INTRODUCTION
Italy is a country of low endemicity for hepatitis A virus (HAV) infection, characterized by low annual incidence rates (around 5/100 000 inhabitants) and in recent decades by a progressive shift of the average age of infection towards adulthood [Reference Mele1]. The epidemiological pattern, based on statutory notifications of HAV, is not homogenous across the country: in southern Italy, especially in the regions of Campania and Puglia, hepatitis A still shows a high incidence, with periodic outbreaks and annual incidence rates over the decade 1993–2003 up to 25/100 000 [2]. The different pattern of infection in southern Italy is also confirmed by HAV seroprevalence rates in young adults (age 18 years) reported to be fourfold higher than in northern Italy [Reference Lopalco3]. In 2004, a community-wide outbreak occurred in the region of Campania, the most densely populated Italian region, with a population of about 5 700 000 inhabitants. An outbreak of hepatitis A was initially suspected at the end of March, when the main regional hospital reported an unusually high number of HAV-related admissions. The regional Epidemiological Observatory immediately recommended offering HAV vaccination to all household contacts of cases. In order to characterize the outbreak and identify appropriate public health actions, an investigation was initiated by local health authorities in collaboration with the Istituto Superiore di Sanità (ISS) (the Italian National Institute of Health). The present paper describes and reports findings of the outbreak investigation.
METHODS
Surveillance and descriptive epidemiology
In Italy, cases of HAV infection are subject to statutory notification. The case definition used includes any patient with clinical acute hepatitis confirmed by detection of HAV IgM antibodies in serum. Diagnosing physicians are responsible for notification of cases while the epidemiological investigation for each case is performed by the Azienda Sanitaria Locale (ASL; the local public health authority) of the patient's area of residence. In urban or densely populated areas, like the Campania region, patients are often diagnosed with HAV in hospitals outside their area of residence. The timeliness of epidemiological investigation, therefore, is dependent on the exchange of information between different ASLs. In order to reduce delays in reporting, collect additional information exposure and improve timely exchange of data between different ASLs, an electronic web-based system called EPOS (EPidemic Observatory System) was promptly established in the region. The system allowed each ASL of the region to notify new cases of HAV resident in Campania, through a password-protected webpage [http://epos.cineca.it, developed with the collaboration of CINECA (Consorzio INtEruniversitario CAlcolo), Bologna, Italy]. Demographic, clinical and potential exposure data, such as shellfish consumption and contacts with other HAV cases, were recorded for each notified case.
The website included the facility to generate tables and epidemic curves both at the regional and local levels. Descriptive epidemiology included computation of attack rates by local geographical area, age and gender.
Case-control study
In order to identify relevant risk factors for acquiring HAV among individuals residing in the region, a case-control study was performed in Ercolano, the town with the highest attack rate. A group of randomly selected resident controls were frequency-matched by age group (0–5, 6–10, 11–20, 21–30 years) to the group of resident cases. Cases were identified from the EPOS surveillance database by extracting all patients residing in Ercolano who had onset of symptoms between 16 February and 30 April 2004, the period of the highest epidemic peak. The general practitioners or paediatricians with whom each case was registered were identified, and controls selected from the list of residents registered with the same physicians. Exclusion criteria for potential controls were: a history of HAV infection or jaundice, previous HAV vaccination, and refusal to participate. Potential controls who could not be traced after three attempts were discarded and replaced.
Individual data of cases and controls was collected through standard questionnaires administered by phone interview and included: (1) demographic characteristics such as age, sex, occupation or school attendance; (2) food and drinking habits and preferences (seafood, fresh vegetables, fresh cheese) with frequency of consumption, site of purchase and, limited to shellfish, storage condition at purchase (i.e. shellfish stored in seawater or dry nets); (3) contact with a HAV case; (4) presence of housemates aged <5 years. Parents were interviewed to obtain the requested information for very young cases and controls, and to consent to the interview of subjects aged <18 years.
Data were entered and analysed using Epi-Info 3.2.2 (Centers for Diseases Control and Prevention, Atlanta, GA, USA). According to the study design, a frequency-matched univariate analysis was run, and Mantel–Haesenzel odds ratio (ORMH) with 95% confidence intervals (95% CI) were calculated. To detect potential effect modifier and confounding factors a multivariate analysis with a logistic regression model was also carried and all variables with an ORMH ⩾1·5 or with a P value ⩽0·10 in the univariate analysis were considered eligible to be included in the multivariate model and retained in the final model, according to a log-likelihood ratio test for goodness-of-fit.
Immunological survey
A survey was undertaken to detect HAV-IgM and HAV-IgG antibodies in saliva samples of children aged <5 years living in the same house of cases or controls, in order to identify any asymptomatic recent infections [Reference Parry4]. Saliva samples were also collected from cases and controls in the identified household. Eligible subjects were asked to provide a saliva sample collected through sterile swabs (ORACOL® – Saliva Collection System, Malvern Medical Developments Ltd, Worcester, UK). Specimens were tested at the Health Protection Agency, Bloodborne and Sexually Transmitted Virology Laboratory, Colindale, UK, for the presence of IgM and IgG antibodies.
Virological investigation on clinical samples
Sera from 33 acute HAV cases admitted to two major hospitals in Campania (one in the town of Caserta and the other in Naples) were collected and analysed for HAV at the ISS.
The RNA of HAV was extracted, purified and amplified by reverse transcription–polymerase chain reaction (RT–PCR) as previously reported [Reference De Paula5]. The region of genome that was amplified corresponded to the junction VP1/2A. Direct sequencing of purified PCR products was performed with an automated sequencer (ABI Prism 301, PerkinElmer, Applied Biosystems, Foster City, CA, USA). Genotyping was performed over sequences obtained using phylogenetic analysis according to the neighbour-joining method and MEGA 2 software.
Virological investigation on shellfish samples
As consumption of shellfish is often reported to be the most relevant risk factor in investigated outbreaks in southern Italy an extensive and systematic sampling from local retailers was performed. From March to July 2004, 72 samples of shellfish were collected from local outlets, fish markets, and breeding sites across the region and analysed for the presence of HAV. The detection of HAV-RNA was performed by nested RT–PCR and an integrated-procedure cell-culture RT–PCR was used to confirm the presence of infectious virus in the positive samples [Reference De Medici6]. The positive samples were further analysed in order to verify whether the detected viral RNA belonged to viruses able to infect cells in culture and show a cytopathic effect [Reference Richards7, Reference Lees8].
RESULTS
Surveillance and descriptive epidemiology
Between 1 January and 1 August 2004, 882 cases of HAV were identified through the EPOS system. According to the historical regional notifications of the same period for the preceding 8 years [2] the number of HAV cases in the first month of 2004 was slightly above the expected and increased steeply afterwards. The epidemic curve by week of onset showed various peaks between February and July (Fig. 1). Areas experiencing different attack rates showed a similar pattern in the epidemic curve, in particular with a peak at about 10–11 weeks of the calendar year.

Fig. 1. HAV cases by week of onset, 1 January to 1 August 2004, Campania, Italy.
The outbreak spread across the region but, as shown in the map, the area around the Gulf of Naples was the most affected (Fig. 2). The overall attack rate in the 7 months considered was 15·9/100 000 inhabitants, with wide variations between ASLs, ranging from a minimum of 2·3/100 000 in the ‘Salerno 3’ ASL in southern Campania, to a maximum of 54·3 cases/100 000 in the ‘Napoli 5’ ASL area, located on the high urbanized southern coast on the Gulf of Naples. The latter ASL reported 341 cases of HAV in this period: 56% (n=188) occurred in the period of the highest peak (between 16 February and 30 April 2004) and about half (n=88) were residents of Ercolano, the town with the highest attack rate in the region.
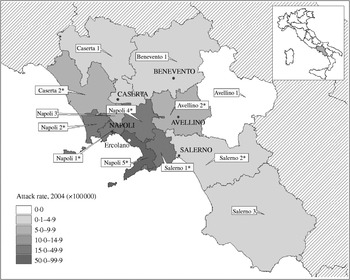
Fig. 2. Map of Campania and attack rate (cases/100 000 inhabitants) of HAV specific for local public health authorities (ASL), from 1 January to 1 August 2004. The case-control study was performed in Ercolano. * Denotes attack rate of the ASL was significantly higher (P<0·05) compared to the average for the same period in the previous four calendar years.
Overall, about 60% of cases of the region were male and the median age was 21 years (range 1–76). About 66% of cases occurred in the age groups 11–20 and 21–30 years, with attack rates respectively of 33·0/100 000 and 37·8/100 000 in these age groups.
The median age of cases reported from the ‘Napoli 5’ ASL was 17 years (range 1–62) and was significantly lower with respect to the median age of cases reported from the entire region (median age 21 years, range 1–76) (Wilcoxon Mann–Whitney test, P<0·0001). More specifically, in this ASL, the most affected ages were 6–10 years and 11–20 years, with incidence rates of about 145 cases/100 000 in both groups.
Information about exposure to shellfish consumption was recorded for 436 cases across the region: 81% (n=355) reported having eaten shellfish and 71% (n=253) raw shellfish at least once in the previous 2 months. Around 80% (708/882) of cases were admitted to hospital.
Case-control study
Of the 88 eligible cases who resided in Ercolano, 57 (65%) consented to an interview. About 19% of cases were in children aged <5 years and 50% aged 11–20 years. There were no statistical difference in gender and age group between participants and non-participants (χ2 test; P=0·26 and P=0·67 respectively). Of 128 potential controls identified from the population of patients assisted by the same general practitioners or paediatricians of the cases, 54 individuals did not have any exclusion criteria and were included in the study. Cases and controls were similar in terms of sex and age distribution respectively (χ2 test; P=0·31 and P=0·77).
According to the univariate analysis (Table), living in a household with >6 members was significantly associated with the infection (ORMH 2·8, 95% CI 1·1–7·2). Cases were more likely to have eaten shellfish than controls (ORMH 3·8, 95% CI 1·7–8·7). Storage condition of shellfish at time of purchase was strongly associated with onset of disease, with storage in seawater representing a significant risk factor (ORMH 4·5, 95% CI 1·8–11·0). Consumption of mussels (ORMH 2·8, 95% CI 1·0–8·0), razor-shell (ORMH 5·6, 95% CI 1·1–27·7), and the typical local seafood fasolari (ORMH 7·8, 95% CI 1·6–38·0) was significantly associated with infection. In addition, 33·3% of cases reported having eaten raw seafood compared to none of controls (ORMH cannot be calculated).
Table. Case-control study. Univariate (Mantel–Haesenzel odds ratio; ORMH) and multivariate (adjusted odds ratio; ORadj) analysis for the association of risk factors with hepatitis A
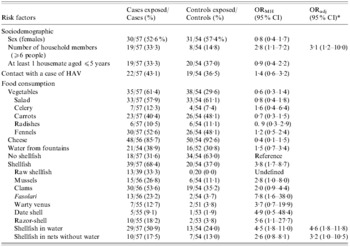
* Variables selected by the multivariate logistic model according to a log-likelihood ratio test for goodness-of-fit.
In the multivariate logistic regression model, the adjusted risk of HAV infection among individuals who had consumed seafood kept in sea-water at the time of purchase remained significant (ORadj 4·6, 95% CI 1·8–11·8), as did the risk for consumers who had eaten shellfish stored in dry nets (ORadj 3·2, 95% CI 1·0–10·5). Living in a household with >6 members remained significantly associated with infection even when adjusted for age and seafood consumption (ORadj 3·1, 95% CI 1·2–10·0).
Immunological survey
The acceptance rate for the saliva survey was limited. Specimens were obtained from six cases and their three housemates and by 29 controls and their 15 young housemates. All cases and two of the three housemates had HAV IgG antibodies. Three out of 29 controls were IgG positive, showing that nearly all controls were still susceptible to HAV infection at the time of the study. Only one housemate of the control group tested was HAV-IgG positive. No IgM-positive specimen was detected.
Virological investigation on clinical samples
A total of 33 serum samples were collected between 31 January and 15 June from patients residing in different areas of the region. Samples were collected after an average period of 9 days after the onset of symptoms. The median age of patients was 20 years (range 2–45 years) and 54·4% (n=33) of patients were males. Thirty-one patients (94%) were positive for the HAV genome, 29 (94%) of them were diagnosed with genotype 1B and two (6%) with genotype 1A.
Virological investigation on shellfish samples
HAV RNA was detected in 11 (15%) of 72 shellfish samples collected. Findings were all confirmed by cell-culture RT–PCR, revealing the presence of infectious HAV. Genotyping analysis of the amplification products showed that three strains belonged to genotype 1B (>99% similarity with the HM175 virus), and eight to genotype 1A (100% similarity with GBM virus) and in one sample both genotypes were present. The positive samples were collected from March to July, and 55% of the positive samples were collected in the area of ‘Napoli 5’ ASL, in which the highest attack rate was reported. Five positive samples originated from breeding farms in Campania, five from breeding farms in other Italian regions and one from a breeding farm in Turkey. Five positive samples were illegally stored in seawater at the time of collection.
DISCUSSION
This outbreak is remarkable because of the high number of affected subjects, the wide geographical area involved, and the extensive investigation performed that involved the use of enhanced surveillance tools, a case-control study and virological characterization of the agent identified from cases and the environment.
The epidemic curve included 882 cases of HAV occurring in Campania between 1 January and 1 August 2004 and showed several peaks of different intensity, spaced 4–5 weeks apart.
As observed in other outbreaks located in southern Italy [Reference Malfait9, Reference Lopalco10], this episode mostly affected younger age groups with attack rates in the 11–30 years group double that observed in the general population. Cases were particularly young in the ‘Napoli 5’ ASL, the area with the highest attack rate, where the median age (17 years, range 1–62) was significantly lower in respect to that of the entire region (median 21 years, range 1–76, P<0·001).
Findings from the case-control study strongly suggested that consumption of shellfish, particularly if stored in seawater prior to purchase and eaten raw, was the most likely source of infection. The epidemic curve showed a first peak at the beginning of February, compatible with the start of the circulation of HAV infection around Christmas, when consumption of seafood is traditionally more frequent in the affected area.
The subsequent cases could be partially attributable to persistent transmission supported by local factors: in fact, nearly all cases tested for the presence of HAV were found to be infected by similar viral strains belonging to the same 1B genotype. This genotype, considered relatively unusual in Italy, was also recently described in a cluster of 26 cases observed in another region in southern Italy [Reference Chironna11] but had never before been reported in a large community-wide outbreak. HAV of the 1B genotype was also detected in several shellfish samples collected from local retailers across the region. The frequency of genotypes of strains isolated from food samples was different from those of clinical samples, showing a prevalence of genotype 1A. Many studies have struggled to make a correlation between environmental and clinical samples: shellfish genotyping is in fact extremely difficult and could be affected by the co-presence of different viral strains in the same sample, due to the high capacity of these animals to filter and effectively concentrate all contaminants in seawater [Reference Costa-Mattioli12].
Both environmental and epidemiological investigations reported that the outbreak was not associated with a specific type of shellfish. Most of the positive seafood samples were originally bred either in other Italian regions besides Campania or abroad, suggesting that the shellfish were contaminated locally. This finding is also supported by results of the epidemiological study which attribute a significant role to the traditional, but illegal, habit of local seafood dealers of keeping shellfish alive in seawater probably taken from contaminated shores.
Our investigation could not clearly assess the roles of secondary transmission and of household transmission from asymptomatic children. In fact, the case-control study found that having a contact with a case of HAV was not significantly associated with the disease. On the other hand, living with >5 household members was a risk factor even after adjusting for age and seafood consumption: the significance of this finding, however, is not clear if household transmission is not assumed. The role of silent infections in children aged <5 years also remains unclear, since no association was found to link this exposure and the disease. It should be highlighted that despite the fact that two out of three housemates of cases aged <5 years were HAV-IgG positive (vs. 1/15 in control housemates), the number of samples collected in the saliva survey is too small to draw any conclusions.
Of note, however, is the finding from the saliva survey showing that only 10% of the controls enrolled in the case-control study were HAV-IgG positive. This confirms that most of the controls recruited on the basis of a negative history of jaundice and HAV disease were actually susceptible to the infection and therefore suitable for the study.
Previous HAV outbreaks have occurred in southern Italy: the largest and most recent one lasted over 2 years and involved 5673 cases in 1996 and 5382 in 1997 in the region of Puglia, accounting respectively for annual incidence rates of 138 and 132 cases/100 000. According to the two case-control studies performed at that time, that outbreak was also caused by consumption of raw (or improperly cooked) shellfish, and was sustained over time through person-to-person transmission [Reference Lopalco10].
The major role played by shellfish consumption in HAV transmission in Italy is supported by data from the surveillance system for type-specific acute viral hepatitis (SEIEVA) [Reference Mele13]. Up to 69% of cases occurring in Italy between 1997 and 2004 reported shellfish consumption as the potential exposure. Contact with a case of jaundice, or living in the same household as a child attending day care, as well as travelling to high-medium endemic areas, all appeared to be less important sources of infection, respectively explaining 11%, 14% and 17% of cases in the specified period.
Shellfish is a common food in this part of Italy, where it is traditionally eaten raw or only slightly cooked. Routine vaccination of children is considered the most effective prevention strategy against HAV in endemic areas [14] and is recommended to interrupt person-to-person transmission of the infection, especially in household settings [Reference Sagliocca15]. However, if transmission is sustained by food habits such an intervention may not be fully effective, as the target population for immunization is more difficult to identify. The traditional habit by local retailers of storing shellfish in seawater is still common in the region and every effort should be made to discourage the consumption of raw shellfish, or shellfish not properly stored in dry nets.
During this outbreak, the development of an electronic web-based reporting system (EPOS) was useful for the rapid collection and sharing of relevant data on cases, as well as for assessment of risk in the different areas of the region. The system was developed specifically for this outbreak but could be a useful tool in the investigation of outbreaks due to agents other than HAV. In fact, efficient surveillance tools such as this should be more widely used to identify recent cases and introduce control measures in a timely manner.
ACKNOWLEDGEMENTS
Such an extensive investigation would have not been possible without the efforts of a large number of collaborators. The authors thank all the local public health and hospital staff (Maria Antonietta Ferrara, Paolo D'Argenio, Crescenzo Bove, Angelo D'Argenzio, Andrea Simonetti, Antonino Parlato, Filomena Peluso, Raffaele Palombino, Anna Luisa Caiazzo, Maria Grazia Panico, Giuseppe Di Fluri, Francesco Faella, Evangelista Sagnelli) in particular Dr Renato Pizzuti, Head of Regional Epidemiological Observatory and all the Heads of Prevention Departments of the local health units of Campania. Thanks also to Dr John Parry of the Health Protection Agency London for immunological investigation on saliva samples collected, and to Dr Antonietta Filia for her kind and valuable language review and suggestions.
DECLARATION OF INTEREST
None.





