Early-onset dementias are a fascinating group of disorders that present challenges in diagnosis, management and service provision. It has become accepted practice that ‘early-onset’ refers to dementias that occur before the age of 65 years.Footnote †
The ICD–10 definition of dementia is ‘a syndrome due to disease of the brain, usually of a chronic or progressive nature, in which there is disturbance of multiple higher cortical functions, including memory, thinking, orientation, comprehension, calculation, learning capacity, language and judgement’ (World Health Organization 1992: p. 45). ICD–10 also states that the primary requirement for a diagnosis of dementia is evidence of a decline in both memory and thinking sufficient to impair activities of daily living. However, it should be remembered that dementias, particularly in younger people, often present with symptoms other than memory decline as the predominant feature.
Differences between young-onset dementias and dementias in later life
Early-onset dementia is less common than dementia in later life. The differential diagnosis is broader and younger people are more likely to have a rarer form of dementia. Both the diagnosis and the symptoms of the condition can have a devastating impact on patients, carers and their families. Younger people are likely to have different needs and commitments than older people. They are more likely to be in work at the time of diagnosis, have dependent family or children and have heavy financial commitments (e.g. a mortgage). They are also likely to be physically fit and active, and aware of their problems. As a consequence, they are likely to feel distressed and frustrated. Yet access to information and support can be difficult and limited. Spouses of patients with early-onset dementia have many concerns, especially worries about financial and health matters, a feeling of lack of support and social isolation (Reference Kaiser and PanegyresKaiser 2006).
Prevalence of early-onset dementia
In 1998, an estimated 18 500 people had early-onset dementia in the UK (Reference HarveyHarvey 1998). The disease was more common in men than in women: in people between 30 and 64 years of age there were 78.2 cases per 100 000 men and 56.4 cases per 100 000 women. Incidence increased sharply with age, with two-thirds of patients aged 55 and over (Fig. 1). About a decade later, Reference Knapp and PrinceKnapp & Prince (2007) reported a comparable prevalence, with of early-onset dementias accounting for 2.2% of all people with dementia in the UK. They calculated that there are at least 15 034 people with early-onset dementia. However, this estimate is based on the number of referrals to services, and the true figure may be up to three times higher.

FIG 1 Prevalence of dementia by age (data from Reference HarveyHarvey et al 1998).
Diagnosis of early-onset dementia
Distribution of diagnosis
Comparison of Fig. 2 (a) and (b) shows that the distribution of diagnoses of dementia differs dramatically between older and younger patients. Alzheimer's disease is the most common cause of dementia in both groups, accounting for almost two-thirds of cases in older people, but only a third of cases in younger people. Frontotemporal dementia occurs much more commonly in younger than in older populations. Rarer causes of dementia also occur with greater frequency in the younger population.

FIG 2 (a) Distribution of diagnoses in young-onset dementia (data from Reference Sampson, Warren and RossorSampson et al 2004); (b) distribution of diagnoses of dementia occurring in later life (redrawn from Reference Knapp and PrinceKnapp & Prince 2007, with kind permission of the Alzheimer's Society).
Clinical approach to assessment
Assessment requires input from the multidisciplinary team. Early diagnosis is essential for a number of reasons: so that early treatment can be provided; to enable the person and family to come to terms with the illness; to allow early access to the correct support; and so that appropriate plans for the future can be made.
Clinically, the focus is on identifying early-onset dementia accurately and on differentiating it from illnesses that can also cause cognitive impairment and mimic dementia. The differential diagnosis of early-onset dementia includes various neurological illnesses, psychiatric illnesses, traumatic brain injury and drug misuse (Box 1).
BOX 1 Differential diagnosis of early-onset dementia
-
• Delirium
-
• Amnesic syndromes:
Korsakoff's syndrome
anoxic brain damage
herpes simplex encephalitis
-
• Mild cognitive impairment
-
• Pseudodementia:
depression
dissociative
-
• Traumatic brain injury
-
• Neurological illness (e.g. normal-pressure hydrocephalus, stroke, encephalitis, vasculitis, multiple sclerosis)
-
• General medical conditions (e.g. endocrine, B12 deficiency, systemic lupus erythematosus, sarcoidosis)
-
• Drugs (e.g. alcohol, benzodiazepines)
History
History should be obtained from the patient and collateral information should be gathered from an informant such as a family member. An informant's account is often the key to an accurate diagnosis. Particular areas to concentrate on during history-taking are onset and evolution of symptoms, pattern of deficits, fluctuations in mental state, past or present neurological symptoms, current psychiatric symptoms, past psychiatric symptoms, medical history, family history, use of drugs and alcohol, and risk assessment.
Cognitive testing
Detailed neuropsychological testing is an important component of the diagnostic process. It can reveal the precise nature and location of the cognitive deficits. Neuropsychological testing can also help in teasing out whether there has been any progression of dementia. Using a series of psychological tests, the neuropsychologist establishes the cognitive abilities of the person and compares them with the person's estimated best levels and with those for an average person of the same age. Neuropsychological assessment can also identify where a person's abilities are preserved so that they can be maximised.
Basic bedside testing is also helpful in confirming the nature, degree and location of deficits. The Mini Mental State Examination (MMSE) (Reference Folstein, Folstein and McHughFolstein 1975) is the most widely used screening test for cognitive impairment. It is short, easy to use, has high interrater reliability and is useful for monitoring illness progression. However, it is insensitive to frontal lobe disorders, does not detect focal cognitive deficits and is strongly influenced by previous IQ and education. The Addenbrooke's Cognitive Examination (Reference Mathuranath, Nestor and BerriosMathuranath 2000) is a more comprehensive screening instrument that also tests frontal lobe functioning. It contains a 100-point test battery that assesses six cognitive domains and takes about 15–20 min to complete. It includes the MMSE.
Bedside tests of frontal lobe functioning are described in Table 1.
TABLE 1 Bedside tests of frontal lobe function

| Test | Example | Findings indicative of impairment |
|---|---|---|
| Verbal fluency | ‘Name as many words as you can beginning with the letter “F”, any words except names of people or places’ | Fewer than 13 words a minute |
| Similarities | ‘In what way are a banana and an orange alike?’ | Inability to identify the similarity |
| Cognitive estimates | ‘How tall is the tallest man?’ ‘How fast can a horse gallop?’ |
Estimates will be inaccurate and often wildly wrong |
| Proverb interpretation | ‘What do you understand by the saying “too many cooks spoil the broth”?’ | Concrete (literal) not figurative responses |
| Luria hand sequence | Fist–edge–palm (the patient is asked to tap the table with a fist, open palm, and side of open hand and to repeat the sequence as quickly as possible) | Inability to follow the sequence; perseveration |
| Conflicting instructions | ‘Tap twice when I tap once, and tap once when I tap twice’ | Copying the tapping pattern of examiner rather than doing opposite despite clear instruction |
| Go/No-Go test | ‘Tap once when I tap once. Do not tap when I tap twice’ | Tapping twice when the examiner taps twice |
Physical examination
Physical examination is important both in reaching the diagnosis of the subtype of dementia and in identifying any treatable comorbidity. The physical examination is often normal in the early stages of dementia but the presence of certain symptoms and signs will arouse suspicion of a primary neurological disorder. These include sudden onset of symptoms, focal neurological findings early in the illness, visual hallucinations, incontinence, ataxia and early seizures.
In Alzheimer's disease, patients are typically physically well with no neurological signs in the early stages. Later, there may be akinesia and rigidity. In frontotemporal dementia, neurological signs are also usually absent in the early stages. In later stages of the disorder there may be evidence of primitive reflexes, and with further disease progression there may be akinesia and rigidity. Spontaneous features of Parkinsonism are one of the core features of dementia with Lewy bodies. Huntington's disease is evidenced by the presence of chorea and other pyramidal symptoms. In Creutzfeldt–Jacob disease, myoclonus and other extrapyramidal symptoms are often present.
Further investigations
When arranging for further investigations the priorities are to identify treatable causes of dementia and to guide accurate diagnosis.
We suggest conducting the following investigations routinely: full blood count; erythrocyte sedimentation rate; C-reactive protein; renal, liver and thyroid function; syphilis serology; vitamin B12 and folate levels; bone profile; lipid profile; glucose; structural neuroimaging by computed tomography (CT) or magnetic resonance imaging (MRI). Electrocardiogram or chest X-ray should be carried out as determined by the clinical presentation.
Structural neuroimaging should be carried out to exclude other pathologies and to help establish the subtype of dementia. Magnetic resonance imaging is preferable, as CT may not show focal atrophy well. Functional imaging may be helpful in the detection of focal brain dysfunction. Hexamethylpropyleneamine oxime (HMPAO) single-photon emission computed tomography (SPECT) could help to differentiate the subtype of dementia. Dopaminergic iodine-123-radiolabelled SPECT can be used to confirm suspected Lewy body dementia.
In atypical presentations, particularly when dementia is rapidly progressive with features of encephalopathy or focal neurological signs, patients should be referred for neurological opinion; lumbar puncture may be required. Additional investigations such as HIV testing and CD4 count may be considered, depending on the clinical presentation.
Causes of early-onset dementia
The clinical presentations of the following diseases generally do not differ between older and younger populations.
Alzheimer's disease
Alzheimer's disease accounts for almost a third of early-onset dementia cases. The typical presentation of Alzheimer's disease includes progressive episodic (day-to-day) memory loss and visuospatial and perceptual deficits, but well-preserved language and social functioning. The posterior cortical variant of the disease, with prominent impairment of parietal lobe functions, is more common in younger people.
Alzheimer's disease is more common in women than men. Prevalence increases with increasing age. The average duration of illness is 8 years. Alzheimer's disease mostly occurs as a sporadic disorder, even in the younger population. However, inherited forms are more likely in the younger population.
Inheritance is autosomal dominant. There are many reported genetic mutations, but the three most common ones are in the presenilin 1 gene on chromosome 14, β-amyloid precursor protein on chromosome 21 and the presenilin 2 gene on chromosome 1 (Reference Schott, Fox and RossorSchott 2002).
The preferred diagnostic criteria for Alzheimer's disease are those specified by the NINCDS–ADRDA (National Institute of Neurological and Communicative Disorders and Stroke & Alzheimer's Disease and Related Disorders Association) (Reference McKhann, Drachman and FolsteinMcKhann 1984) (Box 2).
BOX 2 NINCDS–ADRDA criteria for the clinical diagnosis of probable Alzheimer's disease
-
• Dementia established by clinical examination and documented by the MMSE, Blessed Dementia Scale or some similar examination, and confirmed by neuropsychological tests
-
• Deficits in two or more areas of cognition
-
• Progressive worsening of memory and other cognitive functions
-
• No disturbance of consciousness
-
• Onset between ages 40 and 90, most often after age 65
-
• Absence of systemic disorders or other brain diseases that in and of themselves could account for the progressive deficits in memory and cognition
The MRI scan in Fig. 3 illustrates the typical findings in Alzheimer's disease, namely disproportionate bilateral atrophy of the hippocampi (indicated by the white arrow).

FIG 3 MRI scan of Alzheimer's disease. Reproduced with kind permission of Professor Nick Fox, Institute of Neurology, London.
Vascular dementia
Vascular dementia is the second most common cause of dementia in younger people. Diagnosis is based on the clinical picture, identification of risk factors and brain imaging. The clinical presentation is variable and depends on the site and extent of the lesions. The preferred diagnostic criteria for vascular dementia are the NINDS–AIREN (National Institute of Neurological Disorders and Stroke & Association Internationale pour le Recherche et l'Enseignement en Neurosciences) criteria (Reference Roman, Tatemichi and ErkinjunttiRoman 1993), which state that for a clinical diagnosis of probable vascular dementia, both dementia and cerebrovascular disease must be present and there must be a relationship between the two disorders.
Vascular dementia has several syndromes:
-
• multiple cortical infarcts leading to a stepwise deterioration of cognitive functions
-
• small vessel disease leading to a more insidious decline of cognitive functioning
-
• strategic infarcts – small cryptic strokes that cause cognitive impairment
-
• CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy).Footnote †
CADASIL, an uncommon cause of young-onset subcortical strokes and dementia, is caused by mutations of the Notch3 gene on chromosome 19. Magnetic resonance imaging typically reveals diffuse white matter lesions of the cerebral hemispheres, especially the anterior temporal lobes and external capsules (Fig. 4). Electron microscopy of skin biopsies may reveal characteristic granular osmophilic material.

FIG 4 MRI scan of CADASIL.
Frontotemporal dementia
Frontotemporal dementias occur more frequently in the younger population. Men are more often affected than women. The usual age at onset is between 45 and 65 years. The average duration of illness is 8 years. There is a positive family history in up to 50% of patients.
There is an association between frontotemporal dementia and motor neuron disease. The rate of dementia in motor neuron disease is much higher than expected, and indeed a significant minority of patients with frontotemporal dementia develop features of the disease. Up to 10% of patients with motor neuron disease show features of dementia. These individuals usually have an aggressive course of illness.
The hallmark of frontotemporal dementia is the early alteration in personality and social conduct, with relative preservation of memory, perception, visuospatial skills and praxis. Although presentations are variable and subtle, they are often characterised by behavioural disturbance, personality change, reduced motivation, reduced empathy, impaired planning and judgement, and speech and language problems. Box 3 shows clinical criteria for frontotemporal dementia.
BOX 3 Clinical criteria for frontotemporal dementia
-
• The development of behavioural or cognitive deficits manifested by either:
-
• early and progressive change in personality, characterised by difficulty in modulating behaviour, often resulting in inappropriate responses or activities; or
-
• early and progressive change in language, characterised by problems with expression of language or severe naming difficulty and problems with word meaning.
-
-
• The deficits outlined in 1(a) or 1(b) cause significant impairment in social or occupational functioning and represent a significant decline from a previous level of functioning
-
• The course is characterised by a gradual onset and continuing decline in function
-
• The deficits outlined in 1(a) or 1(b) are not due to other nervous system conditions (e.g. cerebrovascular disease), systemic conditions (e.g. hypothyroidism), or substance-induced conditions
-
• The deficits do not occur exclusively during a delirium
-
• The disturbance is not better accounted for by a psychiatric diagnosis (e.g. depression)
As the disease progresses, symptoms of frontal lobe dysfunction may also become apparent. These include behavioural rigidity, disinhibition, loss of social skills and graces, fatuousness, emotional lability, impulsivity, executive dysfunction, reduced verbal fluency, hyperorality, and motor and verbal perseveration.
Frontotemporal dementia can take a number of forms (Fig. 5), which we describe below.
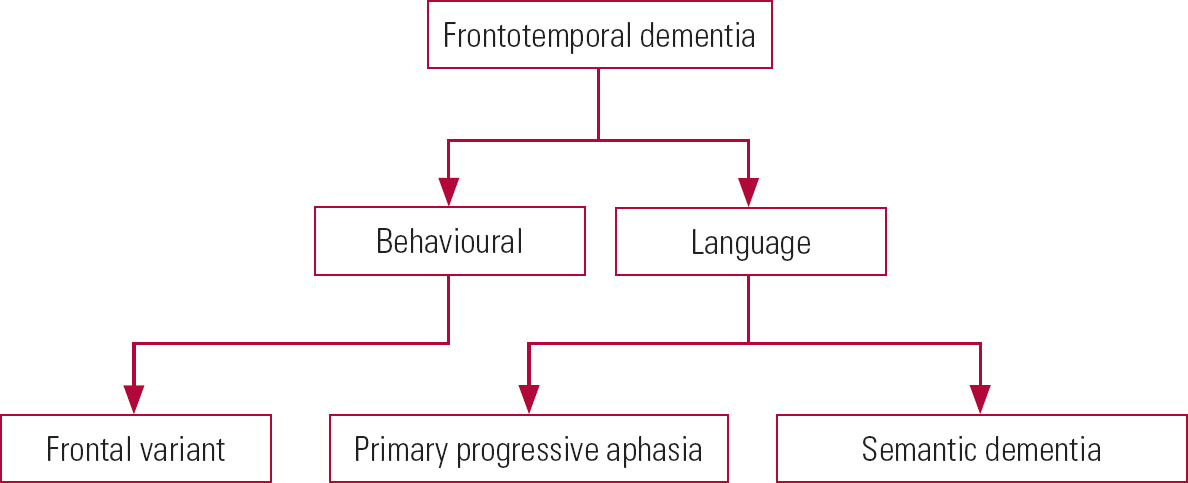
FIG 5 Variants of frontotemporal dementia.
Behavioural form
The frontal variant primarily affects frontal lobes and affected patients typically show symptoms of frontal lobe dysfunction (Box 4) with relative preservation of day-to-day memory. A typical MRI scan of frontal lobe dementia is seen in Fig. 6.

FIG 6 MRI scan of frontal dementia, showing bifrontal atrophy and relative sparing of the temporal lobes. Reproduced with kind permission of Professor Nick Fox, Institute of Neurology, London.
BOX 4 Symptoms of frontal lobe dysfunction
-
• Change in social conduct
-
• Change in behaviour
-
• Behaviour inappropriate to the social situation
-
• Lack of inhibitions
-
• Impulsivity
-
• Poor judgement
-
• Inappropriate sexual behaviour
-
• Repetitive or compulsive behaviours
-
• Hyperorality
-
• Self-neglect
-
• Lack of insight
Language forms
Primary progressive aphasia
Patients have a progressive decline in their language with a relative absence of other cognitive deficits. Speech is non-fluent and effortful. Speech output becomes increasingly difficult and in the later stages the patient may become mute. Behavioural changes may occur later in the disease. There is predominant atrophy of the left perisylvian region.
Semantic dementia
Patients have a variety of language difficulties, including naming difficulties, impaired understanding of word meaning, and use of substitute words. However, speech is fluent and there is preservation of other cognitive domains. Behavioural difficulties may emerge as the disease progresses. Features of semantic dementia include temporal lobe atrophy (of the left more than the right). The characteristic feature is that atrophy of the anterior temporal lobe is more pronounced than that of the posterior temporal lobe (Fig. 7).

FIG 7 MRI scan of semantic dementia, showing disproportionate asymmetric atrophy of the anterior left temporal lobe. Reproduced with kind permission of Professor Nick Fox, Institute of Neurology, London.
Dementia with Lewy bodies
The core features of Lewy body dementia include progressive cognitive decline, fluctuating cognition, the presence of Parkinsonian symptoms, and visual hallucinations that are typically recurrent, well formed and detailed. Other features that are highly suggestive of dementia with Lewy bodies include rapid eye movement (REM) sleep behaviour disorder, severe antipsychotic sensitivity and low dopamine transporter uptake in the basal ganglia demonstrated by SPECT or positron emission tomography imaging (Reference McKeith, Dickson and LoweMcKeith 2005).
A positive history of severe antipsychotic sensitivity suggests a diagnosis of dementia with Lewy bodies. Lewy body dementia should be diagnosed when dementia occurs before or concurrently with Parkinsonism. Dementia that occurs in the context of well-established Parkinson's disease is best described as Parkinson's disease dementia.
People with Lewy body dementia are extremely sensitive to the side-effects of typical and atypical antipsychotics. Approximately 50% of patients with this type of dementia will have an adverse reaction to the administration of antipsychotics.
Alcohol-related dementia
Heavy, prolonged alcohol use can cause damage to the limbic structures and the frontal lobes, leading to memory and executive impairments. Autobiographical memory is often affected and confabulation can occur. The memory loss is often static although may improve following a period of abstinence. Imaging may be non-specific or it may show generalised cortical atrophy with a frontal preponderance.
Huntington's disease
The characteristic triad of clinical features in Huntington's disease are motor disorder, cognitive disorder and emotional disorder. However, there is variation between patients in the time of onset, specific symptoms and the progression of illness. Huntington's disease is an autosomal dominant disorder with complete penetrance caused by expansion of the CAG trinucleotide repeat. Onset is generally in middle life and the typical duration of illness is 15–20 years. Diagnostic and predictive testing is available.
Neuroimaging shows bilateral atrophy of the head of the caudate nucleus and atrophy of the putamen and globus pallidus. Single-photon emission computed tomography scans show cerebral hypoperfusion in the basal ganglia even before atrophy is evident on MRI scan.
Prion diseases
Creutzfeldt–Jakob disease (CJD) tends to occur in middle life. It is rapidly progressive and often fatal within 6 months. Prodromal insomnia, malaise and depression are common. Myoclonus becomes prominent as the disease progresses. Additional symptoms include seizures, cerebellar ataxia, cortical blindness and extrapyramidal symptoms. Electroencephalogram (EEG) is grossly disturbed and shows characteristic periodic triphasic wave complexes. Computed tomography imaging is often normal or shows non-specific atrophy. Magnetic resonance imaging may show high signal changes in the putamen and caudate head and cortical hypersensitivity on fluid-attenuated inversion recovery (FLAIR) sequences. Cerebral spinal fluid proteins including 14-3-3 protein are elevated in both sporadic and new variant CJD (the human form of bovine spongiform encephalopathy).
New variant CJD commonly affects younger people. The average age at onset is 26 years and the average duration of illness is 13 months. The earliest symptoms are often psychiatric and include depression, anxiety and agitation. There are no distinctive changes on EEG. Magnetic resonance imaging shows increased signal in the pulvinar. Prion protein immunostaining is positive in lymphoid tissues. The diagnosis can also be made from a tonsillar biopsy.
Dementia in HIV infection and AIDS
Early features of HIV dementia include changes in behaviour (particularly social withdrawal and impaired activities of daily living), mood (apathy and depression) and cognition. Patients complain of forgetfulness, slowed thinking and poor concentration. Neuropsychological tests reveal abnormalities in spontaneity, motor speed and visual memory, although language is often well preserved. Neurological signs include poor coordination, loss of balance and tremor; later in the illness, hyperreflexia, dysdiadochokinesia, ocular pursuit abnormalities, myoclonus and frontal lobe signs may be observed. As the illness progresses there is a global deterioration in cognitive functioning and incontinence, with seizures, myoclonus and mutism.
Dementia occurs in 7% of patients who are HIV positive and 25% of patients with late-stage AIDS (Reference MitchellMitchell 2004). The severity of the dementia correlates with brain and cerebral spinal fluid levels of HIV-1 RNA. HIV can affect any brain region but the greatest concentrations of the virus are found in the subcortical grey structures. Pathologically, there is neuronal loss, small vessel disease and vacuolar white matter lesions.
Mild cognitive impairments in HIV and AIDS occur much more commonly than full-blown dementias. Such impairments include deficits in attending, slowed information processing and reduced ability to retain new information. Subtle changes are seen in 25–35% of people who are HIV positive under the age of 40, and up to 90% of people with AIDS who are aged over 50 (Reference MitchellMitchell 2004). Although these deficits are often minor, they are not insignificant as they are associated with a negative influence on survival, social problems, unemployment and difficulty in driving.
Management of early-onset dementia
Accurate diagnosis of early-onset dementia based on comprehensive multidisciplinary assessment and full investigation forms the basis for management. It is important to identify treatable causes of dementia and accurately diagnose underlying neurological or psychiatric conditions. Longer-term support is vital to help manage the cognitive, neuropsychiatric and behavioural symptoms that often accompany these disorders.
Management should encompass pharmacological and non-pharmacological strategies. Non-pharmacological management strategies such as psychoeducation, cognitive strategies such as mental exercise, physical therapy and dietary treatment, and drug therapies may be beneficial in reducing the impact or slowing down the progression of the disease. Medications may be needed for delusions, hallucinations, and serious distress or danger from behaviour disturbance or symptoms of depression. Support packages should include appropriate day, respite and intensive home care if required. Support for families and carers is also required especially in cases where there may be dependents such as children.
Pharmacological interventions
Pharmacological management strategies include anti-dementia drugs and other psychotropic drugs. It is important to treat any comorbid medical or psychiatric illnesses. The underlying degenerative disorder should also be treated if appropriate. In addition, vascular risk factors should be addressed.
Anti-dementia drugs that are currently licensed in the UK for patients of any age are donepezil, rivastigmine, galantamine and memantine (Table 2). The available evidence base confirms that the acetylcholinesterase inhibitors are effective across the spectrum of Alzheimer's disease (mild, moderate and severe disease) and they also lead to improvements in non-cognitive symptoms (Reference Burns, O'Brien and AmesBurns 2005). Results of trials in other types of dementia (vascular dementia, dementia with Lewy bodies and Parkinson's disease dementia) are also positive, but the inhibitors are not currently licensed in the UK for treatment of all of these conditions. Acetylcholinesterase inhibitors should be prescribed with caution in patients with sick sinus syndrome or other supraventricular conduction abnormalities, those who are susceptible to peptic ulcers, and in asthma or chronic obstructive pulmonary disease. Memantine, licensed in the UK for use in moderate to severe Alzheimer's disease, is an N-methyl-d-asparate receptor antagonist, which may reduce glutamate-mediated neuronal excitotoxicity.
TABLE 2 Licensed uses of the anti-dementia drugs in the UK

| Drug | Licensed use |
|---|---|
| Donepezil | Mild to moderate dementia in Alzheimer's disease |
| Rivastigmine | Mild to moderate dementia in Alzheimer's disease or Parkinson's disease |
| Galantamine | Mild to moderate dementia in Alzheimer's disease |
| Memantine | Moderate to severe dementia in Alzheimer's disease |
The National Institute for Health and Clinical Excellence (NICE) has produced guidelines on the use of anti-dementia agents (National Institute for Health and Clinical Excellence 2007) (Box 5).
BOX 5 NICE guidelines for Alzheimer's disease
Donepezil, galantamine and rivastigmine are recommended for the adjunctive treatment of moderate Alzheimer's disease in those whose MMSE score is 10–20, under the following conditions:
-
• Alzheimer's disease must be diagnosed in a specialist clinic. The clinic should also assess cognitive, global and behavioural functioning, activities of daily living and likelihood of adherence
-
• treatment should be initiated by specialists, but continued by the general practitioner under a shared care protocol
-
• the carers' views should be sought before and during the treatment
-
• the patient should be reassessed every 6 months
-
• the drug treatment should only continue if the MMSE remains at or above 10 and if it is considered to have a beneficial effect on functioning and behaviour.
NICE does not recommend memantine for moderately severe to severe Alzheimer's disease except as part of clinical trials.
(National Institute for Health and Clinical Excellence 2007)
Antipsychotic agents
Symptoms that may respond to antipsychotic medication include physical aggression and psychotic symptoms. They should be used for the shortest time possible, at the lowest dose possible.
Antidepressants
Depression occurs frequently in early-onset dementias. Symptoms of mood disturbance should always be asked about. Selective serotonin reuptake inhibitors are used as the first-line treatment because of their favourable side-effect profile and because an antidepressant with strong anticholinergic effects may worsen cognition.
Psychological and non-pharmacological interventions
Symptoms that may respond to non-pharmacological interventions include mild depressive symptoms, wandering and repetitive questioning. The ideal environment for someone with dementia is one that is constant, familiar and non-stressful. Other therapies that have been shown to help in the management of behavioural and psychological symptoms in dementia include aromatherapy (Reference Holmes and BallardHolmes 2004), music therapy, recreation, bright light therapy, behaviour therapy, reality orientation, reminiscence therapy and art therapy (Reference Douglas, James and BallardDouglas 2004; Reference WoodsWoods 2004).
Services for people with early-onset dementia
The ongoing support and care of people with early-onset dementia is complex and requires input from a multidisciplinary team. Patients and their families also benefit from the support offered by voluntary organisations such as those listed in Box 6. However, there are few specific statutory services in the UK and there is limited awareness and understanding of people who develop dementia at an early age, thus making it difficult to access support. In the absence of specific services, 38 referral pathways and 4 separate gateways to specialist investigation and care were identified in the city of Leeds in the UK (Reference Williams, Dearden and CameronWilliams 2001). Another UK study reported 11 different referral pathways (Reference FuhrmannFuhrmann 1997). Patients can be referred to two to five different consultants sequentially, leading to significant delays in diagnosis (Reference Williams, Dearden and CameronWilliams 2001). This is clearly unsatisfactory service provision and causes high rates of patient and carer dissatisfaction.
BOX 6 Organisations and further reading
Organisations
-
• Alzheimer's Society (www.alzheimers.org.uk)
-
• Alzheimer's Society's information officer for younger people with dementia (www.alzheimers.org.uk/Younger_People_with_Dementia/index.htm)
-
• Pick's Disease Support Group (www.pdsg.org.uk)
-
• CJD Support Network (www.cjdsupport.net)
-
• The Lewy Body Society (www.lewybody.org)
Further reading
Hodges J (ed) (2001) Early-Onset Dementia: A Multidisciplinary Approach. Oxford University Press.
Jefferies K, Agrawal N (2006) Early onset dementias. CPD online, Royal College of Psychiatrists (www.psychiatrycpd.co.uk/learningmodules/earlyonsetdementias.aspx).
Murray M, Baldwin RC (eds) (2003) Younger People with Dementia: A Multidisciplinary Approach. Taylor & Francis.
Younger people with dementia have different needs than older people with the disease. General dementia services are often inappropriate for use by younger people and may not be able to meet their needs. Age can often be a barrier to accessing services, so in some parts of the UK younger people may not be eligible to receive care from general dementia services. We believe the best solution is the development of regional specialist early-onset dementia services in every area and for services to be flexible; some people over the age of 65 may be better suited to services for younger people, whereas some people under the age of 65 may be better catered for by the general dementia services.
National recommendations
Various recent publications have ratified the need for specialist services for people with early-onset dementia in the UK. The National Institute for Health and Clinical Excellence & Social Care Institute for Excellence (2006) joint guidelines state that specialist multidisciplinary services allied to existing dementia services should be developed for the assessment, diagnosis and care of younger people with dementia. The Royal College of Psychiatrists & Alzheimer's Society report (2006) states that there should be a named person responsible for planning services for younger people with dementia and a named consultant to provide clinical input. They suggest that for populations of half a million or more a specialist multidisciplinary team is justified.
To adequately address the needs of these patients and their carers there needs to be cooperation and collaboration across all the relevant statutory and voluntary services. Carers regularly report that they would like to see better coordination of services and to receive welfare advice, support and respite care. However, it is often unclear how services should be accessed, with subsequent delay in diagnosis, treatment and provision of appropriate support.
MCQs
-
1 The prevalence of early-onset dementia in the UK is:
-
a 5000–10 000
-
b 10 000–15 000
-
c 15 000–20 000
-
d 20 000–25 000
-
e 25 000–30 000.
-
-
2 The most common cause of dementia in people under the age of 65 years is:
-
a alcohol-related dementia
-
b Alzheimer's disease
-
c dementia with Lewy bodies
-
d frontotemporal dementia
-
e vascular dementia.
-
-
3 Among people with dementia, more younger people than older people have:
-
a Alzheimer's disease
-
b dementia with Lewy bodies
-
c frontotemporal dementia
-
d mixed dementia
-
e vascular dementia.
-
-
4 Of people with frontotemporal dementia, the proportion with a positive family history of the disease is:
-
a 0%
-
b 25%
-
c 50%
-
d 75%
-
e 100%.
-
-
5 The following is not a test of frontal lobe function:
-
a cognitive estimates
-
b Luria sequence
-
c proverb interpretation
-
d serial sevens
-
e verbal fluency.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | f | a | f | a | f | a | f | a | f |
| b | f | b | t | b | f | b | f | b | f |
| c | t | c | f | c | t | c | t | c | f |
| d | f | d | f | d | f | d | f | d | t |
| e | f | e | f | e | f | e | f | e | f |












eLetters
No eLetters have been published for this article.