Introduction
Photochemical reactions in snow have recently attracted strong interest (e.g. Reference Dominé and ShepsonDominé and Shepson, 2002, and references therein). Several field studies at different locations in polar and mid-latitude regions have demonstrated that reactive nitrogen oxides are produced in the surface layers of irradiated snow covers and are subsequently emitted to the atmosphere (Reference Honrath, Peterson, Guo, Dibb, Shepson and CampbellHonrath and others, 1999, Reference Honrath, Guo, Peterson, Dziobak, Dibb and Arsenault2000a, Reference Honrath2002; Reference Jones, Weller, Wolff and JacobiJones and others, 2000, Reference Jones2001; Reference RidleyRidley and others, 2000; Reference DavisDavis and others, 2001; Reference Beine, Honrath, Dominé, Simpson and FuentesBeine and others, 2002). Moreover, other reactive compounds like hydrogen peroxide (H2O2) and formaldehyde (HCHO), which are present in the snow and the atmosphere, can effectively be transferred in both directions between both compartments (Reference Hutterli, McConnell, Stewart, Jacobi and BalesHutterli and others, 2001, Reference Hutterli, Bales, McConnell and Stewart2002; Reference JacobiJacobi and others, 2002). Such processes modify the chemical composition and the oxidation capacity of the boundary layer over snow-covered regions (Reference RidleyRidley and others, 2000; Reference ChenChen and others, 2001; Reference YangYang and others, 2002).
In addition, H2O2, HCHO and nitrate concentrations in ancient atmospheres would constitute important constraints for investigations of the chronological development of the atmospheric composition. Thus, a better understanding of the transfer processes of reactive species is needed to calculate atmospheric concentrations from profiles in surface snow, firn and ice cores. Transfer models for H2O2 and HCHO including physical processes in the atmosphere and the surface layers of the snow have so far been developed (Reference McConnell, Bales, Stewart, Thompson, Albert and RamosMcConnell and others, 1998; Reference Hutterli, Röthlisberger and BalesHutterli and others, 1999, Reference Hutterli, Bales, McConnell and Stewart2002). However, these models potentially suffer from the lack of consideration of photochemical processes taking place in irradiated snow.
Recent laboratory experiments have shown that the photolysis of nitrate incorporated in snow is responsible for producing nitrogen oxides (Reference Honrath, Peterson, Dziobak, Dibb, Arsenault and GreenHonrath and others, 2000b; Reference Dubowski, Colussi and HoffmannDubowski and others, 2001, Reference Dubowski, Colussi, Boxe and Hoffmann2002; Reference Cotter, Jones, Wolff and BaugitteCotter and others, 2003). Other species absorbing solar radiation in the troposphere are compounds like ozone, nitrate radical, organic peroxides and aldehydes (e.g. Reference Finlayson-Pitts and PittsFinlayson-Pitts and Pitts, 2000). However, among these species only hydrogen peroxide (H2O2) and formaldehyde (HCHO) are incorporated in natural snow in considerable amounts. Indeed, Reference Haan, Zuo, Gros and BrenninkmeijerHaan and others (2001) suggested that the HCHO photolysis could be responsible for observed CO production in sunlit snow. Therefore, we investigated whether H2O2 and HCHO can undergo photochemical reactions in snow. Consequently, we performed experiments with laboratory-made snow samples containing only H2O2 or HCHO to prevent further photochemical reactions, which possibly would lead to the concurrent production of both compounds.
Experimental
Solutions for the generation of artificial snow were prepared by adding 30% H2O2 (Merck, Darmstadt, Germany) or 37% HCHO (Merck) solutions to Milli-Q water. The diluted solutions were transferred into a stainless-steel tank. The tank was pressurized to 2–3×105 Pa and the liquid was forced through a brass nozzle producing a fine spray, which was collected in a wide-mouth Dewar flask filled with liquid nitrogen. The ice produced in this way was transferred into a walk-in cold room at –20˚C, where it was collected on aluminum foil. After evaporation of the remaining liquid nitrogen, small amounts of the ice were ground with an electric mill and passed through a stainless-steel sieve with a mesh size of 0.5 mm. Following sieving, the snow was stored overnight in 1 L Schott bottles covered with aluminum foil and sealed with zero-air traps, which were filled with Hopcalite (Aero-Laser, Garmisch-Partenkirchen, Germany) to allow further degassing of nitrogen and to prevent the condensation of H2O2 or HCHO on the snow.
The experimental set-up for the photolysis experiments is shown in Figure 1. A 1000W mercury-arc lamp (Oriel Instruments, Stratford, CT) installed in the cold room was used as a light source. The emission intensity was regulated by the output of the lamp’s power supply, which was set to 440 W. A water filter consisting of a cuvette filled with Milli-Q water and equipped with quartz windows was directly coupled to the output of the lamp housing condenser. The water absorbed the infrared radiation, which was sufficient to keep the water in the liquid state and to prevent the snow sample from melting. The transmittance of the water filter was >80% between 250 and 700 nm. The snow samples were filled into cylindrical Teflon cells equipped with quartz windows. Since the Teflon cell has the same inner diameter (4.6 cm) as the liquid cell, the snow sample was completely illuminated by the light beam. Two different cells with path lengths of 1 and 10.5 cm, respectively, were used.
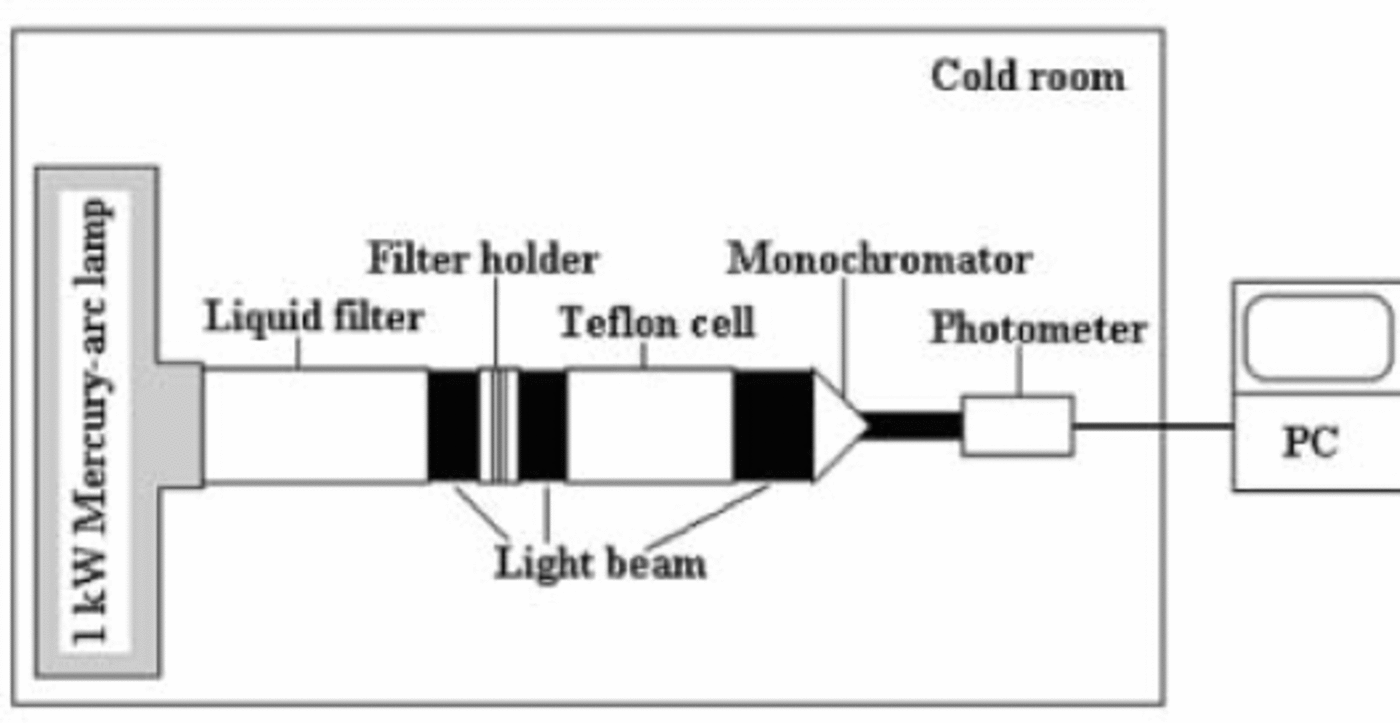
Fig. 1. Experimental system used for the photolysis experiments.
The intensity of the irradiance transmitted through the cell was measured with a photodetector (SED 400, International Light, Newburyport, MA) connected to a recently calibrated radiometer (IL1700, International Light). The photodetector captured data over the range ~280–385 nm with a maximum sensitivity at 350 nm. The spectral resolution of the irradiance was obtained using a monochromator (Oriel Instruments, Stratford, CT) with a bandwidth of 2 nm.
The wavelength range of the emitted light was varied with two long-pass filters. The 50% cut-on points of these filters were either 295 nm (WG 295, Schott Glas, Mainz, Germany) or 360 nm (WG 360, Schott Glas). The output of the lamp shows two bands with maximum intensities around 310 and 370 nm. The long-pass filter WG 295 reduces only the intensity of the band at the lower wavelength and has a negligible influence on the intensity of the second band. The second long-pass filter, WG 360, entirely blocks the radiation of the first band and reduces the intensity of the second band at 370 nm by approximately 50%. However, the radiation in the visibility range remains almost unchanged.
H2O2 and HCHO concentrations in the snow were determined before and after each experiment. When the cell was filled for a new experiment, a sample of the same batch of snow was kept in an airtight bottle. After the experiment the snow from the 1 cm cell was transferred into a single bottle. The snow from the 10.5 cm cell was sampled in several layers and stored in different bottles. The snow samples were melted and immediately analyzed for H2O2 by titration with potassium permanganate solution or for HCHO by iodometric titration. Initial concentrations in the freshly prepared snow were approximately 9.4 mM for H2O2 and 12–69mM for HCHO. Detection limits of the titrations were 0.25 mM for H2O2 and 0.5 mM for HCHO.
Results
All experiments with the 1 cm cell show that H2O2 and HCHO in the artificial snow samples are decomposed during the irradiation with light in the ultraviolet (UV) and visible range. Figure 2 shows the logarithm of the ratio of the final and initial concentrations as a function of the irradiance time. It demonstrates that increasing the durations of the irradiation results in more effective decomposition of both compounds.
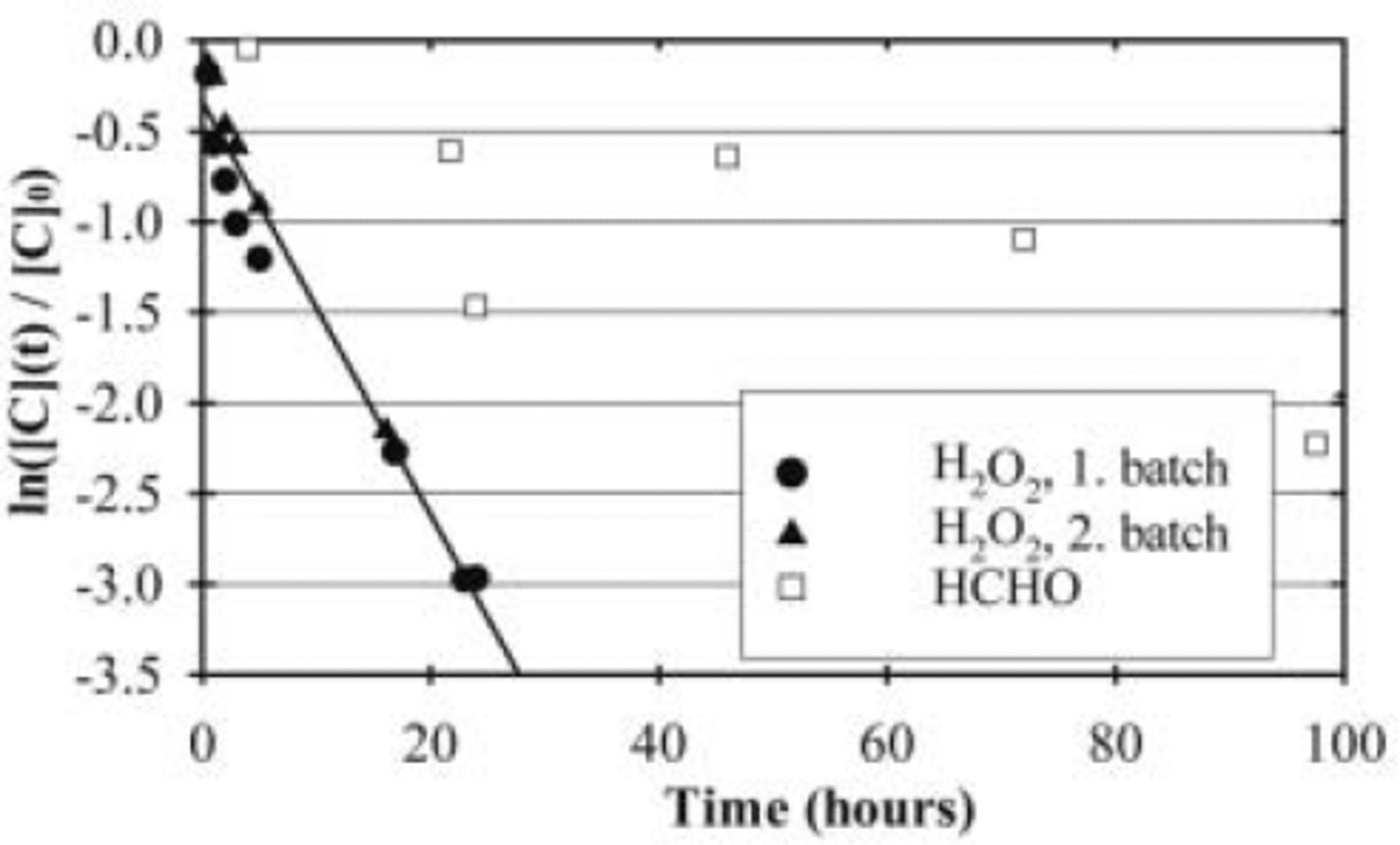
Fig. 2. Logarithm of relative H2O2 and HCHO concentrations in artificial snow as a function of irradiation time. [H2O2]0=9.5– 9.8 μM, [HCHO]0=12–69 μM. The experiments were made with the 1 cm cell.
Assuming a first-order decay due to a photolysis reaction as described in Equation (1), the decrease of the concentrations can be expressed by Equation (2).
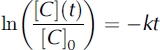
with C = H2O2 or HCHO, [C](t) the concentration after the experiment, [C]0 the initial concentration, k the first-order reaction rate constant, and t the duration of the experiment.
In the case of H2O2, experiments were performed with two different batches of artificial snow. Separate regressions of the results of the two batches resulted in slopes of –(0.11 ± 0.01) h–1 and –(0.13 ± 0.01) h–1, respectively. These results are in excellent agreement and indicate that the results are reproducible and independent of the batches.
Regression lines for the decay of both compounds are shown in Figure 2, resulting in calculated slopes of –(0.11 ± 0.01) h–1 for H2O2 including all experiments and –(0.017 ± 0.007) h–1 for HCHO. These values show that under comparable conditions the decay of H2O2 occurs more than a factor of 5 faster than the decay of HCHO. Moreover, the regression coefficients of R 2 = 0.97 and 0.62 for H2O2 and HCHO indicate that the decomposition of both compounds can well be described by a first-order reaction.
Results of the experiments with the 10.5 cm cell are shown in Figure 3. As expected, the decomposition of both compounds is more effective in the surface layers that were directly exposed to the irradiation. Deeper layers show very small or even no decomposition of both compounds due to the effective attenuation of the irradiation intensities in the snow (e.g. Reference Peterson, Barber and GreenPeterson and others, 2002). In order to investigate the decay as a function of the wavelength, additional experiments with long-pass filters were performed. To compare the results of the different experiments with different duration, decomposition rates in per cent per hour were calculated (Fig. 4). Adding the long-pass filters obviously leads to smaller decomposition rates for both compounds, demonstrating that the decay of both compounds is more sensitive to irradiation in the UV than in the visible range. As in the experiments with the 1 cm cell, the decomposition of H2O2 is more effective than the decomposition of HCHO under comparable conditions.
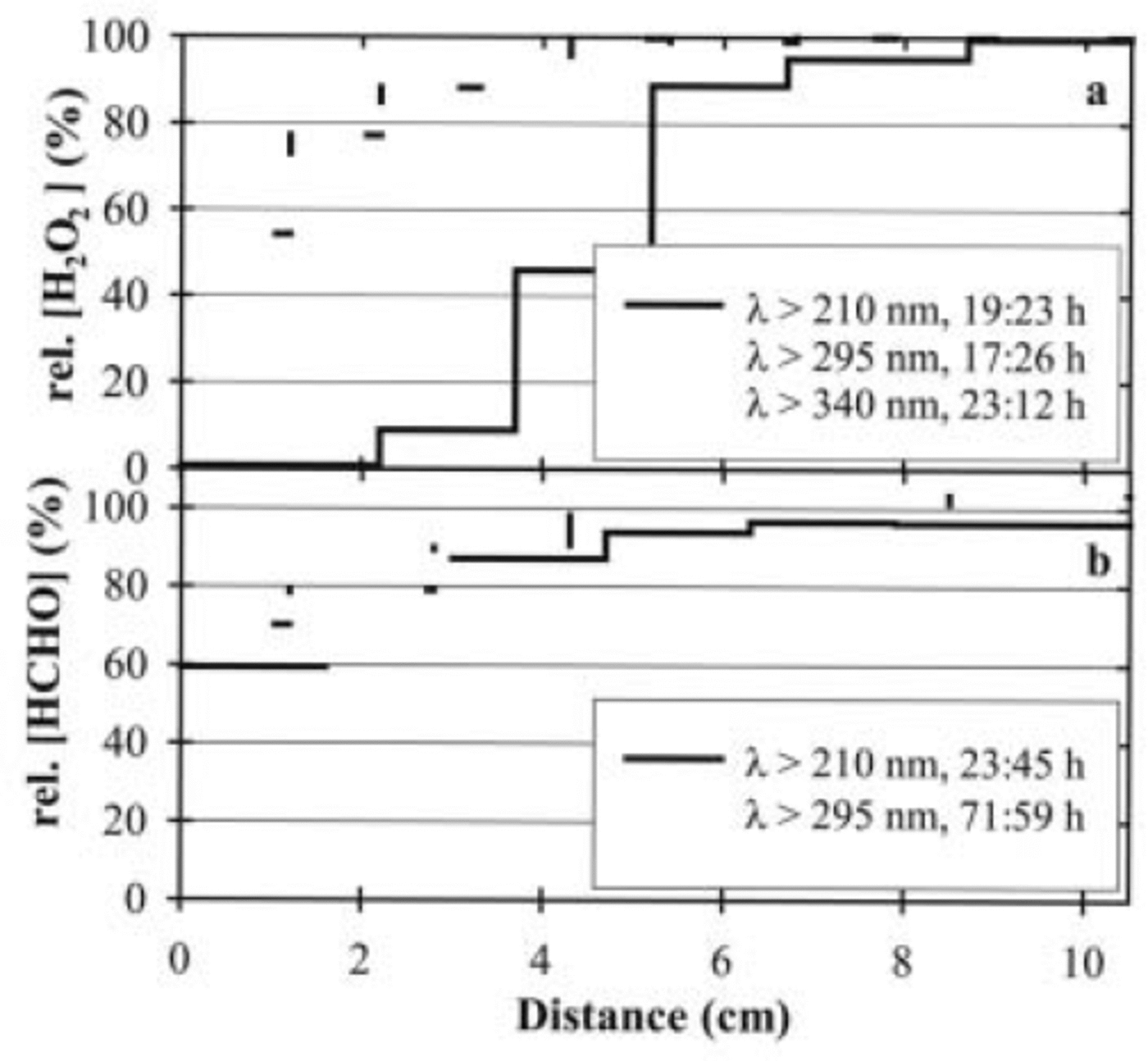
Fig. 3. Percentage of remaining (a) H2O2 and (b) HCHO in artificial snow samples after irradiation experiments. Samples were illuminated from the left; applied wavelengths and durations are indicated.

Fig. 4. Decomposition rates of H2O2 and HCHO during photolysis experiments with artificial snow.
Discussion
H2O2 in the gas and liquid phase as well as HCHO in the gas phase significantly absorb solar radiation (e.g. Reference Finlayson-Pitts and PittsFinlayson-Pitts and Pitts, 2000). Therefore, photolytic reactions are well-known sinks for both compounds in the atmosphere. For the first time, our results demonstrate that H2O2 and HCHO incorporated in artificial snow can also be destroyed if the snow is illuminated by radiation in the UV and visible range. These reactions can constitute additional pathways for the destruction of reactive compounds in natural snow covers due to photochemical reactions. Currently, only the photolysis of nitrate in snow has been investigated (Reference Honrath, Peterson, Dziobak, Dibb, Arsenault and GreenHonrath and others, 2000b; Reference Dubowski, Colussi and HoffmannDubowski and others, 2001, Reference Dubowski, Colussi, Boxe and Hoffmann2002; Reference Cotter, Jones, Wolff and BaugitteCotter and others, 2003) as a key reaction for the photochemical activity of surface snow layers. However, the results of this study imply that the photochemical activity of illuminated snow is possibly more diverse than previously thought.
The decays follow first-order kinetics as expected for the direct photolysis of both compounds. Such photolysis reactions also occur in the liquid phase according to reactions (3) and (4):
In liquid solutions, HCHO is only present in the hydrated form H2C(OH)2 (Reference BellBell, 1966). Since the artificial snow is produced from liquid solutions, which are very rapidly frozen, we assume that in the artificial snow HCHO also exists in the hydrated form. Absorption of the hydrated HCHO in the aqueous phase in the UV range is much smaller than the absorption of H2O2 in aqueous solution. Therefore, in contrast to the H2O2 photolysis, this reaction is not included in tropospheric liquid-phase chemical models (e.g. Reference Herrmann, Ervens, Jacobi, Wolke, Nowacki and ZellnerHerrmann and others, 2000). Accordingly, the decay of HCHO in artificial snow also occurs more slowly than the decay of H2O2. If the HCHO photolysis proceeds via the fission of one of the C-H bonds as shown in reaction (4), H atoms are produced, which can react further with oxygen to form HO2. Assuming that reactions (3) and (4) also occur in natural snow covers, they would constitute new pathways for the production of the highly reactive radicals OH and HO2. These radicals could initiate further reactions in the snow or could be released into the interstitial air of the snowpack, where they could enhance the oxidation capacity of the interstitial air.
However, several difficulties exist in seeking to apply the experimental results with artificial snow samples to snow covers under natural conditions. These deficiencies are discussed in more detail.
The properties of the artificial snow samples are not directly comparable to natural snow, mainly due to the different production processes. In the atmosphere, ice crystals are formed by freezing of supercooled droplets or nucleation on ice-forming nuclei (Reference Petrenko and WhitworthPetrenko and Whitworth, 1999). The incorporation of H2O2 and HCHO into ice crystals is probably governed by co-condensation (Reference Sigg, Staffelbach and NeftelSigg and others, 1992). Although this mechanism should lead to rather uniform concentrations throughout the ice crystal, it has been shown that, in fresh precipitating snow and aged snow in the snowpack, large fractions of both compounds are located on the surface or within the surface layer of the crystals (Reference Hutterli, Bales, McConnell and StewartHutterli and others, 2002; Reference JacobiJacobi and others, 2002). In contrast, the shock freezing of the artificial snow leads most probably to ice crystals with evenly distributed H2O2 and HCHO. Due to the low diffusion coefficients of both compounds in ice of <10–10cm–2 s–1 (Reference McConnell, Bales, Stewart, Thompson, Albert and RamosMcConnell and others, 1998; Reference Perrier, Sassin and DominéPerrier and others, 2003), the molecules would require >100 days to diffuse from the middle to the surface of the artificial snow crystal. Therefore we assume that, in our experiments, H2O2 and HCHO were always rather uniformly distributed in the ice lattice. Nevertheless, H2O2 and HCHO molecules, which are present in a quasi-liquid surface layer, are most likely more effectively photolyzed than molecules incorporated in the ice lattice. Thus, we assume that under natural conditions, with larger fractions of both impurities present at the grain surfaces, the decay of both compounds could be even more effective than in our experiments.
The H2O2 and HCHO concentrations in the artificial snow samples were considerably higher than concentrations found in natural snow samples. Concentrations determined in snow samples from Antarctica and Greenland range from 1 to 35 μM for H2O2 (e.g. Reference McConnell, Bales, Stewart, Thompson, Albert and RamosMcConnell and others, 1998; Reference Hutterli, McConnell, Stewart, Jacobi and BalesHutterli and others, 2001) and 0.1 to 2 μM for HCHO (e.g. Reference Riedel, Weller and SchremsRiedel and others, 1999; Reference Hutterli, Bales, McConnell and StewartHutterli and others, 2002). In addition, the irradiation intensities in the experiments were also considerably higher than the intensities of the solar radiation reaching the Earth’s surface. To estimate this difference, we measured the radiation intensity in our experiments and compared it to measured intensities at the German research station Neumayer (70˚39' S) in Antarctica, where radiation in the wavelength range 300–370nm is continuously measured with a total UV radiometer (TUVR; Eppley, Newport, RI). The measured radiation data can be retrieved via the Internet (http://www.awi-bremerhaven.de/MET/Neumayer/radiation.html). Highest daily averages of the radiation intensities occur in December and reach values of 23–27Wm–2. In contrast, in our experiments the radiation intensity in the same wavelength range corresponded to a value of approximately 1200Wm–2 at the surface of the artificial snow samples. Thus, we assume that the concentration levels of the produced OH and HO2 radicals in the snow were significantly higher in our experiment than in natural surface snow. These high levels may initiate further reactions, leading to an additional destruction of the impurities. Further experiments with lower H2O2 and HCHO concentrations and lower radiation intensities are needed.
Post-depositional loss processes may have important implications for the interpretation of H2O2 and HCHO profiles in surface snow and ice cores. Understanding and accounting for such reactions are essential for reconstructing past atmospheric composition. However, current transfer models of H2O2 and HCHO do not include photochemical reactions in the surface snow layer (Reference McConnell, Bales, Stewart, Thompson, Albert and RamosMcConnell and others, 1998; Reference Hutterli, Röthlisberger and BalesHutterli and others, 1999, Reference Hutterli, Bales, McConnell and Stewart2002). Interestingly, such transfer models based only on physical processes have successfully been applied to model measured H2O2 and HCHO properties at different locations (e.g. H2O2 and HCHO profiles retrieved from shallow ice cores; relationships between atmospheric and surface snow concentrations; fluxes between the snow surface and the atmosphere).
The discrepancy between measured and modeled field measurements, on the one hand, and our laboratory experiments, on the other, has several possible explanations. The photolysis of H2O2 and HCHO in natural snow may not be significant. If we compare again the radiation intensities in our experiment with the intensity measured at Neumayer (see above), we applied intensities which were roughly a factor of 50 higher than the maxima of daily means of the measured solar radiation intensities. For the comparison, we use the results of the experiments with the 295 nm long-pass filter, since the filter strongly reduces the radiation below 295 nm, comparable to the spectrum of the solar radiation reaching the Earth’s surface. In these experiments, we obtained decomposition rates of 2.6 and 0.4%h–1 for H2O2 and HCHO in the surface layer of the snow (Fig. 4). Divided by a factor of 50, these decomposition rates correspond to 1.2% d–1 or 8.4% week–1 for H2O2, and 0.19% d–1 or 1.3%week–1 for HCHO. These rates are not negligible and would lead to observable losses in natural snow covers, at least in the case of H2O2. However, such losses can be obscured either by the deposition of H2O2 and HCHO from the atmosphere or by the photochemical production in the surface snow. Field measurements have shown that the transfer of both compounds between the atmosphere and the underlying surface snow occurs (Reference Hutterli, McConnell, Stewart, Jacobi and BalesHutterli and others, 2001, Reference Hutterli, Bales, McConnell and Stewart2002; Reference JacobiJacobi and others, 2002). Although bidirectional fluxes have been observed, deposition occurred normally during the night (Reference JacobiJacobi and others, 2002), and in summer the net fluxes were directed from the snow to the atmosphere (Reference Hutterli, McConnell, Stewart, Jacobi and BalesHutterli and others, 2001, Reference Hutterli, Bales, McConnell and Stewart2002; Reference JacobiJacobi and others, 2002). Therefore, such fluxes are not able to balance a photochemical decay, which potentially occurred in the surface snow layers. The possibility that the photochemical decay of H2O2 and HCHO is matched by a concurrent photochemical production is intriguing. Such mechanisms were proposed by Reference Sumner and ShepsonSumner and Shepson (1999) and were further elaborated by Reference Dominé and ShepsonDominé and Shepson (2002). Organic material is quite abundant even in the snow in polar regions (Reference DassauDassau and others, 2002) and could act as a precursor for the photochemical formation of H2O2 and HCHO, which are typical products during the oxidation of organic compounds in the atmospheric gas and liquid phase (Reference Finlayson-Pitts and PittsFinlayson-Pitts and Pitts, 2000). If similar photochemical mechanisms also take place in the surface snow, the production of H2O2 and HCHO is possible.
Conclusions
An effective decomposition of H2O2 and HCHO in laboratory-made snow was observed under the influence of highly intense UV and visible radiation. Further experiments with lower radiation intensities comparable to the solar radiation are needed before the results can be applied to processes occurring in natural snow covers. Additionally, lower trace compound concentrations should be applied and product studies should be performed.
However, if such reactions take place in natural snow, our experiments demonstrate that the decomposition rates are not negligible. The photochemical activity of the surface snow would depend not only on the photolysis of nitrate, but also on the photolysis of H2O2 and HCHO, which are always present in natural snow due to their solubility. Further laboratory and modeling studies are needed to better characterize photochemical processes in the snow and their effects on the composition of the atmospheric boundary layer above natural snow covers as well as on the concentration profiles of H2O2 and HCHO conserved in snow, firn and ice cores.
Acknowledgement
Financial support by the Deutsche Forschungsgemeinschaft (DFG), grant JA 932/5-1, is gratefully acknowledged.






