Colorectal cancer (CRC) is the third most common cancer among men and the second most common cancer among women worldwide, contributing to >200 000 deaths in 2018 in Europe(Reference Bray, Ferlay and Soerjomataram1). There is accumulating evidence that up to 50 % of all CRC cases may be preventable through diet, physical activity and being a healthy body weight(2) and that diet could be an important predictor of CRC recurrence and survival(Reference Rock, Doyle and Demark-Wahnefried3,Reference Ulrich, Himbert and Holowatyj4) . To date, there are no evidence-based dietary guidelines for CRC survivors and it is recommended to follow the guidelines for cancer prevention(2), including reducing processed meat and alcohol consumption. Evidence is limited for other nutrients and foods(2). Few studies have evaluated dietary intake after CRC diagnosis, whether by dietary recall or dietary biomarkers, nor its association with CRC outcomes. Nonetheless, emerging data suggest that dietary factors implicated in colorectal carcinogenesis may not have the same impact on CRC progression and invasion; thus, further research is needed to support the evidence base for CRC dietary guidance post-diagnosis(Reference van Zutphen, Kampman and Giovannucci5). With the increasing rates of CRC among younger adults <50 years and the increasing prevalence of CRC survivors in Europe(Reference Ait Ouakrim, Pizot and Boniol6), the development of dietary recommendations for this population is of major public health relevance.
Evaluating the association of dietary factors with biological pathways implicated in CRC cancer progression among CRC patients may shed light on potential strategies for intervention to improve prognosis. Important pathways in the development, promotion and progression of CRC include inflammation(Reference Drew, Cao and Chan7–Reference Landskron, De la Fuente and Thuwajit11) and angiogenesis(Reference Albini, Bruno and Noonan12,Reference Tonini, Rossi and Claudio13) . Inflammatory bowel diseases and obesity, two strong CRC risk factors, promote inflammation, whereas non-steroidal anti-inflammatory drugs are associated with lower CRC risk(Reference Drew, Cao and Chan7–Reference Ulrich, Bigler and Potter9). Inflammatory chemokines are immune modulators that promote angiogenesis, a key feature of tumour progression, supported by vascular endothelial growth factors (VEGF)(Reference Albini, Bruno and Noonan12,Reference Tonini, Rossi and Claudio13) . Modulation of one-carbon metabolites that serve many biological functions(Reference Locasale14) (e.g. vitamin B6 and folate) has been shown to lower circulating inflammatory biomarkers in non-cancer populations(Reference Abbenhardt, Miller and Song15), though the role of B vitamins in angiogenesis is less clear.
The interrelationship of inflammation, angiogenesis and components of one-carbon metabolism with colorectal carcinogenesis among CRC patients and survivors remains inconclusive. Indeed, the role of nutrient metabolism in cancer is complex. For example, a U-shaped relationship between micronutrients and cancer is evident where supplementation among those with deficiency is associated with reduced cancer risk, but among those with sufficient or higher micronutrient status may promote cancer growth(Reference Mayne, Ferrucci and Cartmel16). Considering this complexity, we sought to better characterise the nature of the relationship between one-carbon metabolites and pathways integral to cancer progression. Few studies have evaluated post-diagnosis circulating one-carbon metabolites in relation to cancer prognostic pathways among CRC patients(Reference Lochhead, Nishihara and Qian17).
Our objective was to evaluate associations between inflammation- and angiogenesis-related biomarkers and one-carbon metabolites among CRC survivors. Based on prior findings(Reference Abbenhardt, Miller and Song15,Reference Zschabitz, Cheng and Neuhouser18–Reference Shen, Lai and Mattei20) , we hypothesised that pyridoxal-5’-phosphate (PLP) is inversely associated with pro-inflammatory biomarkers C-reactive protein (CRP), serum amyloid A (SAA), TNFα and IL-6 and IL-8 among CRC patients. We further hypothesised that 5-methyl-tetrahydrofolate (mTHF) inversely associates with inflammatory biomarkers corresponding to higher production of folate catabolites para-aminobenzoylglutamic acid (pABG) and acetyl-pABG (apABG) in this population.
Subjects and methods
Study design
This is a cross-sectional analysis of baseline data from the ColoCare Study, a multicentre, international prospective cohort initiated at the Fred Hutchinson Cancer Research Center (Seattle, WA, USA), described in detail in prior publications(Reference Gigic, Boeing and Toth21–Reference Ulrich, Gigic and Böhm23). ColoCare recruits newly diagnosed CRC patients prior to surgery, with the goal to investigate predictors of cancer recurrence, survival, treatment toxicities and health-related QoL.
Patients (eligibility: newly diagnosed primary invasive CRC, aged 18–89 years, German-speaking and able to provide informed consent) from the ColoCare Heidelberg site were included in this study, recruited between October 2010 and December 2014 at the University Hospital of Heidelberg, Germany (ClinicalTrials.gov Identifier: NCT02328677). Participants were staged according to the American Joint Committee on Cancer (AJCC) system based on histopathological findings. Both patients with colon (ICD-10 C18) and rectal or recto-sigmoid junction cancer (ICD-10 C19/C20) were included. The ColoCare Study has been approved by the ethics committee of the Medical Faculty at the University of Heidelberg. All study participants provided written informed consent.
Study population
In total, 356 stage I–IV CRC patients have been recruited into the ColoCare Study in Heidelberg (online Supplementary Fig. S1). For this project, we excluded patients who did not have all measured biomarkers of interest, with 238 CRC patients included in the current analysis. Patients with a history of hereditary nonpolyposis CRC or familial adenomatous polyposis were not excluded from the study. According to estimates(Reference Gryfe24), approximately 1 % of our participants were likely to have familial adenomatous polyposis and 2–4 % to have hereditary nonpolyposis CRC. The fact that non-steroidal anti-inflammatory drugs intervention prevents tumours among individuals with genetic predisposition to CRC (e.g. in the case of Lynch syndrome)(Reference Labayle, Fischer and Vielh25,Reference Burn, Gerdes and Macrae26) suggests that inflammatory pathways remain an important target for this population, and therefore the relationship of inflammation with other cancer-related molecular pathways remains of interest among individuals with or without germline mutations. ColoCare participants were also part of the international FOlate-dependent one-carbon metabolism in Colorectal cancer recUrrence and Survival (FOCUS) Consortium.
Circulating inflammation and angiogenesis biomarkers
Non-fasting blood samples were collected at baseline prior to surgery at the University Hospital Heidelberg. Blood samples were collected 1·3 (sd 1·25) d before surgery, on average. Samples were frozen 6·3 (sd 7·1) h following blood draw and stored for approximately 5·5 years. Serum was extracted within 4 h of blood draw, and aliquots were stored at –80°C until analysis. Aliquots (500 µl) were shipped on dry ice from Heidelberg to the Huntsman Cancer Institute, Salt Lake City, UT, USA. Ten inflammation biomarkers, including CRP, SAA, TNFα, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), soluble intercellular adhesion molecule 1 (sICAM-1) and soluble vascular cell adhesion molecule 1 (sVCAM-1), and angiogenesis-related biomarkers, including VEGF-A and VEGF-D were measured in serum. Previously established assays on the Mesoscale Discovery Platform (MSD), on the Sector 2400A, were used in the Ulrich laboratory at the Huntsman Cancer Institute(Reference Himbert, Ose and Nattenmüller27). Blinded patient samples, intraplate and interplate quality control samples were run on the U-PLEX Proinflammatory Combo 1 for MCP-1, IL-6, IL-8, and TNFα, on the V-PLEX Vascular Injury Plate 2 for CRP, SAA, sICAM-1 and sVCAM-1, and on the V-PLEX Angiogenesis Panel 1 for VEGF-A and VEGF-D. Blinded serum samples were diluted 1:2 (pro-inflammatory panel), 1:1000 (vascular injury plate) and 1:8 (angiogenesis panel). For the vascular injury and angiogenesis panels, the samples were freeze-thawed once and the pro-inflammatory panel was freeze-thawed twice. The plates were read with an MSD sector 2100A, and data analyses were carried out with MSD Discovery Workbench 4.0 software (Meso Scale Diagnostics). The resulting overall interplate CV was 9·9 % (range 5·1–15·5 %) and intraplate CV was 4·6 % (range 1·8–6·4 %).
B vitamins and one-carbon metabolites
Twelve B vitamins and one-carbon metabolites were measured in non-fasting blood samples collected at baseline prior to surgery, including PLP, pyridoxal (PL), pyridoxic acid (PA), mTHF, apABG, pABG, folic acid (FA), thiamine, thiamine monophosphate, riboflavin, cobalamin and homocysteine. After serum extraction, aliquots were stored at −80°C. Assays were run at the laboratory BEVITAL A/S, Bergen, Norway (www.bevital.no), as part of analyses run for the FOCUS consortium. Biological analyses were performed on seven complementary analytical platforms, using liquid chromatography-tandem MS-based assays (LC-MS/MS) and GC-MS/MS(Reference Midttun, Kvalheim and Ueland28). Within-day and between-day CV ranged between 1–9 and 2–16 %, respectively.
Statistical analysis
Descriptive statistics of the study population were calculated for clinical characteristics. Means and standard deviations were computed for normally distributed continuous variables, medians and interquartile ranges for right-skewed variables (inflammation, angiogenesis and one-carbon metabolism biomarkers) and frequencies (n, (%)) for categorical variables. Categorical variables with more than two classes were considered in our analyses as indicator variables. Distributions of variables were analysed using q-q plots and histograms along with measurement of skewness and kurtosis. One-carbon metabolism, inflammation- and angiogenesis-related biomarkers were log2-transformed to meet the normality assumption for parametric statistical tests. No outliers, defined as three times SD above or below the mean value, accounting for multivitamin intake, were found. For data flagged as missing by the laboratory due to biomarker concentrations below and above the detection limit, half-minimum and maximum values were imputed, respectively.
For the primary hypothesis, sequential multivariable linear regression models were used to study associations between pre-specified one-carbon metabolites (exposure) and inflammation- and angiogenesis-related biomarkers (outcome). Models were adjusted for (1) sex and age group (<60, 60–70, >70 years); (2) model 1 plus BMI category (<25, 25–30, ≥30 kg/m2), smoking status (current, former, never smoker), regular multivitamin intake in the month prior to surgery weekly (yes, no), CRC site (colon, rectum), CRC stage (I, II, III) and physical activity (min/week). Validity checks (tests for heteroscedasticity and multicollinearity) for the multivariable models were performed. Inclusion of alcohol in the model did not modify effect estimates more than 10 %; thus, it was excluded from the final models. We did not adjust for race/ethnicity owing to our homogeneous non-Hispanic white population.
In secondary analyses, regression models were stratified by PLP sufficiency defined as ≥ 20 nmol/l(29), BMI and CRC site (colon v. rectum). Effect modification was evaluated by testing for the significance of an interaction term in the linear regression models. We further calculated the PA:(PLP + PL) ratio (PAr index), an integrated measure of vitamin B6 metabolism that has been proposed as a systemic inflammation indicator(Reference Ueland, McCann and Midttun30,Reference Ulvik, Midttun and Pedersen31) , and evaluated its association with inflammation- and angiogenesis-related biomarkers. Moreover, we calculated the ratio of 3-hydroxykynurenine (HK):xanthurenic acid (XA), both involved in tryptophan metabolism, (HK:XA ratio), as a functional marker of vitamin B6 status(Reference Ulvik, Theofylaktopoulou and Midttun32). Finally, we stratified the analyses of the association of inflammation biomarkers with PLP and mTHF by BMI and by disease stage.
In exploratory analyses, we computed Spearman partial correlation coefficients between inflammation-, angiogenesis-related, B vitamins and one-carbon metabolism biomarkers, adjusting for covariates. A Gaussian graphical model (GGM) was generated to visualise correlated pathways among the analysed biomarkers, conditioned on the presence of all other biomarkers. Conditional correlations ≥|0·20| are displayed with an edge, connecting biomarker nodes. The GGM is a powerful tool for visualising metabolomics datasets and identifying relationships between biological pathways(Reference Krumsiek, Suhre and Illig33). We further generated a hierarchical clustering heat map to visualise pathway inter-relationships.
All statistical tests were two-sided. Statistical significance was set at P < 0·05 for the primary and secondary hypotheses, and to account for multiple testing in Spearman partial correlation analyses, we considered significance at the Benjamini and Hochberg’s False Discovery Rate (FDR) <0·05(Reference Benjamini and Hochberg34). With a sample size of 238, eighteen partial variables, and α = 0·0033 (to account for analyses of three one-carbon metabolites with five inflammatory biomarkers for the primary and secondary hypotheses), we estimated about 80 % statistical power to detect partial correlations of r ~ 0·25. The statistical power calculations were conducted using the Proc Power Multreg statement in SAS 9.4 (SAS Institute). Descriptive statistics, multivariable linear regression analyses and Spearman partial correlation analyses were performed using SAS 9.4 (SAS Institute). GGM was created using R (R studio) and visualised using Cytoscape 3.6.1 (Cytoscape Consortium) and the hierarchical clustering heat map with BioVinci 1.1.5 (BioTuring Inc.).
Results
Study population
Baseline clinical and demographic characteristics of the study population are summarised in Table 1. CRC patients in this study (n 238) had a mean age of 64·4 (sd 12·1) years, were predominantly male (65 %), overweight (63 %) and ever smokers (63 %). Only 6 % of participants regularly used multivitamin supplements and 23 % used non-steroidal anti-inflammatory drugs in the month prior to surgery, at least weekly. Approximately 50 % of CRC tumours were in the rectum or recto-sigmoid junction, 24·0 % of individuals were diagnosed with stage I, 40·3 % stage II and 35·7 % stage III disease.
Table 1. Baseline clinicopathological and demographic characteristics of 238 colorectal cancer patients enrolled in the ColoCare Study*
(Mean values and standard deviations; median values and interquartile ranges (IQR); numbers and percentages)

* Percentages may not add to 100 % due to missing values.
† Multivitamin intake is defined as 1+ times/week of multivitamin use in the past 1 month.
‡ Twenty patients had unknown information on multivitamin intake; twenty-three patients had unknown smoking history.
§ Former smoker is defined as stopped smoking within the last 2 years.
¶ Moderate physical activity refers to activities, which require moderate effort, such as walking or bicycling. Vigorous physical activity includes, for example, jogging or fast swimming.
Mean values and standard deviations and medians and interquartile ranges of inflammation- and angiogenesis-related biomarkers, B vitamins and one-carbon metabolites are reported in Table 2. Mean CRP and SAA concentrations were elevated (27·5 (sd 51·7) and 42·5 (sd 73·0) mg/l, respectively) according to clinical reference ranges (<10 mg/l)(Reference Kushner, Rzewnicki and Samols35), indicative of active systemic inflammation in our study population. The median concentration of circulating PLP was 26·5 nmol/l (interquartile range 17·1–40·8 nmol/l); 66 % of participants had sufficient PLP concentrations (≥20 nmol/l)(29).
Table 2. Biomarkers of inflammation, angiogenesis and one-carbon metabolism among 238 colorectal cancer patients enrolled in the ColoCare Study
(Mean values and standard deviations; median values and interquartile ranges (IQR))
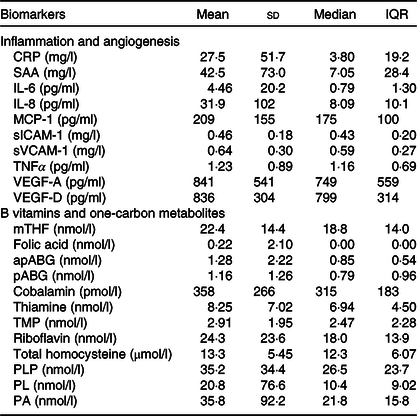
CRP, C-reactive protein; SAA, serum amyloid A; MCP-1, monocyte chemoattractant protein 1; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; mTHF, 5-methyl-tetrahydrofolate; apABG, acetyl-para-aminobenzoylglutamic acid; pABG, para-aminobenzoylglutamic acid; TMP, thiamin monophosphate; PLP, pyridoxal-5’-phosphate; PL, pyridoxal; PA, pyridoxic acid.
Pyridoxal-5’-phosphate and inflammation
To evaluate our primary and secondary hypotheses, we performed multivariable linear regression analyses (Table 3, Fig. 1). Magnitude of association differed for minimally v. fully adjusted models, but the directions of association were unchanged. In fully adjusted models, PLP was significantly inversely associated with inflammation biomarkers CRP (r –0·33, β = –0·86, P linear < 0·0001), SAA (r –0·23, β = –0·56, P linear = 0·003), IL-6 (r –0·39, β = –0·69, P linear < 0·0001), IL-8 (r –0·20, β = –0·26, P linear = 0·02) and TNFα (r –0·12, β = –0·15, P linear = 0·045).
Table 3. Associations between one-carbon metabolites and inflammation biomarkers among 238 colorectal cancer patients enrolled in the ColoCare Study*
(β-Coefficients and 95 % confidence intervals)

PLP, pyridoxal-5’-phosphate; mTHF, 5-methyl-tetrahydrofolate; CRP, C-reactive protein; SAA, serum amyloid A; apABG, acetyl-para-aminobenzoylglutamic acid; pABG, para-aminobenzoylglutamic acid.
* Biomarker values were log2-transformed to meet the normality assumptions for linear regression models.
† Simple model: multivariable linear regression model, adjusted for: age group (<60, 60 to <70 and ≥70 years) and patient sex.
‡ Full model: multivariable linear regression model, adjusted for: age group, patient sex, BMI category, cancer stage and site, physical activity, multivitamin intake and smoking status.

Fig. 1. Scatter plots of one-carbon metabolite pyridoxal-5’-phosphate (PLP) and inflammation biomarkers C-reactive protein (CRP) (a), IL-6 (b), IL-8 (c) and serum amyloid A (SAA) (d) based on univariate linear regression. The represented biomarkers were log2-transformed to meet the normality assumption for linear regression. Single biomarker values are visualised in grey and the regression line in black. This figure was created with GraphPad Prism8.
Folate species and inflammation
mTHF showed significantly inverse associations with CRP (r –0·14; β = –0·71, P linear = 0·004), SAA (r –0·14; β = –0·68, P linear = 0·003) and TNFα (r –0·15; β = –0·20, P linear = 0·02). Folate catabolite apABG was positively correlated with IL-6 (r 0·27; β = 0·93, P linear < 0·0001), and pABG was positively correlated with IL-8 (r 0·21; β = 0·58, P linear < 0·0001). Folic acid, the synthetic form of folate, was not associated with any inflammation biomarker (P > 0·05 for all associations). In sensitivity analyses, we additionally adjusted the primary analyses for non-steroidal anti-inflammatory drugs use in the month prior to surgery, with similar results.
Secondary analyses
In stratified analyses by serum PLP sufficiency (≥20 nmol/l), PLP remained inversely associated with IL-6 among those with insufficient PLP concentrations (r –0·40; β = –1·14, P linear = 0·03), while the association was attenuated among those with PLP sufficiency (r –0·22; β = –0·48, P linear = 0·03). Magnitudes of association were similar for PLP and IL-8, stratified by PLP sufficiency (PLP-insufficient: r –0·23; β = –0·28, P linear = 0·5; PLP-sufficient: r –0·19; β = –0·37, P linear = 0·05). Moreover, the results suggested that the associations of PLP with CRP may differ by BMI and CRC stage, but the P values for heterogeneity did not reach statistical significance (P heterogeneity = 0·07 and 0·09, respectively) (Table 4). For example, there was a stronger inverse association between PLP and CRP among normoweight (r –0·39; β = –1·45, P linear = 0·0008), compared with overweight participants (r –0·19; β = –0·54, P linear = 0·03, P het = 0·02). The magnitude of inverse associations between PLP and CRP was stronger among patients with stage I (r –0·53; β = –2·02, P linear < 0·0001), compared with stage II (r –0·31; β = –0·72, P linear = 0·02, P het = 0·20) and stage III (r –0·20; β = –0·44, P linear = 0·20, P het = 0·03) disease. Moreover, there was evidence for effect modification of mTHF associations with TNFα by BMI; the association was stronger for overweight individuals (P het = 0·009), but the same was not observed for under/normal-weight or obese individuals (Table 5). The inverse association of mTHF with both CRP and SAA appeared to be stronger among those with earlier stage disease (P het = 0·02).
Table 4. Associations of active vitamin B6 (pyridoxal-5’-phosphate) with inflammation biomarkers, stratified by BMI and cancer stage among 238 colorectal cancer patients enrolled in the ColoCare Study*
(β-Coefficients and 95 % confidence intervals)

CRP, C-reactive protein; SAA, serum amyloid A.
* Biomarker values were log2-transformed to meet the normality assumptions for linear regression models.
† Multivariable regression analysis adjusted for: age group (<60, 60 to <70 and ≥70 years), sex, BMI category, cancer stage and site, physical activity, multivitamin intake and smoking status.
Table 5. Associations of active folate species (5-methyl-tetrahydrofolate) with inflammation biomarkers, stratified by BMI and cancer stage among 238 colorectal cancer patients enrolled in the ColoCare Study*
(β-Coefficients and 95 % confidence intervals)

CRP, C-reactive protein; SAA, serum amyloid A.
* Biomarker values were log2-transformed to meet the normality assumptions for linear regression models.
† Multivariable regression analysis adjusted for: age group (<60, 60 to <70 and ≥70 years), sex, BMI category, cancer stage and site, physical activity, multivitamin intake and smoking status.
The PAr index showed positive associations with IL-6 (r 0·18; β = 0·57, P linear = 0·004), IL-8 (r 0·18; β = 0·36, P linear = 0·02) and TNFα (r 0·15; β = 0·20, P linear = 0·04); associations with CRP were positive but not statistically significant (r 0·11; β = 0·36, P linear = 0·20) or SAA (r 0·08; β = 0·26, P linear = 0·30). The HK:XA ratio was positively associated with CRP (r 0·46; β = 1·16, P linear < 0·0001), SAA (r 0·38; β = 0·83, P linear < 0·0001) and IL-6 (r 0·43; β = 0·86, P linear < 0·0001); associations with IL-8 were also positive but not statistically significant (r 0·21; β = 0·13, P linear = 0·20) or TNFα (r 0·13; β = 0·06, P linear = 0·40).
Exploratory analyses
In exploratory analyses, we evaluated partial correlations between the full panel of circulating biomarkers (online Supplementary Table S1). Forty-two partial correlations reached the FDR significance threshold of <0·05. For example, PL was inversely correlated with CRP (r –0·29, P < 0·001), IL-6 (r –0·30, P < 0·001) and VEGF-D (r 0·23, P = 0·002). We also noted positive correlations between pABG and VEGF-D (r 0·27, P = 0·0003) and between total homocysteine and VEGF-D (r 0·31, P < 0·001). Thiamine and thiamine monophosphate were inversely correlated with CRP (r –0·20, P < 0·001 and r –0·35, P < 0·001) and IL-6 (r –0·38, P < 0·001 and r –0·33, P < 0·001), while thiamine monophosphate was inversely correlated with SAA and monocyte chemoattractant protein 1 (r –0·27, P < 0·001 and r –0·18, P = 0·01, respectively). In contrast, correlations between cobalamin (vitamin B12) and biomarkers of inflammation were positive, but not statistically significant at FDR < 0·05.
Biomarker intercorrelations were visualised using a hierarchical clustering heat map (Fig. 2) and GGM (Fig. 3). The GGM showed a single interconnected network between B vitamins, one-carbon metabolites and angiogenesis- and inflammation-related biomarkers, including inverse conditional correlations of PLP with IL-6, PL and SAA. The GGM visualises correlations conditional on the presence of all other biomarkers, with a threshold (r |0·20|) for connecting nodes with edges.

Fig. 2. Hierarchical clustering heat map, based on Spearman partial correlations of B vitamins and one-carbon metabolites with inflammation and angiogenesis biomarkers, adjusted for age group, patient sex, BMI category, cancer stage and site, physical activity, multivitamin intake and smoking status. Biomarkers involved in the same or similar pathways (such as pyridoxal (PL), pyridoxic acid (PA) and pyridoxal-5’-phosphate (PLP)) cluster together in the heat map. Blue indicates inverse correlations, for example, between PLP and C-reactive protein (CRP). Orange and red represent positive correlations, for example, between CRP and serum amyloid A (SAA). This heat map was created with BioVinci 1.1.5 (BioTuring Inc.). sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; TMP, thiamin monophosphate; Thi, thiamin; pABG, para-aminobenzoylglutamic acid; Ribo, riboflavin; apABG, acetyl-pABG; mTHF, 5-methyl-tetrahydrofolate; FA, folic acid; tHcy, total homocysteine; VEGF, vascular endothelial growth factor; Cob, cobalamin.

Fig. 3. Gaussian graphical model (GGM) of biomarker correlations, conditioned on the presence of all other biomarkers (conditional r ≥|0·20|). Biomarkers are represented by nodes (circles), and conditional correlations are connected by edges (lines). Orange nodes represent inflammation, yellow nodes represent angiogenesis biomarkers and green nodes represent B vitamins and one-carbon metabolites. The line width represents the strength of conditional correlation. Red lines indicate positive correlations, and blue lines represent inverse correlations. Vascular endothelial growth factor A (VEGF-A) conditional r < 0·20. The GGM was created with Cytoscape 3.6.1 (Cytoscape Consortium). sICAM-1, soluble intercellular adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; sVCAM-1, soluble vascular cell adhesion molecule 1; PLP, pyridoxal-5’-phosphate; PA, pyridoxic acid; PL, pyridoxal; apABG, acetyl-para-aminobenzoylglutamic acid; mTHF, 5-methyl-tetrahydrofolate; pABG, para-aminobenzoylglutamic acid; FA, folic acid; tHcy, total homocysteine; Thi, thiamin; TMP, thiamin monophosphate; Ribo, riboflavin; Cob, cobalamin; CRP, C-reactive protein; SAA, serum amyloid A.
Discussion
In this study, we measured associations between one-carbon metabolites, inflammation- and angiogenesis-related biomarkers in a cross-sectional analysis among CRC patients participating in the ColoCare Study. We found that vitamin B6 species, PLP, PL and PA, were inversely associated with inflammatory biomarkers CRP, SAA, IL-6 and IL-8. Thiamine and active folate were also inversely associated with inflammatory biomarkers, and folate catabolites were positively associated with inflammation, indicating higher folate utilisation.
PLP is the bioactive form of vitamin B6, an essential vitamin that may be obtained from many sources including fortified cereals, milk products, beans, nuts and vitamin supplements(29,Reference Ueland, McCann and Midttun30) . PL represents the dephosphorylated form of PLP, and PA derives from PLP in an oxidation reaction with aldehyde oxidase I(Reference Ulvik, Midttun and Pedersen31). PLP serves as a coenzyme in over 4 % of human metabolic reactions(Reference Ueland, McCann and Midttun30), including preventing the formation of reactive oxygen species(Reference Ueland, McCann and Midttun30). Inverse associations of vitamin B6 intake and circulating PLP with CRC risk have been reported(Reference Zschabitz, Cheng and Neuhouser18,Reference Gylling, Myte and Schneede36,Reference Larsson, Orsini and Wolk37) . The relationship of PLP with CRC outcomes is less established(Reference Je, Lee and Ma38).
An inverse association between PLP and inflammatory biomarkers was previously reported among postmenopausal women in the Women’s Health Initiative Observational Study(Reference Abbenhardt, Miller and Song15), among healthy adults in the Boston Puerto Rican Health Study(Reference Shen, Lai and Mattei20) and Framingham Heart Study(Reference Friso, Jacques and Wilson19), and in patients with chronic inflammatory conditions, such as depression and inflammatory bowel disease(Reference Hvas, Juul and Bech39,Reference Saibeni, Cattaneo and Vecchi40) . In a prior intervention trial among rheumatoid arthritis patients, vitamin B6 supplementation suppressed IL-6 and TNFα concentrations(Reference Huang, Wei and Wu41). In our study, we also noted that participants with increased CRP concentrations (>10 mg/l) were less PLP-sufficient than those within the physiological range of CRP. Similar findings were described among 2686 adults in the US National Health & Nutrition Examination Survey(Reference Morris, Sakakeeny and Jacques42). Our results corroborate prior findings and show that these processes additionally apply to CRC patients. Furthermore, we provide evidence of an inverse association between other one-carbon metabolites and inflammation among CRC patients.
Our observations may reflect a modified distribution and utilisation of PLP in serum during inflammatory processes, rather than hypovitaminosis(Reference Ueland, McCann and Midttun30,Reference Paul, Ueland and Selhub43) . PLP is stored predominantly in muscle and liver. During inflammation, PLP is preferentially mobilised from the liver. Due to the high demand of PLP by enzymes involved in the inflammatory response, circulating concentration may decrease(Reference Paul, Ueland and Selhub43). Alternately, a recent study suggested that vitamin B6 species could inhibit the activation of NF-κB, which is involved in the inflammatory response, and nucleotide-binding oligomerisation domain, Leucine-rich Repeat and Pyrin domain containing-mediated caspase-1 activation, which suppresses cytokine production(Reference Zhang, Tsuchiya and Kinoshita44). Postulated anti-inflammatory mechanisms relevant for CRC patients include activation of p53 and p21 tumour suppressor gene expression in the colon(Reference Abbas and Dutta45,Reference Zhang, Suidasari and Hasegawa46) . Moreover, human colon carcinoma cell line studies demonstrated that vitamin B6 induced a reduction in inflammatory cytokine (including IL-6 and TNFα) secretion from peripheral blood mononuclear cells(Reference Bessler and Djaldetti47).
Our findings do not support strong effect modification of the cross-sectional relationship between PLP and inflammation by BMI or CRC stage, but it is possible that our study was underpowered to observe effect modification. Nonetheless, a trend was apparent suggesting that advancing stage of CRC and increasing BMI weakened the association between markers of inflammation and B vitamins/one-carbon metabolites. Specifically, the inverse relationship of PLP with inflammation biomarkers appeared stronger among normal-weight compared with overweight or obese participants. Similarly, it appeared stronger among those with earlier stage disease, although results were not statistically significant. The same heterogeneity according to disease stage was apparent for the association of mTHF with CRP and SAA. This might suggest that normal associations become increasingly perturbed with advancing disease and adiposity. Indeed, both obesity and cancer stage have been shown to promote systemic inflammation(Reference Ulrich, Himbert and Holowatyj4,Reference Park, Morley and Kim48–Reference Nicklas, Ambrosius and Messier50) .
We observed positive associations between the PAr index (PA/(PL + PLP)) and inflammation biomarkers, supporting prior findings(Reference Ueland, McCann and Midttun30,Reference Ulvik, Midttun and Pedersen31,Reference Zuo, Tell and Ueland51,Reference Zuo, Ueland and Midttun52) . As a systemic inflammation indicator(Reference Ulvik, Midttun and Pedersen31), the PAr index is an independent predictor of stroke(Reference Zuo, Tell and Ueland51) and cardiovascular mortality(Reference Ulvik, Pedersen and Svingen53). Furthermore, we observed strong positive associations between HK:XA ratio, a marker of functional vitamin B6 status(Reference Ulvik, Theofylaktopoulou and Midttun32), and several inflammation biomarkers (in particular the acute-phase proteins CRP and SAA). PAr index and HK:XA ratio may represent alternative biomarkers for identifying those who might benefit from anti-inflammatory dietary intervention.
Our findings suggest increased folate catabolism during inflammation among CRC patients given that elevated inflammatory state was associated with higher folate catabolite concentrations in parallel with lower mTHF concentration. Prior interventions of mTHF infusion among haemodialysis patients demonstrated reduced circulating CRP(Reference Cianciolo, La Manna and Coli54). As excretion products, pABG and apABG are indicators of folate catabolism and status(Reference Hannisdal, Svardal and Ueland55) and would be expected to increase in response to higher folate utilisation during inflammatory processes. This observation of increased folate metabolism in relation to inflammation may be important for informing future studies designed to understand folate metabolism in the context of CRC. A meta-analysis of nine randomised controlled FA intervention trials (n 1179 adults) reported decreased serum CRP post-intervention(Reference Fatahi, Pezeshki and Mousavi56). These data were partially supported by an analysis within the Women’s Health Initiative Observational Study cohort that showed a positive association of folate status with SAA, but not with CRP(Reference Abbenhardt, Miller and Song15). However, the Aspirin/Folate Polyp Prevention Trial did not observe a significant decrease of CRP, IL-6 or TNFα among participants receiving FA(Reference Ho, Xue and Cushman57). Whether folate’s influence on inflammation plays a causal role in CRC is unclear. Rapidly proliferating cancer cells have higher folate requirements to accommodate accelerated DNA synthesis(Reference Suh, Herbig and Stover58), though a dual modulatory effect on cancer risk has been observed according to dosage and timing(Reference Kim59–Reference Ulrich62). Folate may prevent CRC among subjects with a healthy colon, but increase risk among those with colon premalignant lesions(Reference Kim59–Reference Ulrich and Potter63), although these findings have been inconsistent(Reference Lochhead, Nishihara and Qian17).
Unlike in other countries, food products in Germany are not fortified with FA. Moreover, the prevalence of supplement use in our population was very low. We did not observe synthetic FA associations with inflammation biomarkers. In contrast to dietary folate, FA is not metabolised immediately to active mTHF. The role of circulating unmetabolised FA in health remains under debate(Reference Ebbing, Bonaa and Nygard64). Future studies among CRC populations should consider the roles of synthetic FA in comparison with the active form mTHF in inflammatory processes.
We observed positive correlations of PA, PL and PLP with angiogenesis biomarker VEGF-D, a cytokine involved in blood vessel formation(Reference Tonini, Rossi and Claudio13). Prior studies have found an inverse association between these metabolites and angiogenesis biomarkers(Reference Matsubara, Mori and Matsuura65,Reference Matsubara, Mori and Akagi66) . Hypothesised mechanisms include inhibition of DNA topoisomerases and DNA polymerase by PLP(Reference Matsubara, Mori and Matsuura65,Reference Matsubara, Mori and Akagi66) . In a study measuring mRNA expression levels of VEGF-A, VEGF-C and VEGF-D and their receptors VEGFR-2 and VEGFR-3 in seventy CRC (thirty-five with paired mucosae) and twenty adenomatous polyps, it was found that VEGF-D mRNA expression was significantly lower in both polyps and CRC compared with normal mucosa but VEGF-A and VEGF-C were significantly raised in CRC. It was postulated that decreased VEGF-D may enable higher levels of VEGF-A and VEGF-C to bind readily to the VEGF receptors, promoting tumour growth(Reference George, Tutton and Janssen67). Therefore, our observation that vitamin B metabolites were associated with VEGF-D but not VEGF-A might suggest that PLP associates with an anti-angiogenic microenvironment.
We observed inverse correlations between thiamine (vitamin B1) and its metabolite thiamine monophosphate with inflammation and angiogenesis biomarkers. Vitamin B1 plays a key role in supporting enzymes involved in cellular and carbohydrate metabolism and has been shown to inhibit cell growth(Reference Zastre, Sweet and Hanberry68). Thiamine supplementation trials have shown mixed results with respect to inflammation biomarkers(Reference Alaei-Shahmiri, Soares and Zhao69,Reference González-Ortiz, Martínez-Abundis and Robles-Cervantes70) , possibly due to the short intervention duration (1 month)(Reference González-Ortiz, Martínez-Abundis and Robles-Cervantes70), or thiamine deficiency at baseline(Reference Page, Laight and Cummings71). Preclinical studies have demonstrated elevated TNFα in thiamine-deficient mice(Reference de Andrade, Gayer and Nogueira72) and reduced TNFα and IL-6 after thiamine administration(Reference Menezes, Godin and Rodrigues73). Hypothesised mechanisms for thiamine’s anti-inflammatory potential include inhibition of mitogen-activated protein kinase (MAPK) signalling, a key inflammatory pathway(Reference Yadav, Kalariya and Srivastava74).
Total homocysteine was positively correlated with TNFα and VEGF-D. These findings support prior observations of homocysteine associations with inflammation(Reference Ross75). The inverse association of total homocysteine with IL-6 is not in line with prior studies(Reference Araki, Hosoi and Orimo76,Reference Gori, Corsi and Fedi77) and warrants investigation. However, IL-6 has been suggested to play both anti- and pro-inflammatory roles(Reference Scheller, Chalaris and Schmidt-Arras78,Reference Schett79) .
Our study utilised rigorously collected data from the well-established ColoCare Study, including state-of-the-art and highly reproducible biomarker measurements. Our results support prior findings of associations between some B vitamins and inflammation biomarkers but apply these findings to a CRC patient population. The current study also expands on prior work by measuring a more comprehensive panel of one-carbon and inflammatory biomarkers, in addition to angiogenesis biomarkers. Although cross-sectional, our observations of a relationship between B vitamins and angiogenesis biomarkers are novel and warrant further investigation in future prospective studies. The dietary intake of CRC patients might change after diagnosis depending on cancer treatment and on lifestyle modification. Considering that blood was drawn in our study within days of surgery and that B-vitamin half-lives are relatively long (e.g. 25 d for PLP(80)), it is unlikely that dietary intake changed substantially in this period to affect circulating vitamin status. We note some study limitations. Study participants were predominantly non-Hispanic white; thus, studies in diverse populations are warranted to enhance generalisability. Our inflammatory biomarker panel did not include potentially anti-inflammatory cytokines like IL-10. IL-10 has been shown to both associate with CRC tumour progression and to inhibit tumour growth depending on host immunity(Reference Evans, Morrison and Heriot81). Further investigation of the relationship between both IL-10 and CRC progression, and between IL-10 and B vitamins like PLP, may shed further light on this relationship. Finally, the cross-sectional study design precludes causal inference; therefore, results should be interpreted with caution and further studies are needed to determine the relevance of our observations to CRC outcomes.
In conclusion, our study showed that PLP and folate species within the one-carbon metabolism pathway are associated with both inflammation and angiogenesis pathways among CRC patients. These findings reinforce the notion that B vitamins involved in the one-carbon metabolism may be correlated with carcinogenic processes. Prospective studies are needed to determine whether modulation of B-vitamin status leads to changes in inflammation or angiogenesis pathways among CRC patients, and the influence on CRC prognosis to further reinforce B-vitamin status as a target for future dietary guidance in this population.
Acknowledgements
The authors thank all the study participants for their commitment and time to the ColoCare Study and the entire Heidelberg ColoCare team.
This work was supported by grants from the National Institutes of Health/National Cancer Institute (U01 CA206110, R01 CA189184 and R01 CA207371 to C. M. U.), the German Consortium of Translational Cancer Research (DKTK) and the German Cancer Research Center, the Matthias Lackas Foundation, Stiftung LebensBlicke, and Claussen-Simon Stiftung (Germany), and the Huntsman Cancer Foundation. This work was also supported by the ERA-NET, JTC 2012 call on Translational Cancer Research (TRANSCAN), JTC2013-FOCUS, project FOCUS I2104-B26 and the Federal Ministry of Education and Research, Germany (01KT1503). A. N. H. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute. E. H. R. was financially supported by Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund International grant program (grant number 2016/1620). J. L. K. was supported by a grant from Kankeronderzoekfonds Limburg as part of Health Foundation Limburg (Grant No. 00005739). The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA042014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the funding institutions had any role in the design, analysis or writing of this article.
R. K., C. M. U. and M. P. conceived and designed the study, wrote the manuscript and had primary responsibility for the final content; C. A. W., C. H. and R. K. conducted research; R. K., T. L. and M. P. analysed data; R. K., A. N. H., B. G., J. O., M. P. and C. M. U. participated in interpreting the data. All authors provided critical intellectual content to review the manuscript and read and approved the final manuscript.
C. M. U. has Cancer Center Director oversight over research funded by several pharmaceutical companies, but has not received funding directly herself. The other authors declare that there are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520000422











