Introduction
Amphibians are the most threatened vertebrate class, with 41% of assessed species in danger of extinction (IUCN, 2020). They are threatened by multiple processes that may act synergistically to drive population declines. One of the most significant threats is habitat loss (Gallant et al., Reference Gallant, Klaver, Casper and Lannoo2007). However, some amphibian species have disappeared from intact or suitable habitats (Daszak et al., Reference Daszak, Berger, Cunningham, Hyatt, Green and Speare1999; Hirschfeld et al., Reference Hirschfeld, Blackburn, Doherty-Bone, Gonwouo, Ghose and Rödel2016), with such declines usually having been mediated by disease (Skerratt et al., Reference Skerratt, Berger, Speare, Cashins, McDonald and Phillott2007; Stegen et al., Reference Stegen, Pasmans, Schmidt, Rouffaer, van Praet and Schaub2017) or related to overexploitation (Stuart et al., Reference Stuart, Rhodin, Grismer and Hansel2006; Phimmachak et al., Reference Phimmachak, Stuart and Sivongxay2012).
The Chinese giant salamander is a Critically Endangered aquatic cryptobranchid salamander. It has been identified as a global priority for conservation based on the evolutionary distinctiveness of its so-called living fossil lineage (Gumbs et al., Reference Gumbs, Gray, Wearn and Owen2018), and was designated as a State 2 protected animal in China in 1998, with this national legislation making hunting illegal (Liang et al., Reference Liang, Geng and Zhao2004). It has traditionally been interpreted as the single geographically wide-ranging species Andrias davidianus, distributed across multiple montane ecoregions and river basins (Yangtze, Yellow, Pearl, and south-east river drainages), but has recently been shown to constitute a complex of at least three species, including the South China giant salamander A. sligoi and other undescribed taxa (Yan et al., Reference Yan, Lü, Zhang, Yuan, Zhao and Huang2018; Liang et al., Reference Liang, Chen, Wang, Zhang, Wang and He2019; Turvey et al., Reference Turvey, Marr, Barnes, Brace, Tapley and Murphy2019). Giant salamanders were formerly widespread over much of China (Liang et al., Reference Liang, Geng and Zhao2004; Fei et al., Reference Fei, Hu, Ye and Huang2006), but in recent decades many populations have undergone dramatic declines or extirpations (Turvey et al., Reference Turvey, Chen, Tapley, Wei, Xie and Yan2018), even in habitats that appear suitable and support abundant prey (Tapley et al., Reference Tapley, Okada, Redbond, Turvey, Chen, Lü and Cunningham2015). The range-wide decline of giant salamanders across China has been attributed to overexploitation for the luxury food market (Liang et al., Reference Liang, Geng and Zhao2004; Wang et al., Reference Wang, Zhang, Wang, Ding, Wu and Huang2004; Feng et al., Reference Feng, Lau, Stuart, Chanson, Cox and Fishman2007; Dai et al., Reference Dai, Wang and Liang2009; Cunningham et al., Reference Cunningham, Turvey, Zhou, Meredith, Guan and Liu2016; Turvey et al., Reference Turvey, Chen, Tapley, Wei, Xie and Yan2018), and to habitat loss and degradation through anthropogenic modification of freshwater habitats, including through pollutant emissions and the alteration of flow regimes and water turbidity from damming (e.g. Liang et al., Reference Liang, Geng and Zhao2004; Wang et al., Reference Wang, Zhang, Wang, Ding, Wu and Huang2004; Dai et al., Reference Dai, Wang and Liang2009). However, there has been no systematic attempt to determine the extent to which these two threat processes have each contributed to giant salamander declines in China. This limitation is compounded by a general lack of published data on the habitat requirements of giant salamanders in China, especially with regard to water parameters.
Using environmental data associated with historical locality records, Chen et al. (Reference Chen, Cunningham, Wei, Yang, Liang and Wang2018) developed a habitat suitability model for Chinese giant salamanders based on elevation, forest cover, mean annual temperature and mean annual precipitation, which was broadly congruent with the estimated IUCN range map for Andrias davidianus (Liang et al., Reference Liang, Geng and Zhao2004). Anecdotally, Chinese giant salamanders are thought to inhabit clear, cool, slow to swift flowing streams of pH 6–7 in steep-sided, well-vegetated valleys that have caves in rocky banks (Wang et al., Reference Wang, Zhang, Wang, Ding, Wu and Huang2004). Water parameters, such as dissolved oxygen, alkalinity and nitrate, are among the known predictors of cryptobranchid salamander distribution in the USA (Pugh et al., Reference Pugh, Hutchins, Madritch, Siefferman and Gangloff2016). Siltation is known to cause the loss of specific cryptobranchid microhabitats by embedding boulders (under which these salamanders find refugia) in stream substrate (Fobes, Reference Fobes1995); turbidity could therefore be an additional predictor of suitable habitat for cryptobranchid salamanders (Quinn et al., Reference Quinn, Gibbs, Hall and Petokas2013). Water temperature (Buckley & Jetz, Reference Buckley and Jetz2007; Grant et al., Reference Grant, Wiewel and Rice2014), pH (Freda & Dunson, Reference Freda and Dunson1986; Grant et al., Reference Grant, Wiewel and Rice2014), ammonia concentration (Weyrauch & Grubb, Reference Weyrauch and Grubb2004), and flow rate (Welsh & Olivier, Reference Welsh and Ollivier1998) are known to influence the distribution and/or survivorship of all life stages of other salamander species.
Robust understanding of the microhabitat requirements of Chinese giant salamanders and of the drivers of their decline in China is essential if appropriate conservation strategies are to be formulated and implemented. We therefore gathered a novel large-scale dataset during a multi-year field survey programme in China, obtaining data from both ecological surveys and community-based surveys of local ecological knowledge. Together, these multidisciplinary and independent data provide an important new baseline for understanding Chinese giant salamander microhabitat requirements, and for identifying the significance of habitat degradation and/or overexploitation as the primary drivers of population declines across their range.
Methods
We randomly selected 50 Chinese counties containing historical giant salamander records (Fei et al., Reference Fei, Hu, Ye and Huang2006), and 50 further counties from a sample of all counties lacking historical records that contained > 50% predicted suitable giant salamander habitat based on the habitat suitability model of Chen et al. (Reference Chen, Cunningham, Wei, Yang, Liang and Wang2018). These sites were distributed across 16 Chinese provinces or equivalent administrative units (Fig. 1; Supplementary Table 1). We selected sites with intact natural habitat (i.e. fast-flowing mountain streams with riparian forest cover and rocky substrates) for surveys in all counties, with specific site selection for those most likely to be occupied by Chinese giant salamanders determined through discussion with local government fisheries offices or protected area managers. Logistical issues prevented survey work in three of the randomly selected counties, so only 97 of the 100 selected sites were surveyed. In addition, we opportunistically surveyed an additional site within Guangwushan-Nuoshuihe Geopark National Nature Reserve in Nanjiang County, Sichuan Province, which contained > 50% predicted suitable habitat and had recent reports of giant salamanders.
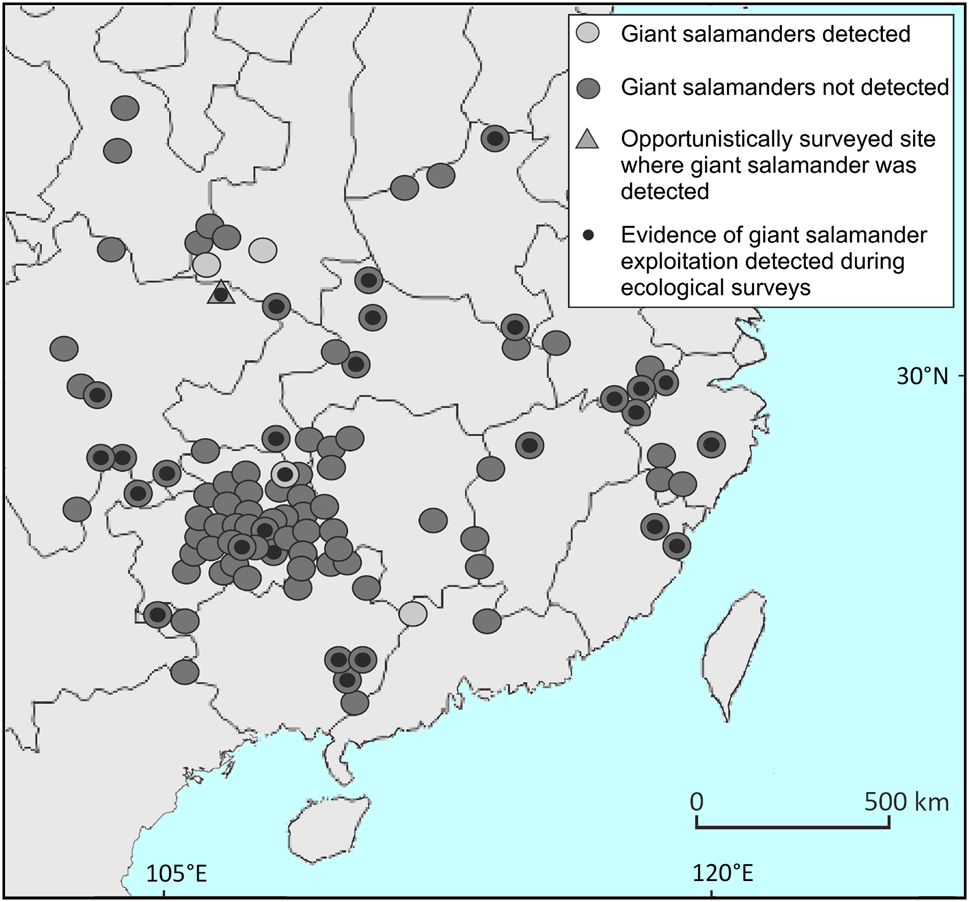
Fig. 1 Sites in China surveyed for Chinese giant salamanders Andrias spp., with sites where salamanders were detected and not detected, the opportunistic survey site where a giant salamander was detected, and sites where direct evidence of giant salamander exploitation was detected.
Fieldwork was conducted during 2013–2016 by teams trained to undertake standardized field survey methodology (Tapley et al., Reference Tapley, Chen, Turvey, Redbond, Okada and Cunningham2017), with surveys during May–October each year to coincide with inferred peak activity periods of Chinese giant salamanders (Okada et al., Reference Okada, Utsunomiya, Okada, Felix and Ito2008). Ecological surveys covered a cumulative 1 km transect of suitable habitat at each site. At some sites, geographical barriers (e.g. waterfalls) prevented the completion of a continuous 1 km transect; in such cases, the survey team continued the survey beyond the obstruction until a cumulative transect of 1 km had been completed. Surveys included trapping (passive searching) using baited crab traps set for a 48-hour period (see Tapley et al., Reference Tapley, Chen, Turvey, Redbond, Okada and Cunningham2017 for details), and active survey techniques, including daytime and night-time snorkeling and rock turning. Cloacal and skin swabs were taken from giant salamanders (see Tapley et al., Reference Tapley, Chen, Turvey, Redbond, Okada and Cunningham2017 for details), and tested for chytrid infection (Batrachochytrium dendrobatidis) using molecular methods described by Boyle et al. (Reference Boyle, Boyle, Olsen, Morgan and Hyatt2004), and for ranaviruses following Cunningham et al. (Reference Cunningham, Turvey, Zhou, Meredith, Guan and Liu2016). During each ecological survey, physical evidence of giant salamander exploitation (e.g. bow hooks, traps, electrofishing) was also recorded if detected. Ecological surveys following our standardized methods (excluding the opportunistically surveyed Nanjiang County site) represented 2,675 person-days of passive searching and 50 cumulative person-days of active searching (Turvey et al., Reference Turvey, Chen, Tapley, Wei, Xie and Yan2018).
We recorded environmental parameters at each site. Elevation and geographical coordinates were recorded using a GPS; water temperature, dissolved oxygen and salinity were measured using a Micro 800 Meter (Palintest, Gateshead, UK); pH was measured using a Micro 600 pH Meter (Palintest); and ammonia (the sum of ammonia and ammonium), nitrite, nitrate, alkalinity and dKH (carbonate hardness) were measured with aquarium test kits (Salifert, Duiven, The Netherlands). Flow rate was measured using a locally produced unbranded flow meter, and indirect measurements of turbidity were taken using a Secchi disc. Water parameters were recorded once at each site, at the start of the first daytime survey in the middle of the stream being surveyed. All parameters were recorded either in the stream (temperature, salinity, dissolved oxygen, flow rate, turbidity) or within 15 min for tests that required reagents (ammonia, nitrite, nitrate, alkalinity and hardness).
We also conducted standardized questionnaire-based interviews (Tapley et al., Reference Tapley, Chen, Turvey, Redbond, Okada and Cunningham2017) at the 97 selected survey sites (but not at the opportunistically surveyed site in Nanjiang County), to collect local ecological knowledge on Chinese giant salamanders, including respondent sighting experience, last-sighting records and information on exploitation. All interviews were conducted in Chinese (either standard Mandarin Chinese or a local dialect), in communities situated within 1 km of the surveyed rivers. Full details of interview methods, including respondent selection and interview protocols, are given in Tapley et al. (Reference Tapley, Chen, Turvey, Redbond, Okada and Cunningham2017), Chen et al. (Reference Chen, Cunningham, Wei, Yang, Liang and Wang2018) and Turvey et al. (Reference Turvey, Chen, Tapley, Wei, Xie and Yan2018).
As data were not normally distributed, we used two-tailed Mann–Whitney U tests, with Social Science Statistics (2020), to compare each of the recorded water parameter values between grouped sets of sites, as follows: (1) Sites where giant salamanders were directly detected during ecological surveys vs sites where salamanders were not detected. Because salamanders were detected in only four of the 97 randomly selected counties, data from the opportunistically surveyed site in Nanjiang County, where a giant salamander was detected, were also included in this analysis, to fulfil the minimum number of sites required for statistical analysis. This site was excluded from further analyses as it was not chosen by our standardized site selection method. (2) Sites where giant salamanders were detected during ecological surveys and/or where mean reported last-sighting date (based on last-sighting data from all respondents in each county) was within the previous 5 years vs sites where giant salamanders were not detected and where mean reported last-sighting date was > 5 years ago.
We performed principal component analyses (PCA; Hammer et al., Reference Hammer, Harper and Ryan2001) using varimax rotation for water parameters from three categories of sites: (1) where giant salamanders were detected directly during ecological surveys (including the opportunistically surveyed site in Nanjiang County); (2) where giant salamanders were not detected directly, but with mean last-sighting date within the previous 5 years; and (3) sites where giant salamanders were not detected directly, and with mean last-sighting date > 5 years ago. We examined each variable that contributed moderately (> 0.50) to factor loadings. We also performed PCAs using varimax rotation for water parameters from sites grouped by river basin (Yellow, Yangtze, Pearl, and south-east rivers), again examining each variable that contributed moderately (> 0.50) to factor loadings.
Results
We recorded water parameters at all survey sites (Table 1, Supplementary Table 1). Shallow water depth at all sites precluded measurement of turbidity as the water was too clear for using a Secchi disc, and we suggest that turbidity meters are used for future studies. We detected 25 giant salamanders (Plate 1a) in four of the 97 selected survey counties and at the opportunistically surveyed site in Nanjiang: Liannan (Guangdong), 17 individuals; Jiangkou (Guizhou), 1; Lüeyang (Shaanxi), 5; Zhouzhi (Shaanxi), 1; Nanjiang (Sichuan), 1. Of these, 20 were captured and were swabbed for the amphibian chytrid fungus Batrachochytrium dendrobatidis and for ranaviruses. We did not detect the presence of either pathogen in any sample. Five animals that were recorded in Liannan (Guangdong) were not accessible as they were deep within caves. Releases of farmed giant salamanders had occurred shortly beforehand at two sites where they were detected during ecological surveys (Liannan and Lüeyang), so a proportion or all of the giant salamanders detected at these two sites could have been released animals. There was no statistically significant difference between any of the water quality parameters at sites where giant salamanders were detected by survey teams and/or had been recently seen by local respondents, and sites where they were not detected and/or from which they had recently been extirpated (Table 2). Evidence of illegal giant salamander exploitation was found at 26 of the 97 survey sites (illegal traps at 10 sites; bow hooks at six sites; evidence of electrofishing at six sites; evidence of poison fishing at nine sites; evidence of multiple illegal activities at some sites), including within protected areas (Plate 1b).

Plate 1 (a) Wild giant salamander Andrias sp. detected in Shaanxi Province, and (b) bow hooks used for poaching giant salamanders, discovered within a protected area in Guizhou Province.
Table 1 Mean (and range) of environmental and water parameters collected at 98 sites surveyed for giant salamanders Andrias spp. in China, where the species were directly detected (i.e. during ecological surveys) and not directly detected (i.e. not encountered during ecological surveys).

Table 2 Results of two-tailed Mann−Whitney U tests comparing recorded water parameter values between grouped sets of sites: (1) sites where giant salamanders were directly detected during surveys vs sites where salamanders were not detected; and (2) sites where giant salamanders were detected during surveys and/or where mean reported last-sighting date was within the previous 5 years vs sites where giant salamanders were not detected and where mean reported last-sighting date was > 5 years ago.

A total of 2,842 people were interviewed (Supplementary Table 2). Mean reported giant salamander last-sighting dates fell within the previous 5 years in only six of the 97 counties, and did not include any of the counties in which we detected giant salamanders. Ongoing hunting by respondents was directly reported across 14 of the 16 surveyed provinces or equivalent administrative areas (Anhui, Chongqing, Fujian, Gansu, Guangdong, Guangxi, Guizhou, Hubei, Hunan, Jiangxi, Shaanxi, Sichuan, Yunnan, Zhejiang). At least one respondent in 45 of the 97 counties admitted to hunting giant salamanders (205/2,842, 7.2%). Furthermore, 936/2,842 respondents (32.9%) thought that overharvesting (either by local people or by outsiders specifically hunting salamanders) was a problem for wild giant salamander populations, with 806/2,842 (28.4%) stating that it was the top threat to wild populations, and with hunting by outsiders considered by respondents to be the primary threat to giant salamanders in three of the surveyed counties.
Between-group PCAs of water parameters across sites grouped by different giant salamander detection histories (Table 3) revealed that most of the variance (57.61%) was explained by PC1 (eigenvalue = 1.67), which had a strong loading for nitrate. PC2 had strong factor loadings for temperature, and explained 22.51% of the variance (eigenvalue = 0.65). PC3 had strong factor loadings for dKH, and explained 19.88% of the variance (eigenvalue = 0.58). Sites where giant salamanders had recently been detected did not cluster separately from sites where they had not recently been detected (Fig. 2a).
Table 3 Rotated factor loadings of principal component analysis of water parameters between sites grouped into three categories: (1) sites where giant salamanders were detected directly during surveys; (2) sites where giant salamanders were not detected directly, but with mean last-sighting date within the previous 5 years; and (3) sites where giant salamanders were not detected directly, and with mean last-sighting date > 5 years ago. Factor loadings > 0.50 are highlighted in bold.

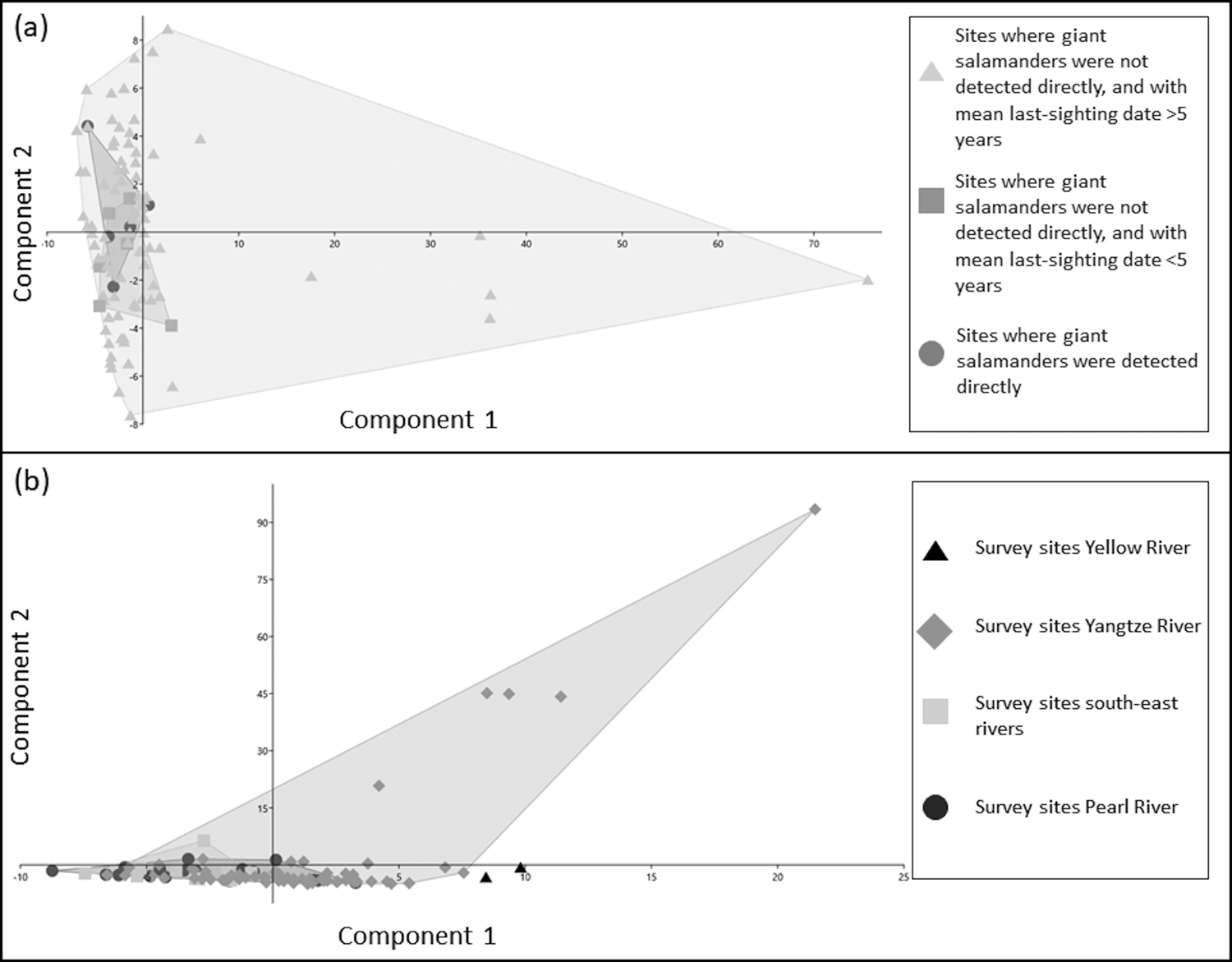
Fig. 2 Scatterplots of first and second principal components from principal component analyses of water parameters between sites, grouped by (a) giant salamander detection history, and (b) river basin. Shaded areas are the smallest convex polygons that enclose all the points in each grouped data set.
PCA of water parameters across sites grouped by river basin (Table 4) revealed that most of the variance (87.62%) was explained by PC1 (eigenvalue = 177.38), which had a strong loading for nitrate. PC2 had a strong loading for temperature, but explained just 6.63% of the variance (eigenvalue = 13.42). Different river basins did not form separate clusters (Fig. 2b), although only a small number of samples were collected from the Yellow River basin.
Table 4 Rotated factor loadings of principal component analysis of water parameters from sites grouped by river basin (Yangtze, Yellow, Pearl, and south-east rivers). Factor loadings > 0.50 are highlighted in bold.

Discussion
Our multi-year survey effort to detect Chinese giant salamanders provides a new baseline both for assessing the current status of giant salamander populations across China (Turvey et al., Reference Turvey, Chen, Tapley, Wei, Xie and Yan2018), and also for identifying the likely drivers of their decline, knowledge of which is essential to inform effective conservation management interventions. To investigate possible drivers of giant salamander decline, we explored associations between environmental factors and indicators of potential species presence across our survey sites. These indicators comprised both direct detection of Chinese giant salamanders and metrics of their possible presence based on sighting histories reported by local respondents, and local knowledge of exploitation. We chose to assess a range of indicators of giant salamander presence, to minimize the effect of potential error (either commission or omission error) in any specific signal. In addition, mean reported last-sighting date is more robust to error associated with factors such as misidentification or poor recall by non-trained respondents, compared to metrics that are more dependent upon single sighting accounts (cf. Hermoso et al., Reference Hermoso, Kennard and Linke2013; Turvey et al., Reference Turvey, Trung, Quyet, Nhu, Thoai and Tuan2015).
We found no significant differences in any of the water quality parameters that we tested between sites with no recent signal of giant salamanders, and those with either a direct signal (our detection) or a recent indirect signal (local respondent reports). Our PCA analyses are congruent with these findings, as sites where giant salamanders were detected either directly or indirectly do not cluster separately from other sites. This lack of any statistical differences suggests that water quality is still suitable for giant salamanders at the survey sites where the species was not detected. This conclusion is supported by the fact that we encountered diverse amphibian species assemblages, often including other aquatic salamanders, at most survey sites, which typically included amphibian genera known to be primarily associated with large intact forest patches and/or clear streams (e.g. Leptobrachium, Megophrys; Dring, Reference Dring1979; Malkmus et al., Reference Malkmus, Manthey, Vogel, Hoffmann and Kosuch2002; Bickford et al., Reference Bickford, Ng, Qie, Kudavidanage and Bradshaw2010) or that are threatened by water pollution (e.g. Batrachuperus, Paramesotriton; Fei & Ye, Reference Fei and Ye2004; Gu et al., Reference Gu, Geng and Yuan2004; Zhao & Yuan, Reference Zhao and Yuan2004).
Some freshwater landscapes with historical records of Chinese giant salamanders, including the type locality for Andrias davidianus in Zhongba, Sichuan, are now heavily degraded as a result of industrial, agricultural and other anthropogenic activities, and are unable to support giant salamander populations (Dai et al., Reference Dai, Wang and Liang2009). Habitat suitability modelling based on available environmental parameters, however, suggests that considerable suitable habitat for giant salamanders still exists across China (Chen et al., Reference Chen, Cunningham, Wei, Yang, Liang and Wang2018). Our analyses of water quality parameters at a large number of sites containing such apparently suitable habitat did not reveal any environmental differences between sites with and without giant salamanders, further validating the habitat suitability model developed by Chen et al. (Reference Chen, Cunningham, Wei, Yang, Liang and Wang2018). Because of the ambitious scale of this study, water samples were collected only once at each site, and our surveys were undertaken during May–October over a 3-year period. Both temperature and nitrate accounted for much of the variance between sites in some of the PCA analyses. Temperature and nitrate are likely to vary between seasons, with nitrate increasing after periods of heavy rainfall (e.g. Zhu et al., Reference Zhu, Wang, Kuang, Luo, Tang and Xu2009). We recommend that future assessment of water quality should analyse multiple water samples, with data collected at the same time of year across survey sites if possible.
Habitat loss and degradation remains a threat to giant salamander populations across China, and we did not investigate water pollutants such as heavy metals, phosphates or persistent organic pollutants, which are likely to affect giant salamanders and other amphibians (Dai et al., Reference Dai, Wang and Liang2009). However, the results of our large-scale multi-year survey suggest that habitat loss is not the main driver of giant salamander population decline in China. Principal component analysis of water parameters between river basins demonstrates a high degree of similarity and there was overlap in principal components 1 and 2. Our results support the hypothesis that illegal overexploitation is an important driver of giant salamander decline in areas where suitable habitat remains. A substantial proportion of local respondents reported that hunting was a major threat to giant salamanders, and at least one respondent from each of nearly half of the survey sites reported hunting the species, with these figures likely to be underestimates of true hunting levels as a result of the sensitive and illegal nature of this activity. Even if respondents did not hunt giant salamanders themselves, they often reported that other people from outside their community hunted salamanders locally. The prevalence of illegal overexploitation is further demonstrated by direct evidence of ongoing poaching of giant salamanders observed by field teams at over a quarter of our survey sites. Although observed illegal fishing activities could also be targeting other aquatic species, the methods deployed are non-species specific and could result in capture, death or injury of giant salamanders.
The Chinese giant salamander complex comprises at least three species, including the recently described South China giant salamander A. sligoi (Yan et al., Reference Yan, Lü, Zhang, Yuan, Zhao and Huang2018; Liang et al., Reference Liang, Chen, Wang, Zhang, Wang and He2019; Turvey et al., Reference Turvey, Marr, Barnes, Brace, Tapley and Murphy2019). The ecological survey methods deployed in this study have been successfully used to detect other cryptobranchid salamander species in both Japan and the USA (Browne et al., Reference Browne, Li, Mcginnity, Okada, Wang and Bodinof2011), and therefore it is unlikely that the existence of multiple giant salamander species across our survey region confounds our results, or that different giant salamander species differ in their susceptibility to hunting. Speciation of Andrias in China was associated with uplift of the Qinghai–Tibet Plateau and geographical isolation of populations in different montane ecoregions rather than with niche differentiation (Turvey et al., Reference Turvey, Marr, Barnes, Brace, Tapley and Murphy2019), and giant salamanders are likely to be generalist species with respect to their macrohabitat and microhabitat requirements. Hybrids between Chinese giant salamanders and Japanese giant salamanders (Andrias japonicus) are now invasive in parts of Japan (Fukumoto et al., Reference Fukumoto, Ushimaru and Minamoto2015), and wide-scale intentional releases of giant salamanders across China has resulted in genetic homogenization of some local populations (Yan et al., Reference Yan, Lü, Zhang, Yuan, Zhao and Huang2018).
Other possible factors could also have driven the observed range-wide decline of Chinese giant salamanders. Disease is known to cause significant mortality on giant salamander farms in China (Geng et al., Reference Geng, Wang, Zhou, Li, Wang and He2011; Meng et al., Reference Meng, Ma, Jiang, Zeng and Xiao2014) and effluent from farms is discharged into river systems without treatment across the range of Chinese giant salamanders (Cunningham et al., Reference Cunningham, Turvey, Zhou, Meredith, Guan and Liu2016). Furthermore, the widespread practice of releasing farmed salamanders into rivers across China is not informed by pathogen screening (Cunningham et al., Reference Cunningham, Turvey, Zhou, Meredith, Guan and Liu2016), and pathogen pollution could constitute a potential risk to wild populations. We were unable to quantify the potential impact of the farming industry on the health of wild populations, but the presence of diverse amphibian species communities, including other salamander species, at most of our survey sites further indicates that disease is not likely to be a primary driver of wild giant salamander population declines.
Our investigation of environmental parameters associated with giant salamander presence and absence in China provides a new evidence-base to guide conservation planning for this Critically Endangered species complex of the largest amphibians. It appears likely that giant salamander populations have been largely extirpated across our surveyed sites by poaching rather than habitat degradation. This highlights the pressing need for existing protective legislation prohibiting the hunting of giant salamanders to be better implemented and more strictly enforced, together with strict habitat protection, if Chinese giant salamanders are to persist in the wild.
Our increased knowledge of the environmental conditions under which giant salamanders occur is invaluable for informing ex situ conservation breeding programmes, which have been suggested as a necessary component of the conservation strategy for Chinese giant salamanders (Turvey et al., Reference Turvey, Chen, Tapley, Wei, Xie and Yan2018, Reference Turvey, Marr, Barnes, Brace, Tapley and Murphy2019). A robust understanding of the environmental requirements of the target species is a prerequisite for the establishment of captive breeding programmes (Michaels et al., Reference Michaels, Gini and Preziosi2014). To our knowledge, there are no existing Chinese giant salamander captive breeding programmes that can produce offspring suitable for subsequent release into the wild. Although Chinese giant salamanders have been bred to several generations on commercial breeding farms, the often unknown provenance of founding stock, presence of multiple pathogens, and often suboptimal biosecurity makes farms inappropriate for conservation breeding (Cunningham et al., Reference Cunningham, Turvey, Zhou, Meredith, Guan and Liu2016). Our water parameter data should therefore be used to inform the development of husbandry protocols and water quality management for Chinese giant salamander conservation breeding programmes in dedicated conservation breeding facilities in China. These data have already been used successfully for the management of an ex situ population of Andrias davidianus at the Zoological Society of London. We hope that stakeholders and decision makers in China will act upon our findings and will strengthen both in situ and ex situ conservation actions for giant salamanders while there is still time to save these remarkable species.
Acknowledgements
We thank all of the field assistants for participating in surveys. Funding was provided by the Darwin Initiative (Project No. 19-003), the National Natural Science Foundation of China (31360144), Ocean Park Conservation Foundation Hong Kong, and the Zoological Society of London's EDGE of Existence programme.
Author contributions
Study design, data collection, writing; BT, STT, AAC; coordination of field activities, study design, data collection: SC; study design, data collection: SO, JR, GW, FX, MW, JC; data collection: JY, ZL, HT, JW, JL, FZ, HZ, JX, TB.
Conflicts of interest
None.
Ethical standards
This study adhered to the code of ethics developed and endorsed by the British Sociological Foundation. All methods, including a biosecurity protocol to prevent the spread of pathogens between sites, were approved by the Zoological Society of London's Ethics Committee and comply with the Oryx guidelines on ethical standards.









