylation is abundant in the small and large bowel epithelium as well as in the brain. Crotonyl-CoA modification of histones is linked to the cell cycle regulation. Moreover, several studies have shown that butyrate also modifies DNA and protein methylation and phosphorylation levels(Reference Boffa, Gruss and Allfrey74–Reference Parker, de Haan and Gevers76).
Novel role of butyrate as a ligand of transcription factors
Besides the extensive described effects of SCFA on host physiology through GPR and post-translational modifications, a novel role emerged for butyrate as a ligand of two transcription factors, expanding our knowledge on microbial–host crosstalk. By exploring the mechanisms involved in the microbial modulation of angiopoietin-like protein 4, Alex and co-workers demonstrated that SCFA induce angiopoietin-like protein 4 transcription and secretion through a novel role as the selective modulator of PPARγ in colonic cell lines(Reference Alex, Lange and Amolo77). In this study, Alex and co-workers showed that butyrate promotes, similarly to PPARγ ligands, the interactions between PPARγ and multiple coactivators and binds into PPARγ binding pocket with a conformation similar to the known PPARγ agonist, decanoic acid(Reference Alex, Lange and Amolo77). The evidence suggests, for the first time, an original function of butyrate as a ligand for a transcription factor. This original mechanism was also reported for another nuclear transcription factor, the aryl hydrocarbon receptor in human colonic cell lines(Reference Marinelli, Martin-Gallausiaux and Bourhis78). This latter study described a ligand-dependent activation of human aryl hydrocarbon receptor by butyrate in synergy with its role as a HDAC inhibitor. By using selective ligand antagonists and structural modelling, it emerges that butyrate activates human aryl hydrocarbon receptor by binding into its ligand binding pocket similarly to the aryl hydrocarbon receptor ligand FICZ(Reference Marinelli, Martin-Gallausiaux and Bourhis78). Together, these reports provide an expanded view of the possible mechanisms for butyrate to modulate human transcription factor activity that might apply to other transcription factors (Fig. 1b).
Functional impact of SCFA on the host
SCFA, regulators of the gut metabolism, proliferation and differentiation
SCFA are efficiently taken up from the gut lumen by the intestinal epithelial cells (IEC) with different fates (Fig. 1b). Butyrate is the primary energy source of IEC, being oxidised via β-oxidation in the mitochondria. This catabolic process represents from 73 to 75 % of oxygen consumption by human colonocytes, by which part of butyrate is converted into ketone bodies(Reference Fleming, Choi and Fitch79–Reference Roediger81). The main substrates of colonocytes are by order of preference, butyrate > ketone bodies > amino acid > glucose. By using a high level of oxygen, the colonocyte metabolism maintains epithelial hypoxia with an oxygen partial pressure <1 % oxygen (7⋅6 mmHg), thus favouring anaerobic commensals(Reference Furuta, Turner and Taylor82). The capacity to produce ketone bodies and oxidise butyrate is a crucial difference between the small and large bowel. Epithelial cell butyrate oxidative capacity has been determined in vitro to be between 1 and 5 mm butyrate, therefore when a greater concentration is available, SCFA can affect cell functions such as K/HDAC inhibition(Reference Donohoe and Bultman69,Reference Andriamihaja, Chaumontet and Tome83) . Moreover, butyrate absorption increases the pyruvate dehydrogenase kinases which negatively regulates the pyruvate dehydrogenase complex. The pyruvate dehydrogenase decarboxylates pyruvate to produce acetyl-CoA and NADH, both necessary to tricarboxylic acid(Reference Blouin, Penot and Collinet84). This dual action pushes the colonocyte metabolism from glycolysis to β-oxidation. After transport into the cells, butyrate enhances oxidative phosphorylation, which consumes oxygen(Reference Andriamihaja, Chaumontet and Tome83). Similarly, it has been demonstrated that fatty acid oxidation is reduced in germ-free mice compared to conventional mice(Reference Donohoe, Garge and Zhang85). Butyrate is not the only fatty acid metabolised. Acetate is a substrate for cholesterol and fatty acid synthesis and is metabolised in muscles. Propionate is a precursor for the synthesis of glucose in the liver(Reference Dalile, Van Oudenhove and Vervliet20,Reference Louis, Hold and Flint25,Reference Donohoe, Garge and Zhang85) . Acetate and butyrate are also major substrates for lipogenesis in rat colonocytes(Reference Zambell, Fitch and Fleming86).
Through the production of SCFA, gut microbiota actively communicates with host cells and strongly modulates a variety of cellular mechanisms. Two of the main functions influenced by SCFA and thus gut microbiota are cell proliferation and differentiation. Indeed, the proliferative activity of crypt epithelial cells as well as the migration of mature epithelial cells along the crypt–villus axis are greatly attenuated in antibiotic-treated and germ-free mice(Reference Park, Kotani and Konno87). In the physiological state, butyrate favours cell differentiation and inhibits proliferation. First, evidences on IEC were demonstrated on cell lines(Reference Augeron and Laboisse88,Reference Barnard and Warwick89) . In these studies, long-term incubation of intestinal cancerous cell lines with SCFA resulted in differentiated phenotypes coupled to decreased cell proliferation. High concentration of butyrate is associated with inhibition of stem cells and proliferative cells in the crypts, through a HDAC inhibition-dependent binding of Foxo3 to promoters of key genes in the cell cycle(Reference Kaiko, Ryu and Koues90). Butyrate concentration near the crypt base is estimated to be 50–800 μm dose equivalent(Reference Donohoe, Garge and Zhang85,Reference Csordas91,Reference Sengupta, Muir and Gibson92) . These studies indicate that butyrate concentration is low in the deep crypts and increasing in a gradient along the lumen-to-crypt axis. Butyrate metabolisation by differentiated cells on the epithelium plateau may result in a protective depletion in the crypts that is protective for stem cell proliferation. Hence, the crypt structure is suggested to be an adaptive mechanism protecting the gut epithelial stem cells of butyrate high concentration found in the lumen(Reference Kaiko, Ryu and Koues90).
Interestingly, butyrate has a dual role in epithelial cellular metabolisms: it supports healthy cells as primary energy source for IEC and represses cancerous cell expansion. This is known as the butyrate paradox or Warburg effect(Reference Donohoe, Collins and Wali66). This is explained by a metabolic shift occurring in cancerous cells using preferentially glucose as the energy source. The inhibition of cell proliferation is generally characterised by an increase in reactive oxygen species production, DNA damages and cell cycle arrest, suggesting that SCFA initiate apoptosis signalling in cancer cells(Reference Arun, Madhavan and Reshmitha93–Reference Verma, Agarwal and Das96). Indeed, through the activation of the pro-apoptotic protein BAX, the upregulation of apoptosis-inducing factor-mitochondria associated 1 isoform 6 and the reduction of mitochondrial membrane potential, SCFA stimulate the cytochrome c release which drives caspase 3 activation(Reference Arun, Madhavan and Reshmitha93). Coherently, the induction of the cyclin-dependent kinase inhibitors p21 and p27 and the downregulation of heat-shock cognate 71 kDa protein isoform is observed, leading to growth arrest(Reference Kim, Kwon and Ryu97,Reference Wang, Chiang and Chu98) .
Another mechanism for propionate to inhibit cell proliferation is suggested to involve its role as GPCR agonist. In human monocyte and lymphoblast cancer cell lines, Bindels and colleagues observe that the effect on cell proliferation is dependent on GPR43 activation(Reference Bindels, Porporato and Dewulf99). GPR43 displays a dual coupling through Gi and Gq protein families. While phospholipase C blockage does not influence cell proliferation, increase in cAMP, mediated by the inhibition of Gi subunit, slightly reduces the propionate anti-proliferative effect, suggesting a mechanism dependent on cAMP levels(Reference Bindels, Porporato and Dewulf99).
Considering the important metabolic shift occurring in cancer cells, the production and availability of a large variety of metabolites are modified among which acetyl-CoA. Acetyl-CoA is crucial in several metabolic pathways and a fundamental cofactor for histone acetyltransferases. Consequently, different cell metabolites are produced, such as a large amount of lactate, which in turn could stimulate the growth of commensal bacteria and partially explain the anti-tumorigenic effect of some probiotics(Reference Casanova, Azevedo-Silva and Rodrigues100).
Regulation of gut endocrine functions, importance on host physiology
Among IEC, EEC play an important role in host physiology by secreting hormones that regulate food intake, insulin secretion and gut functions in response to a variety of stimuli(Reference Gribble and Reimann101). Among these stimuli, fibre-rich diets or infusion with SCFA have been associated with increased circulating levels of gut hormones(Reference Cani, Amar and Iglesias102,Reference Samuel, Shaito and Motoike103) . Supporting these results, expression of butyrate receptors GPR43, GPR41 and GPR109a have been reported in EEC(Reference Karaki, Tazoe and Hayashi104–Reference Nohr, Pedersen and Gille106). Acute stimulation of EEC by SCFA is shown to trigger hormone secretion such as glucagon-like peptide (GLP)-1 and PYY. The mechanism involves GPR43 activation leading to increased intracellular calcium, corresponding to the activation of a Gq-coupled receptor(Reference Tolhurst, Heffron and Lam107). Several studies have confirmed the role of GPR43 in the EEC response to SCFA using additional knockout models or agonists(Reference Bolognini, Tobin and Milligan108–Reference Psichas, Sleeth and Murphy110). In particular EEC, the L-cells, GPR41 is also involved in the GLP-1 secretory response as suggested by the results in GPR41 knockout animals or GPR41 agonists(Reference Nohr, Pedersen and Gille106,Reference Tolhurst, Heffron and Lam107) . However, GPR41 stimulation also inhibits glucose insulinotropic polypeptide secretion from glucose insulinotropic polypeptide -producing EEC(Reference Lee, Zhang and Miyamoto111). This inhibition of glucose insulinotropic polypeptide-producing cells could correspond to the activation of Gi/o pathways which are mainly resulting in inhibitory responses. The exact role of GPR41 in GLP-1 secretion remains to be fully understood. The possibility of GPR41 hetero-dimerisation with GPR43 has been recently highlighted and could explain a role of GPR41 in GLP-1 stimulatory activity(Reference Ang, Xiong and Wu112). Additionally, species differences are described in response to the different SCFA. If propionate and acetate are strong stimuli for PYY and GLP-1 secretion in rodents at low concentrations, much higher concentrations are required to induce secretion in human subjects(Reference Psichas, Sleeth and Murphy110,Reference Chambers, Morrison and Frost113) . These divergences can be explained both by the variation of SCFA affinities to the receptor families as well as the different receptor expression levels. Indeed, GPR41 is expressed in fewer EEC in human subjects compared to rodents(Reference Nohr, Pedersen and Gille106,Reference Tazoe, Otomo and Karaki114) . The role of other SCFA receptors GPR109a, GPR42, OR51E1 and OR51E2, is still to be deciphered but some studies show that they are also enriched in some EEC subpopulations(Reference Fleischer, Bumbalo and Bautze62,Reference Roberts, Larraufie and Richards115) .
In addition to the SCFA-dependent acute stimulation of gut hormone secretion, it emerged that SCFA also tune EEC identity and consequently long-term hormonal production. Indeed, animals fed with fibre-rich diets have, in addition to a higher circulating gut hormone levels, an elevated number of EEC(Reference Cani, Amar and Iglesias102). Supporting this result, an increase in the differentiation of epithelial cells into L-cells by SCFA has been reported, with a higher GLP-1, PYY and serotonin production(Reference Samuel, Shaito and Motoike103,Reference Larraufie, Martin-Gallausiaux and Lapaque116–Reference Zhou, Martin and Tulley120) . GPR43 and GPR41 play important but different roles in the differentiation of EEC. GPR43 stimulation increased the number of the PYY-producing cells and PYY expression but not the number of GLP-1-positive cells which is dependent on GPR41(Reference Larraufie, Martin-Gallausiaux and Lapaque116,Reference Brooks, Viardot and Tsakmaki117) .
Moreover, receptor-independent pathways are also involved in the expression regulation of gut hormone genes. Indeed, butyrate HDAC inhibitory activity highly increased PYY expression in human L-cells with a much stronger effect compared to GPR43 stimulation(Reference Larraufie, Martin-Gallausiaux and Lapaque116). The modulation of PYY gene expression is associated with increased production and secretion both under basal and stimulated conditions and could explain the long-term effects of SCFA on circulating gut hormone levels seen with fibre-enriched diets. Butyrate also impacts EEC responses to external stimuli by regulating the expression of receptors sensing exogenous molecules deriving from the microbiota. In particular, butyrate increases Toll-like receptor expressions in L-cells leading to an amplified stimulation by Toll-like receptor ligands and a consequent higher NF-κB activation and butyrate-dependent PYY expression(Reference Larraufie, Dore and Lapaque121).
Due to their important functions on host, gut hormones link SCFA and the modulation of other gut functions such as electrolyte absorption. Indeed, PYY is strongly associated with the modulation of electrolyte and water absorption functions due to the expression of neuropeptide Y receptors on epithelial cells and neuronal cells(Reference Cox122,Reference Pais, Rievaj and Larraufie123) . As SCFA stimulate PYY release, they impact electrolyte absorption(Reference Okuno, Nakanishi and Shinomura124). Similarly, serotonin is also important in water and electrolyte absorption. SCFA also increase serotonin production, and blockade of serotonin receptors decreases butyrate-dependent electrolyte absorption(Reference Reigstad, Salmonson and Rainey119,Reference Fukumoto, Tatewaki and Yamada125) . These results indicate that the regulation of electrolyte absorption by SCFA is mediated by multiple pathways including gut hormone modulations.
SCFA have also been associated with tuning of intestinal transit(Reference Fukumoto, Tatewaki and Yamada125). Acute effect of SCFA on gut motility is hormone dependent with an important role of PYY(Reference Cherbut, Ferrier and Roze126,Reference Cuche, Cuber and Malbert127) . Moreover, germ-free animals have decreased gut motility which is partially restored by SCFA infusion in the colonic lumen, with butyrate having the highest effect(Reference Vincent, Wang and Parsons128). The gut motility dysfunction in germ-free mouse could be partially explained by the highly dysregulated gut endocrine functions. However, no difference could be found in non-producing serotonin mouse model using TPH1 knockout mice(Reference Vincent, Wang and Parsons128). This suggests that serotonin might not play an important role in the SCFA-dependent regulation of gut motility and effects previously described could be minor compared to other pathways(Reference Fukumoto, Tatewaki and Yamada125). Interestingly, SCFA, and mostly butyrate, have a direct effect on gut motility through the regulation of enteric neurons(Reference Cherbut, Ferrier and Roze126). Indeed, some enteric neurons express GPR41 and can therefore respond to SCFA(Reference Nohr, Pedersen and Gille106). Additionally, HDAC inhibition by butyrate increases gut motility in the long term by increasing the number of acetylcholine and substance P positive neurons, highlighting the importance of distinct mechanisms triggering similar effects(Reference Soret, Chevalier and De Coppet129).
Butyrate and other SCFA are therefore important regulators of EEC functions, both by acutely stimulating gut hormone secretion, and modulating their production. Indeed, SCFA increase EEC subpopulation cell numbers and regulate gene expression. Different mechanisms including receptor activation and HDAC inhibition are involved in these functions, highlighting the important and diverse roles of SCFA as signalling molecules. Modulations of gut hormones participate in many roles of SCFA on host physiology including gut homoeostasis.
Barrier function and immune responses
In the past decade, SCFA have attracted considerable attention for their impact on host immune responses and barrier functions. SCFA play one of their major roles by maintaining an environment favourable for commensal bacteria and controlling pathogens’ growth. By stabilising the transcription factor HIF, butyrate increases VO2 by IEC favouring the physiologic hypoxia in the colon(Reference Kelly, Zheng and Campbell130). Maintenance of the colonic anaerobic environment is key to favour the anaerobe commensal component of the gut microbiota and control the pathogens’ level such as Salmonella in a virtuous cycle(Reference Faber, Tran and Byndloss131–Reference Rivera-Chavez, Zhang and Faber133). However, enteric pathogens such as Salmonella enterica serovar Typhimurium are highly adapted to the colonic environment and utilise the gut microbiota-derived butyrate to compete with resident bacteria(Reference Bronner, Faber and Olsan134). Besides effect on the O2 level in the intestinal tract, butyrate promotes the epithelial barrier functions by reducing the epithelial permeability via HIF(Reference Kelly, Zheng and Campbell130). Moreover, butyrate reduces epithelial permeability by the regulation of IL-10 receptor, occludin, zonulin and claudins, reinforcing the tight junctions and the trans-epithelial resistance in vitro (Reference Zheng, Kelly and Battista135,Reference Wang, Wang and Wang136) . Another important mechanism involved in the epithelial barrier function is the modulation of the mucus layer thickness protecting the mucosa. In the colon, MUC2 is the predominant mucin glycoprotein produced by the goblet cells. Treatment with butyrate increases MUC2 production both in vitro and in human colonic biopsies(Reference Hamer, Jonkers and Venema32,Reference Gaudier, Jarry and Blottiere137) . SCFA enhance the epithelial barrier functions by modulating antimicrobial peptide secretion by the gut epithelium. Butyrate increases the level of colonic LL-37 in vitro and in vivo (Reference Hase, Eckmann and Leopard138,Reference Raqib, Sarker and Bergman139) . Activation of GPR43 by butyrate induce RegIIIγ and β-defensins expression by the activation of the mTOR pathway and STAT3 phosphorylation in mouse IEC(Reference Zhao, Chen and Wu140). The modulations of β-defensins in epithelial cells rely on the inhibition of HDAC(Reference Fischer, Sechet and Friedman141). Interestingly, SCFA and butyrate in particular, promote antimicrobial peptides targeting both Gram-positive and -negative bacteria.
It is now clear that gut microbiota plays an important role in intestinal homoeostasis by controlling the human immune response notably by the production of SCFA. Indeed, SCFA have a global anti-inflammatory effect by up-regulating both anti-inflammatory and down-regulating pro-inflammatory cytokines by different mechanisms and consequently promoting mucosal homoeostasis(Reference Maslowski, Vieira and Ng142). This anti-inflammatory effect can be mediated by IEC as binding of SCFA to GPR43 and GPR109a induces Ca2+ efflux and membrane hyperpolarisation which activate the inflammasome-activating protein NLRP3 thereby inducing the release of IL-18 with a protective effect on a dextran sulfate sodium colitis mouse model(Reference Macia, Tan and Vieira143). In vitro experiments demonstrate that the increase of protein acetylation by butyrate decreases IL-8 production in IEC(Reference Huang, Katz and Martin144). Moreover, butyrate, and to a lesser extent propionate, upregulate the production of TGFβ1 in IEC, a cytokine promoting anti-inflammatory regulatory T cells (Treg)(Reference Atarashi, Tanoue and Oshima145,Reference Atarashi, Tanoue and Shima146) . Our group has shown that butyrate acts independently of the main GPCR, via its HDAC inhibition property and the SP1 transcription factor present on the human TGFβ1 promoter(Reference Martin-Gallausiaux, Beguet-Crespel and Marinelli28). Moreover, in mice, fibre supplementation promotes vitamin A metabolism in small intestine epithelial cells by increasing RALDH-1. The production of retinoic acid by epithelial cells, the active metabolite of vitamin A, is crucial for the tolerogenic imprinting of dendritic cells (DC)(Reference Goverse, Molenaar and Macia147).
The impact of SCFA goes beyond the epithelial cells, with similar mechanisms reported in macrophages and DC. In mice, macrophage stimulation with butyrate imprints through HDAC3 inhibition, a metabolic reprogramming and elevates antimicrobial peptides. Hence, upon stimulation, antimicrobial peptides belonging to the S100 family, ficolin and lysozyme are increased(Reference Schulthess, Pandey and Capitani148). Here again, butyrate has a stronger antimicrobial effect than propionate and no protective impact is detected with acetate. Butyrate treatment of DC derived from human donors, decreases their capacity to present antigens and increases IL-10 production leading to a tolerogenic phenotype(Reference Liu, Li and Min149). Upon lipopolysaccharide treatment, butyrate induces the IL-23 production by DC thus promoting the differentiation of naive T lymphocytes into pro-inflammatory Th17(Reference Berndt, Zhang and Owyang150). Another study showed that DC treated with butyrate induce the differentiation of naive T lymphocytes into anti-inflammatory Tr1 producers of IL-10(Reference Kaisar, Pelgrom and van der Ham151). By regulating the transcriptional activity, butyrate decreases the inflammatory response of macrophages exposed to inflammatory microbial molecules such as lipopolysaccharide and induces their polarisation through a M2 anti-inflammatory phenotype(Reference Chang, Hao and Offermanns152,Reference Ji, Shu and Zheng153) . Similarly, butyrate-dependent activation of GPR109a increases the tolerogenic response of colonic macrophages and DC reducing colonic inflammation and promoting homoeostasis(Reference Singh, Gurav and Sivaprakasam154). Furthermore, it has been shown that butyrate pre-treatment down-regulates nitric oxide, IL-6 and IL-12 in mice independently of Toll-like receptor and GPCR pathways. Neutrophil migration is increased upon treatment with SCFA, in a GPR43-dependent mechanism(Reference Vinolo, Ferguson and Kulkarni155).
Treg are critical for limiting intestinal inflammation and have thus been subject of considerable attention to improve diseases such as inflammatory bowel disease. Many studies showed that Treg depend on microbiota-derived signals for proper development and function(Reference Atarashi, Tanoue and Oshima145,Reference Atarashi, Tanoue and Shima146,Reference Geuking, Cahenzli and Lawson156,Reference Round and Mazmanian157) . Recently, several groups identified SCFA as key metabolites for promoting differentiation of naive T lymphocytes into Treg cells in the intestine(Reference Arpaia, Campbell and Fan71,Reference Atarashi, Tanoue and Oshima145,Reference Atarashi, Tanoue and Shima146,Reference Chang, Hao and Offermanns152,Reference Singh, Gurav and Sivaprakasam154,Reference Furusawa, Obata and Fukuda158,Reference Smith, Howitt and Panikov159) . By interacting directly with naive T cells, butyrate and propionate increase the acetylation of the promoter of the transcription factor Foxp3 essential for the differentiation of Treg, leading to an increase of Foxp3 expression(Reference Arpaia, Campbell and Fan71,Reference Chang, Hao and Offermanns152,Reference Furusawa, Obata and Fukuda158) . Another group suggested that propionate might induce the same changes via GPR43(Reference Arpaia, Campbell and Fan71,Reference Smith, Howitt and Panikov159) . Moreover, butyrate-dependent activation of GPR109a increases the tolerogenic response of colonic macrophages and DC, promoting Treg and IL-10-producing T cells(Reference Singh, Gurav and Sivaprakasam154). Interestingly, SCFA increase the TGFβ1 production by IEC via its HDAC inhibition property thus promoting the Treg differentiation in the gut(Reference Martin-Gallausiaux, Beguet-Crespel and Marinelli28,Reference Atarashi, Tanoue and Oshima145,Reference Atarashi, Tanoue and Shima146) . Altogether, these studies highlight that the molecular mechanisms induced by SCFA to control Treg-development are complex and involve many cell types involved in the tolerogenic environment such as myeloid cells and IEC.
The impact of SCFA on other lymphocyte populations such as B cells has not been as extensively studied than their Treg counterparts. Acetate supplementation in mice increases intestinal IgA in a GPR43 dependent mechanism(Reference Wu, Sun and Chen160). Dietary fibres and SCFA enhance antibody response to bacteria by supporting B cell differentiation into plasma B cells via the increase of histone acetylation and of B cell metabolism(Reference Kim, Qie and Park161,Reference Sanchez, Moroney and Gan162) . Mechanistically, it is through the downregulation of B cell AID and Blimp1, dependent on their HDAC inhibitory activity that SCFA inhibited class-switch DNA recombination, somatic hypermutation and plasma cell differentiation. Interestingly, SCFA also modulate the fate of B-cell-producing autoantibodies and reduce autoimmunity in lupus-prone mice(Reference Sanchez, Moroney and Gan162).
Conclusion
The past decade of biological research through a combination of translation-focused animal models and studies in human subjects has highlighted the overarching roles that the gut microbiota plays in human health. It has become clear that dysbiotic microbiota is associated with a wide range of pathologies such as obesity, diabetes, CVD, autoimmune diseases and neuronal disorders. Despite the lack of evidence in human subjects, causality has been demonstrated in rodent models. Factors such as antibiotics use, modern sanitation, quality of diet and environmental factors linked with the lifestyle changes that occurred in the past century in developed societies are suggested to contribute to a decrease in the diversity of the human microbiome(Reference Moskowitz and Devkota163).
Diet and nutritional status are important determinants in human health. Numerous studies have shown that diet modulates the composition and functions of the microbiota in human subjects and animal models(Reference David, Maurice and Carmody164–Reference De Filippo, Cavalieri and Di Paola166). These interventional studies showed that microbiota composition is dynamic, can shift rapidly to dietary changes and that this shift is individual dependent and depends on the microbiota diversity of the donor. Thus, the role of diet in shaping microbiota is changing our view of the strategies to take to improve the systemic health. Indeed, it is thought that nutritional interventions could manipulate the microbial ecology and consequently modulate human physiology with beneficial health outcomes. However, what constitutes an optimal health-promoting microbiota and how individuals with distinct microbiota can achieve such a level of diversity are still open questions.
As discussed in this review, the gut microbial metabolites SCFA are well known to exert a wide beneficial impact to the host(Reference Canfora, Jocken and Blaak167,Reference Sanna, van Zuydam and Mahajan168) . Hence, fibre-induced increase of SCFA-producing bacteria has been proposed to play an important role in the prevention and treatment of many diseases. Supporting this idea, clinical studies reported that prebiotics and dietary fibres increased the relative abundance of these beneficial SCFA-producing bacteria and butyrate fermentation, leading to the improvement of type-2 diabetes and ulcerative colitis(Reference Haller169,Reference Zhao, Zhang and Ding170) . However, the microbiota produces a vast number of metabolites that modulate host responses, sometimes in synergy with SCFA(Reference Larraufie, Dore and Lapaque121). Many studies support the benefits of increasing both the amount and the variety of dietary fibres ingested but it is difficult to establish whether it is a direct role of SCFA or the increased bacterial diversity that impact host homoeostasis. As the microbiota is a complex ecosystem, much work remains to be done to investigate fully the functions of SCFA alone or with other beneficial metabolites in physiology and pathophysiology.
Acknowledgements
The authors would like to thank all the members of the ‘functionality of the intestinal ecosystem’ team for helpful discussions.
Financial Support
This work was supported by the by Institut national de recherche pour l'agriculture, l'alimentation et l'environnement and by grants funded by EU-FP7 METACARDIS (HEALTH-F4-2012-305312), by the ANR FunMetagen (ANR-11-BSV6-0013).
Conflict of Interest
None.
Authorship
The authors had joint responsibility for all aspects of preparation of this paper.




