Introduction
Livestock production, a major source of the greenhouse gas nitrous oxide (N2O), comprises 0.3–0.5 of total global N2O emissions (Oenema et al., Reference Oenema, Wrage, Velthof, van Groeningen, Dolfing and Kuikman2005). Nitrous oxide may be emitted during the storage of manure and when the stored manure is applied to pastures, or from excreta deposited while animals graze pasture. In New Zealand, manure collected on dairy farms is stored in anaerobic lagoons from where N2O emissions are assumed to be negligible (Ministry for the Environment, 2016), and hence N2O emissions from manure occur after application to pastures. Manure management and its subsequent application to land are also significant sources of ammonia (NH3) emissions to the atmosphere (Beusen et al., Reference Beusen, Bouwman, Heuberger, Van Drecht and Van Der Hoek2008); for cattle manure it is assumed that 0.35 of manure nitrogen (N) is lost as NH3 during storage, while 0.10 is assumed lost as NH3 if manure is applied to soil (IPCC, Reference Eggleston, Buendia, Miwa, Ngara and Tanabe2006; Hansen et al., Reference Hansen, Sommer, Hutchings and Sørensen2008).
Treatment or management aimed at reducing the emissions of a pollutant may lead to higher emissions of other pollutants, an effect referred to as pollution swapping (Stevens and Quinton, Reference Stevens and Quinton2009; Petersen and Sommer, Reference Petersen and Sommer2011). For example, Amon et al. (Reference Amon, Kryvoruchko, Amon and Zechmeister-Boltenstern2006) found that both separation and aeration of cattle slurry increased NH3 emissions, but reduced N2O emissions resulting from manure storage and field application. Conversely, an increasingly concentrated distribution of cattle slurry and co-fermented slurry was shown to reduce NH3 emissions, but increase N2O emissions (Wulf et al., Reference Wulf, Maeting and Clemens2002). However, there were no differences in NH3 losses from untreated and co-fermented slurry despite a higher pH of the latter, which was assigned to higher infiltration rates (Wulf et al., Reference Wulf, Maeting and Clemens2002). The relative contributions of N2O and NH3 emissions from manure are also influenced by animal feeding regimes, manure management and soil conditions after land application (Sommer et al., Reference Sommer, Génermont, Cellier, Hutchings, Olesen and Morvan2003; Chadwick et al., Reference Chadwick, Sommer, Thorman, Fangueiro, Cardenas, Amon and Misselbrook2011). There remains a need to better understand the processes responsible for N2O and NH3 emissions from manure applied to grazed pastures.
In New Zealand, there has been little research into practices that could reduce gaseous emissions after manure application to grassland, despite a 42% increase in manure collection between 1990 and 2011 (Ministry for the Environment, 2013). To improve management of liquid manure (slurry) the physical separation of the dry matter (DM) component of slurry could be introduced using devices such as screw presses, weeping walls or anaerobic settling ponds. Lowering the DM content of slurry is a practice with a known potential to reduce NH3 emissions due to faster infiltration of the slurry into soil when DM is removed or reduced (Sommer and Olesen, Reference Sommer and Olesen1991). This practice may also affect N2O emissions, which are driven by a complex balance between soil oxygen (O2) demand and O2 supply (Thomsen et al., Reference Thomsen, Pedersen, Nyord and Petersen2010), both of which are influenced by manure composition. For example, reducing liquid manure DM may reduce the potential blockage of soil pores (Bourdin et al., Reference Bourdin, Sakrabani, Kibblewhite and Lanigan2014), which implies that removal of DM by separation could influence N2O emissions by increasing the diffusive supply of O2 to sites of manure carbon (C) and N turnover. Balaine et al. (Reference Balaine, Clough, Beare, Thomas, Meenken and Ross2013) found a well-defined optimum relative gas diffusivity (Dp/Do; where Dp is the diffusion of oxygen through soil (cm2/s) and Do is the diffusion of oxygen through air (cm2/s)) for N2O emissions across a wide range of soil bulk densities and water contents. However, the potential effects of applied manure on Dp/Do and ensuing N2O emissions have not been studied.
The current study hypothesized that N2O emissions from manure-amended grassland soil would be related to Dp/Do near the soil surface, and that Dp/Do would in turn be modified by the manure DM content. This was tested by applying cattle manure (50 t/ha) with either a low DM (LDM) or a high DM (HDM) concentration to grassland soil and determining the NH3 and N2O emissions that ensued, while also measuring Dp/Do.
Materials and methods
Site description and treatments
The study was conducted at the Lincoln University dairy farm in the South Island of New Zealand (172°30′E, 43°38′S), where the soil (6% sand, 65% silt, 29% clay) is classified as a Wakanui silt loam (Udic Dystrochrept) (Kear et al., Reference Kear, Gibbs and Miller1967). The study site, with a soil pH of 6.0 (0–7.5 cm), was a perennial pasture with perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.), with <1% slope, on which cattle grazing was discontinued 2 months before the experiment started in order to avoid antecedent excreta effects. Pasture was cut, with foliage removed, to a height of 1–3 cm at initiation of the experiment. Cattle manure from the commercial dairy farm at Lincoln University, where cattle graze perennial ryegrass-white clover pasture throughout the entire year, was used. To simulate separation, manures were dried (see below) to determine proportion DM. Then one batch of untreated manure, at 0.06 (±0.01, n = 2) proportion DM, was adjusted to 0.16 (±0.01, n = 2) proportion DM, where error term equals standard error of the mean, by addition of solids obtained from the weeping-wall separation at the dairy milking parlour. Manure entering the weeping-wall comprised dung and urine deposited onto the concrete yard while cattle stood waiting to be milked, and water used to wash down the yard at the completion of milking. The experimental design was a randomized complete block consisting of three treatments: slurry at 0.06 DM (LDM), slurry at 0.16 DM (HDM) and a control (equal volume of water), replicated four times. The manure treatments were evenly applied to the 2 × 2 m2 plots using a watering can, with spray rosette removed, at a rate equivalent to 5 mm of irrigation, corresponding to 48 and 173 kg N/ha for the LDM and HDM treatments, respectively.
Manure characterization
Manure sub-samples were centrifuged at 3500 rpm for 20 min and filtered (Advantec 5C, Advantec FMS Inc., Dublin, CA, USA). Total ammoniacal nitrogen (TAN), the sum of NH3 + ammonium (NH4+), nitrate (NO3−) and nitrite (NO2−) concentrations of the manure were determined using standard colorimetric methods and an auto-analyser with detection limits for NO3−-N of 0.10 mg/l, NO2− of 0.01 mg/l and TAN of 0.01 mg/l (Alpkem FS3000 twin channel analyser, EZkem Hood River, OR, USA, application notes P/N A002380 and P/N A002423). Appropriate controls and standards were used to check for colour interference. Total dissolved organic carbon (DOC) concentrations were measured on the filtered samples using a Shimadzu TOC-Analyser (TOC 5000A, Shimadzu, Australia). Total N and C in manure were determined by freeze-drying sub-samples followed by combustion under an oxygen atmosphere in an automated Dumas style elemental analyser linked to a 20–20 stable isotope ratio mass spectrometer (PDZ, Europa Scientific, Crewe, UK). Manure pH was measured with an Inlab Expert Pro pH electrode (Mettler Toledo, Switzerland) and a SevenEasy pH meter (Mettler Toledo, Switzerland). The DM concentrations of the manures were determined gravimetrically after a 24 h drying period at 103 °C.
Soil sampling and soil water composition
During the study, air temperature (1 m) and soil temperatures (surface and 10 cm depth) were measured at the site, while rainfall data were obtained from a nearby (<1000 m) weather station.
From day 1 to 5 the pH of the soil was determined in the field with a portable pH meter (Schott Instruments HandyLab, Mainz, Germany) and a flat-surface pH electrode (Mettler Toledo, Switzerland), with five measurements per plot. When dry, plots were moistened with a drop of deionized water to wet the surface before measuring pH.
Soil was sampled from within the 2 × 2 m2 plots, avoiding the area inside the gas sampling chambers (as described below). Samples of the soil surface were collected by scraping the surface (0–0.2 cm) with a spatula, five random samples per plot, 2 h after slurry application, and then once per day for the first 5 days after application. Thereafter, soil surface samples were collected from the surface once a week. The samples were stored at −18 °C until analysis for TAN. At these same sampling sites, where the soil surface was sampled, further soil samples (0–7 cm) were collected by gently pressing a steel tube (7.3 cm internal diameter) into the soil. Each soil core was split into two fractions corresponding to 0–3.5 and 3.5–7.0 cm depth. Soil water content, NO3−, NO2−, DOC and TAN concentrations were determined as follows: soil water content was measured gravimetrically by drying for 24 h at 104 °C. Soil NO3−, NO2− and TAN were extracted by mixing and shaking soil for 30 min with 2 m potassium chloride (KCl [10 KCl : 1 soil, w/w]); the KCl suspensions were centrifuged at 3200 rpm for 20 min and filtered through Whatman 42 filter paper. The filtered sample was used for determination of NO3−, NO2− and TAN, using standard colorimetric techniques as noted above. Soil DOC determinations were performed using a 30-min cold water extraction (ratio of 1 g soil : 6 ml deionized water) followed by 20 min centrifugation (3500 rpm) and filtering (Advantec 5C, Advantec FMS Inc., Dublin, CA, USA) before analysis on a TOC analyser as described above.
Nitrous oxide emissions
The N2O emissions were determined using a static chamber technique (Hutchinson and Mosier, Reference Hutchinson and Mosier1981) at various times: (1) before slurry application on 4 May 2015, (2) for the first 4 days after slurry application and (3) every second or third day for the remainder of the experiment; monitoring continued until 8 June 2015. Emissions of N2O were determined at 10.00 h as this time has been shown to result in no bias when calculating daily emissions (van der Weerden et al., Reference van der Weerden, Clough and Styles2013). Static chambers were formed by inserting circular (internal diameter 36 cm) stainless steel bases, containing an annular channel for establishing a water seal, 10 cm into the soil. One chamber was placed in each of the 12 plots. During N2O emission measurements, a headspace cover with a rubber septum to enable gas sampling was lowered onto the base, creating a headspace 13 cm high. Water was placed in the annular channel to seal the chamber. Using a syringe, fitted with a stopcock and a hypodermic needle, a 10-ml gas sample was taken and transferred to an evacuated 6 ml Exetainer (Labco, High Wycombe, UK) at 0, 15 and 30 min after headspace closure.
For determination of gas sample N2O concentrations, the samples were injected into a carrier stream of N2 on a SRI-8610 gas chromatograph (GC, Torrance, CA, USA) equipped with a 63Ni capture detector (Pye-Unicam, Cambridge, UK) and a Two Haysep-D packed Column (6′ × 1/8″) Di Vinyl Benzene-DVB. Detector and column temperatures were 310 and 20 °C, respectively. The GC was interfaced to a liquid autosampler (Gilson 222XL, Middleton, WI, USA) which had been modified for gas analysis by substituting a purpose-built double concentric injection needle (PDZ-Europa, Crewe, UK) for the default liquid level detector and needle. This enabled the entire gas sample to be flushed rapidly from the sealed Exetainer onto the GC column. Fluxes of N2O, measured with the static chamber technique, were calculated using the linear regression approach in the free-ware HMR (Pedersen et al., Reference Pedersen, Petersen and Schelde2010), which provides a recommendation on best flux calculation method, as based on the concepts of Hutchinson and Mosier (Reference Hutchinson and Mosier1981).
Soil nitrous oxide concentration measurements
The concentration of N2O in the soil air at 5, 10, 20 and 50 cm depth was determined at 3- to 7-day intervals using a simple and robust diffusion probe (Petersen, Reference Petersen2014). The diffusion probes were inserted prior to manure application and sufficiently far from soil sampling plots and gas chambers to avoid creating artefacts. The probes had a 10-ml diffusion cell with a 3-mm diameter opening covered by a 0.5 mm silicone membrane. At sampling, the diffusion cell was flushed with 10 ml N2 containing 50 µl/l ethylene (C2H4) as a tracer; ethylene was removed immediately after sampling by flushing with nitrogen gas (N2). Tracer recovery was used to calculate sample N2O concentrations using the equations of Petersen (Reference Petersen2014) with correction for dead volumes of connecting tubes and valves.
Ammonia emission estimates
Ammonia emissions during the first 5 days were calculated using the empirical model of Sherlock et al. (Reference Sherlock, Freney, Bacon and van der Weerden1994). For this, wind speed was measured at 1.2 m with a cup anemometer (Sensitive Anemometer No. T16108/2, Casella London Limited, London, UK) with a low stalling speed, and soil temperature at the soil surface was measured with a LM 35 CZ thermometer (R.S. Components, Corby, UK), with all data logged as 60 min averages (CR800, Campbell Scientific Ltd., Shepshed, UK). Since soil pH and TAN were determined at points in time at daily intervals, an average wind speed from 12 h prior to the soil measurements to 12 h after soil measurement was used. This approach assumes that the averaged wind speed is representative of the 24 h period between soil samplings.
The equilibrium concentration of NH3 in the gas phase (NH3(g); μg NH3-N/m3) immediately above the source was calculated using the soil temperature (K), soil surface pH and the TAN concentrations in the 0–0.2 cm depth as follows:
Then, using an empirical equation developed previously for the same grassland site as used in the current study (Sherlock et al., Reference Sherlock, Freney, Bacon and van der Weerden1994), the flux of NH3 for a given plot was determined as follows:
where F is the flux of NH3 from the plot (μg NH3-N/m2/s), NH3(g) is the equilibrium concentration of NH3 in the gas phase immediately above the source, defined above and u is the average (defined above) wind speed (m/s) at 1.2 m. The ammonia equilibrium gas concentration (NH3(g)) was calculated using previously determined equilibrium constants (Petersen et al., Reference Petersen, Markfoged, Hafner and Sommer2014).
Air permeability and relative gas diffusivity
An in-situ method, similar to that described by Iversen et al. (Reference Iversen, Schjønning, Poulsen and Moldrup2001), was used to measure the air permeability (AP) of the soil. The AP was measured on soil cores, taken by inserting 7.3 cm internal diameter stainless steel rings to a depth of 7.4 cm. These were collected on days 7 and 24, giving a total of 24 cores. To create a flow of air through the soil, a cylinder of dry compressed air was connected via a regulator to a variable flow meter (0–60 litres/min capacity). The regulator on the gas cylinder was manipulated until steady flows through the soil ring of 5, 10, 20, 30 and 50 litres/min were reached. The AP of the soil was calculated using the recommended method of Ball and Schjønning (Reference Ball, Schjønning, Dane and Topp2002) where AP (k a) is calculated by solving Eqn (3) for k a, and where q v is the volumetric flow rate of air (L 3/T), ΔP a is the pressure difference across the sample (M/L/T 2), A is the shape factor and h is the gas viscosity (M/L/T), where M, L and T are mass, length and time, respectively:
Values of the shape factor A (L) are derived from cylinder dimensions and insertion depth according to Eqn (4) where D and H are cylinder diameter and insertion depth (L), respectively (Liang et al., Reference Liang, Bowers and Bowen1995):
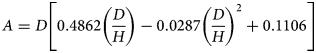 $$A = D\left[ {0.4862\left( {\displaystyle{D \over H}} \right)-0.0287{\left( {\displaystyle{D \over H}} \right)}^2 + 0.1106} \right]$$
$$A = D\left[ {0.4862\left( {\displaystyle{D \over H}} \right)-0.0287{\left( {\displaystyle{D \over H}} \right)}^2 + 0.1106} \right]$$Soil Dp/Do (0–7 cm) was determined using the method of Rolston and Moldrup (Reference Rolston, Moldrup, Dane and Topp2002). In brief, a chamber containing a calibrated O2 sensor (KE-25, Figaro Engineering Inc., Osaka, Japan) was purged with a gas mixture (0.9 argon [Ar] and 0.1 N2) while the base of the soil core, of the same dimensions as described for AP, was isolated from the chamber. Then, after exposing the chamber to the soil surface, O2 diffused through the soil core into the chamber, and over a period of 120–180 min the change in O2 concentration was recorded as a function of time while assuming O2 consumption was negligible (Moldrup et al., Reference Moldrup, Olesen, Gamst, Schønning, Yamaguchi and Rolston2000). A log-plot of the relative O2 concentration v. time enabled Dp (O2 diffusion coefficient in soil) to be calculated according to Rolston and Moldrup (Reference Rolston, Moldrup, Dane and Topp2002). Diffusivity calculations were performed at 25 °C. The value of Do (O2 diffusion coefficient in air) at 25 °C was assumed to be 0.074 m2/h (Currie, Reference Currie1960). Relative gas diffusivity was expressed as Dp/Do.
Data analysis
Statistical analyses were performed using R (R Core Team, 2014). Differences between treatment means at P ⩽ 0.05 were assessed with one-way analysis of variance, and where differences were detected, Tukey's Honest Significant Difference test was applied. Measurements made at different times and depths were analysed separately in order to focus on effects of slurry application. Based on results from graphical tests for normality and homogeneity of variance, the N2O-N flux and DOC data were log10 transformed prior to analysis. Simple and multiple linear regression models were used to test for correlation between soil surface DOC or Dp/Do and N2O-N fluxes.
Results
Manure and soil characteristics
HDM manure contained three times as much DM as the LDM treatment (Table 1) and although there were no significant differences in the proportions of total C and N of the freeze-dried manure samples (Table 1), this resulted in higher N and C inputs. The TAN concentration of applied manures also did not vary statistically (Table 1). However, the manure pH was higher in the HDM treatment (Table 1; P < 0.01).
Table 1. Liquid manure characteristics

Data are means with s.e.m. (n = 3) in brackets, where DM and TAN are dry matter and total ammoniacal nitrogen, respectively.
a Measured on freeze-dried samples.
Soil surface temperatures were typical for the autumn season, with daily averages initially varying from 11 to 18 °C, and later declining to between 4 and 12 °C (Fig. 1). The late summer and autumn of 2015 was very dry, and only 7.8 mm of rain fell during the first 30 days of the study (Fig. 1). It was decided, therefore, to irrigate the plots with 9 mm on day 30, but several days with rain then followed with cumulative rainfall equalling 33.4 mm by day 37 (Fig. 1). Wind speeds ranged from 0.8 to 3.8 m/s.
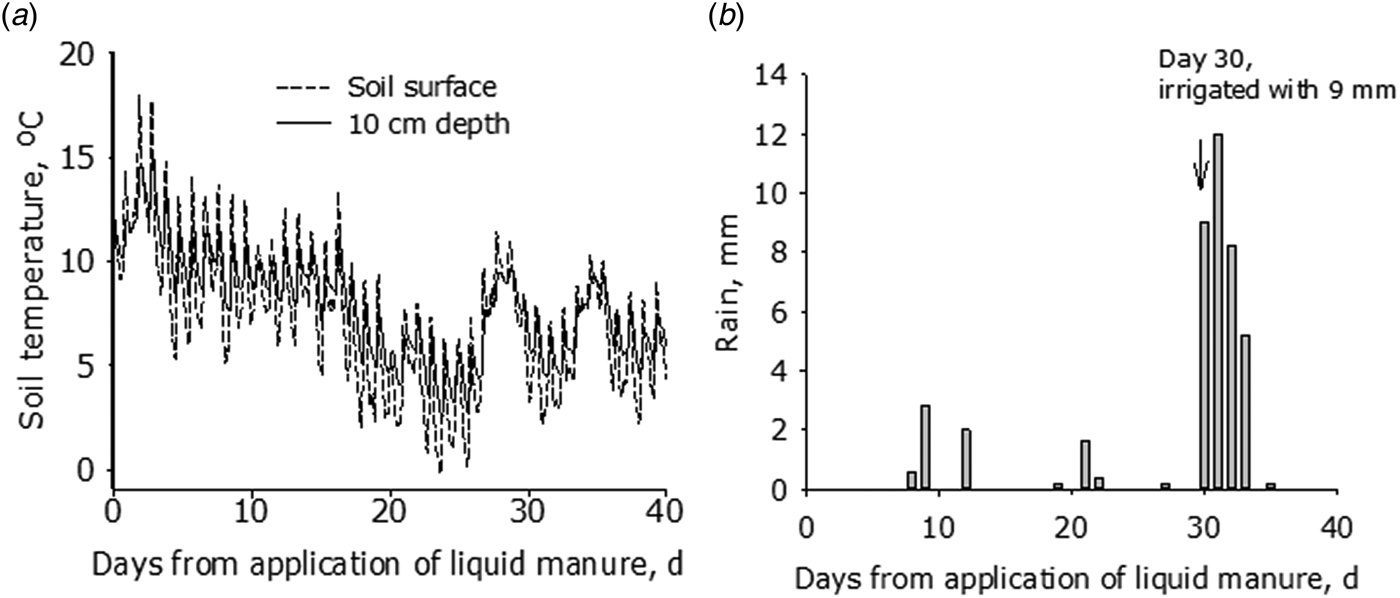
Fig. 1. Meteorological data over the course of the experiment from 4 May to 8 June 2015. (a) Soil temperature at the surface and 10 cm depth and (b) rainfall and irrigation.
Soil air-filled porosity and AP (Fig. 2) were not affected significantly by manure application. In contrast, soil gas diffusivity, Dp/Do, at the soil surface (0–7 cm) had declined (P < 0.05) following the addition of both LDM (0.017) and HDM (0.015) manures, when compared with untreated soil (0.035), 7 days after manure application, with no difference due to manure DM levels. After 24 days, however, Dp/Do did not differ significantly (0.034–0.036) between manure-treated and untreated soil (Fig. 2).
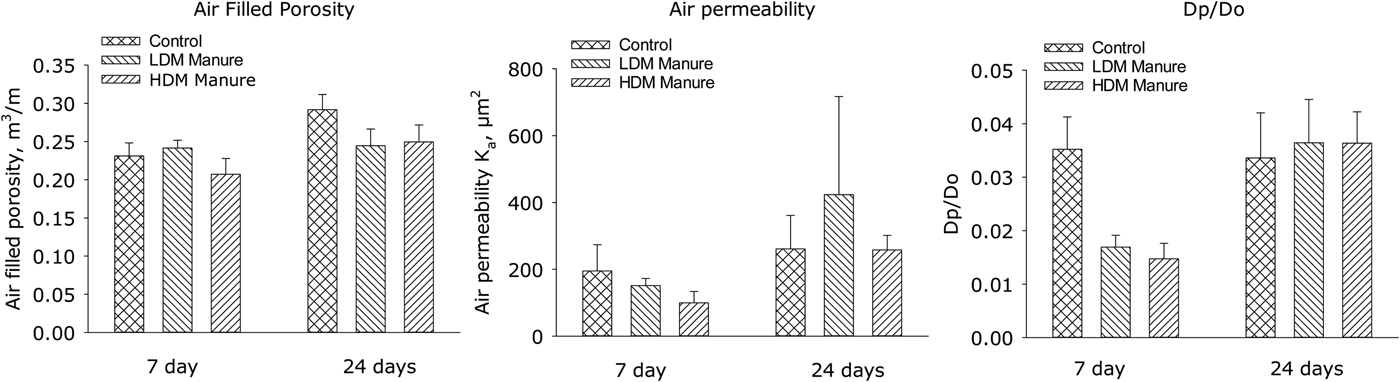
Fig. 2. Air-filled porosity, air permeability and relative gas diffusivity (Dp/Do) in the soil surface (0–7 cm) as affected by liquid manure application (error bars: s.e., n = 4).
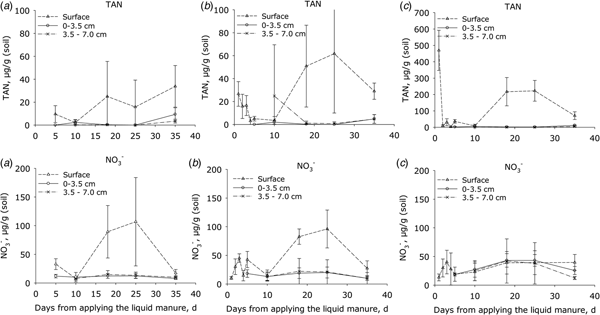
Initially the TAN concentrations (Fig. 3) of manure-amended soil were consistently higher at the soil surface than in the soil below (P < 0.001). The TAN concentrations then declined by day 5 following application but by day 18, concentrations at the surface had increased again in all treatments and peaked after 20–30 days; thereafter, the concentration declined. This trend was similar for both manure treatments, but in plots receiving HDM manure the concentration of TAN at the start and after 20–30 days was 10 and 3 times higher, respectively, at the surface than in plots receiving LDM manure and the control (P < 0.001). There were no differences in the sub-surface TAN concentrations between the LDM-treated and control plots over this period (Fig. 3).
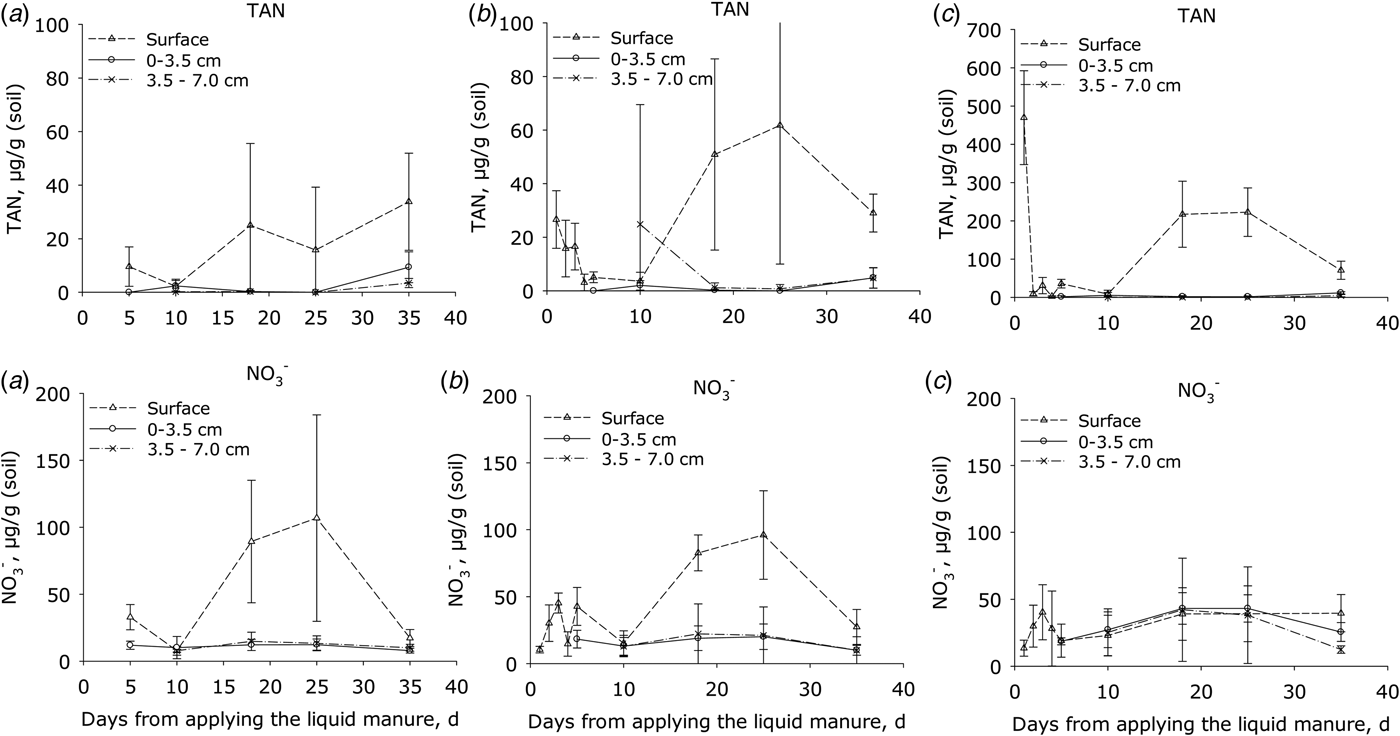
Fig. 3. Concentrations of total ammoniacal nitrogen (TAN = NH4+ + NH3) and nitrate (NO3−) in surface soil samples. (a) Control plots, (b) plots amended with LDM manure and (c) plots amended with HDM manure. Notice that the Y axis scale is larger for the upper right diagram (C; TAN) than in the two other diagrams in the line (error bars: s.e.m., n = 4).
In the surface layer of the control (P < 0.001) and LDM treatment (P < 0.05), soil NO3− concentrations were higher than in the sub-surface (P < 0.001). In both of these treatments, the concentration of NO3− in the surface layer increased after ca. 10 days, peaking by day 25, and then declined to background levels by day 35. In contrast, the plot amended with HDM manure showed no significant differences in NO3− concentrations when comparing surface or sub-surface concentrations (Fig. 3). The surface NO2− concentrations ranged from 0 to 0.6 µg/g in the control and LDM plots. After 3 days, the NO2− concentrations were 2–4 µg/g in the HDM plots (data not shown), higher (P < 0.005) than in LDM plots.
On day 10, DOC concentration at the soil surface was higher in the HDM treatment than in either the LDM or the control treatments (P < 0.001), which did not differ from each other (Fig. 4). Soil depth affected DOC (P = 0.001) in manure-treated plots (Fig. 4).
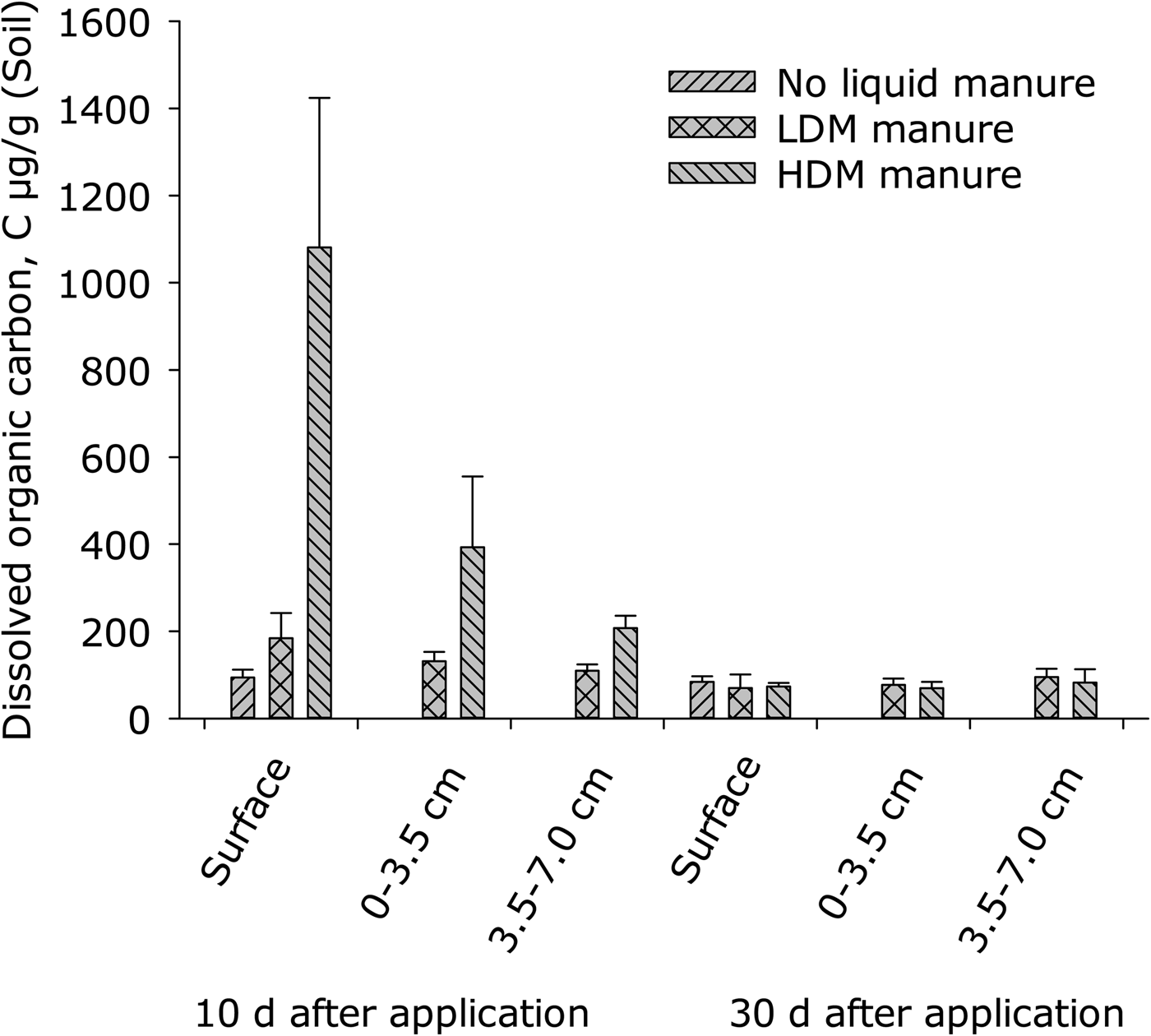
Fig. 4. Dissolved organic carbon (DOC) at day 10 and day 30 after application of slurry (error bars: s.e.m., n = 4). Surface refers to 0–0.2 cm depth. Control values at 0–3.5 cm and 3.5–7.0 cm are not presented.
The pH of LDM and HDM manures were 6.5 and 7.7, respectively, prior to manure application (Table 1). Soil surface pH of the untreated plots was 6.5 during the course of the experiment. The pH of the HDM soil immediately after manure application was 7.8 and this declined to 6.7 during the first 5 days after manure application. The pH values at the soil surface in the LDM-treated plots were initially 6.3 and increased on day two to 6.7 and remained at 6.6 thereafter.
Ammonia and nitrous oxide fluxes
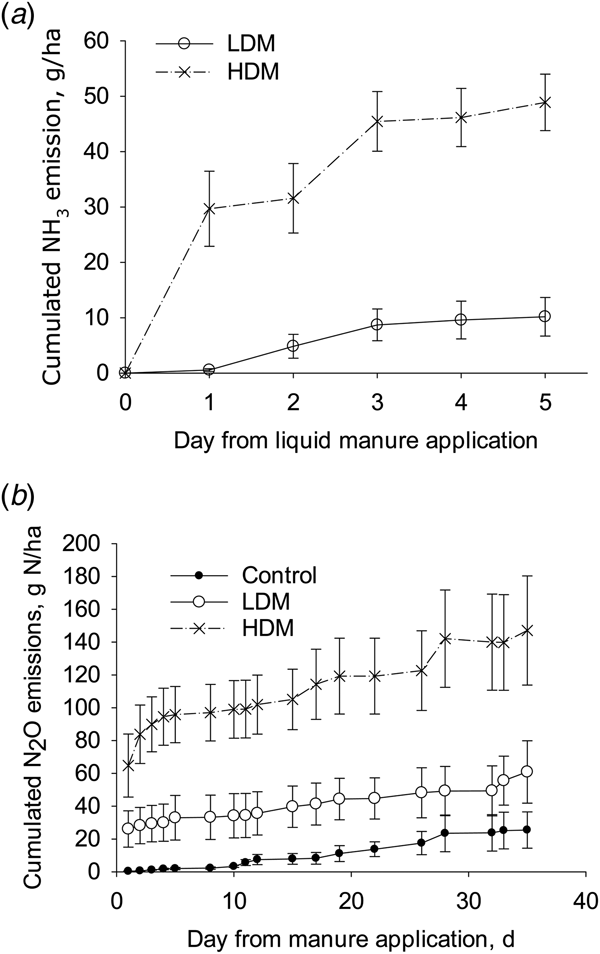
The calculated cumulative NH3 emissions from the HDM treatment (49 g N/ha; s.e. 2.5, n = 4) were higher than those from the LDM plots (10 g N/ha; s.e. 1.7, n = 4) when expressed either as a flux (P < 0.01) or as a proportion of TAN applied (P < 0.01) with mean NH3 fluxes corresponding to 0.050 and 0.012 of TAN applied, respectively. As a proportion of total-N applied, the cumulative NH3 emissions were 0.0003 and 0.0002 of total-N applied in the HDM and LDM treatments, respectively (Fig. 5).
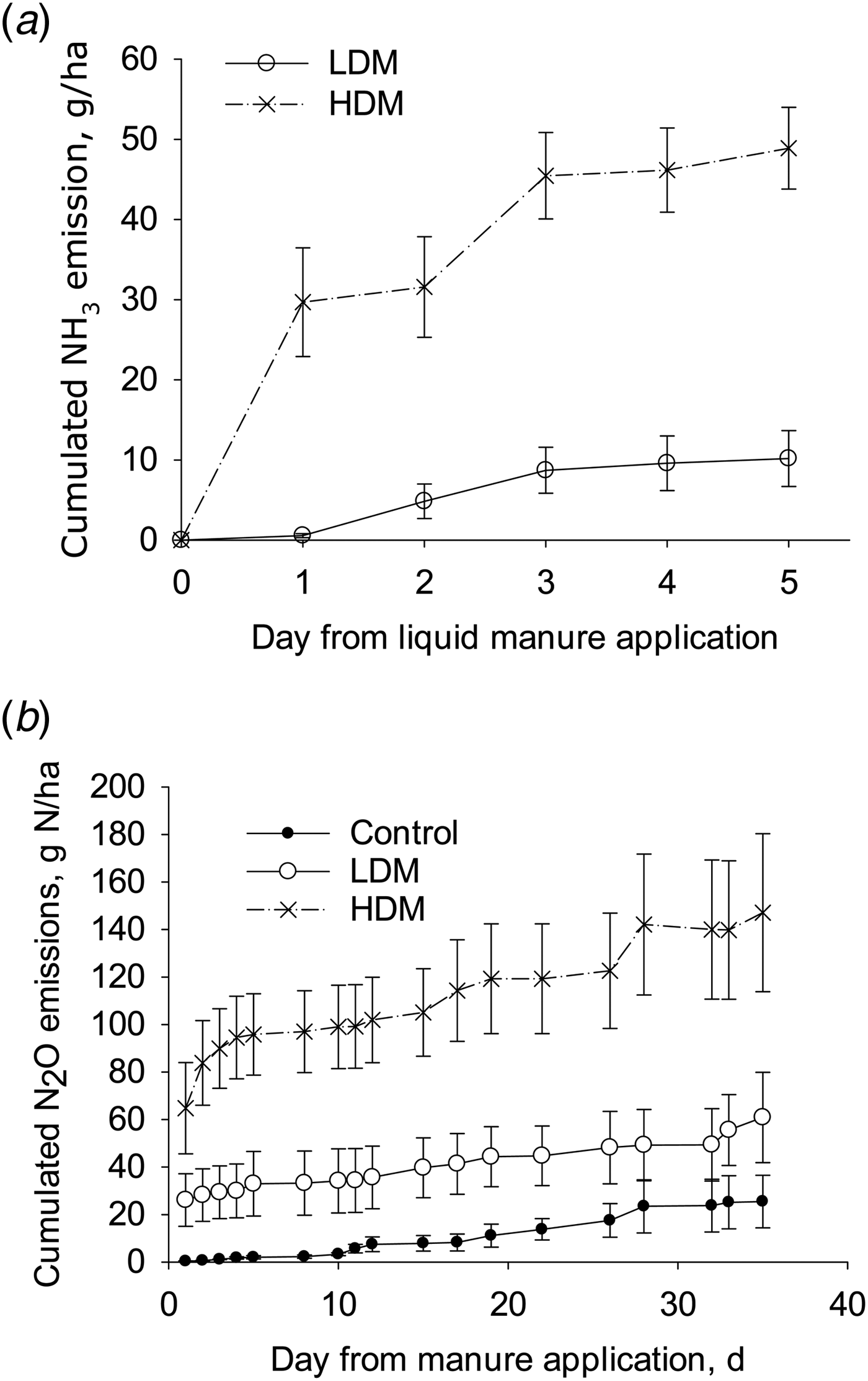
Fig. 5. Cumulative NH3 emissions (a) and cumulative N2O emissions (b) from LDM manure or HDM manure treatments (error bars: s.d., n = 4).
The cumulative N2O emissions from the HDM plots were higher than from the control (P = 0.01), but not from the LDM plots, with no difference in the cumulative N2O emissions when comparing LDM plots with control plots (Fig. 5). After 5 days the daily N2O emission rate was larger from HDM plots than from LDM and control plots. Cumulative N2O emissions from control, LDM and HDM plots equalled 25 g N/ha (s.e. 11, n = 4), 61 g N/ha (s.e. 19, n = 4) and 147 g N/ha (s.e. 34, n = 4), respectively. The cumulative N2O emissions from the LDM and HDM plots did not differ significantly when expressed as a proportion of either TAN or total-N applied. In the LDM and HDM treatments these values corresponded to 0.08 and 0.15 of TAN applied or 0.00094 and 0.00072 of total-N applied, respectively.
Linear regression showed that mean DOC (10 days) explained the variability in the cumulative N2O emissions (R 2 = 0.75) and multiple linear regression analysis showed that N2O emission was significantly (P < 0.05) related to DOC measured on 10 days (Fig. 6). Log N2O fluxes were not linearly related to log(Dp/Do).
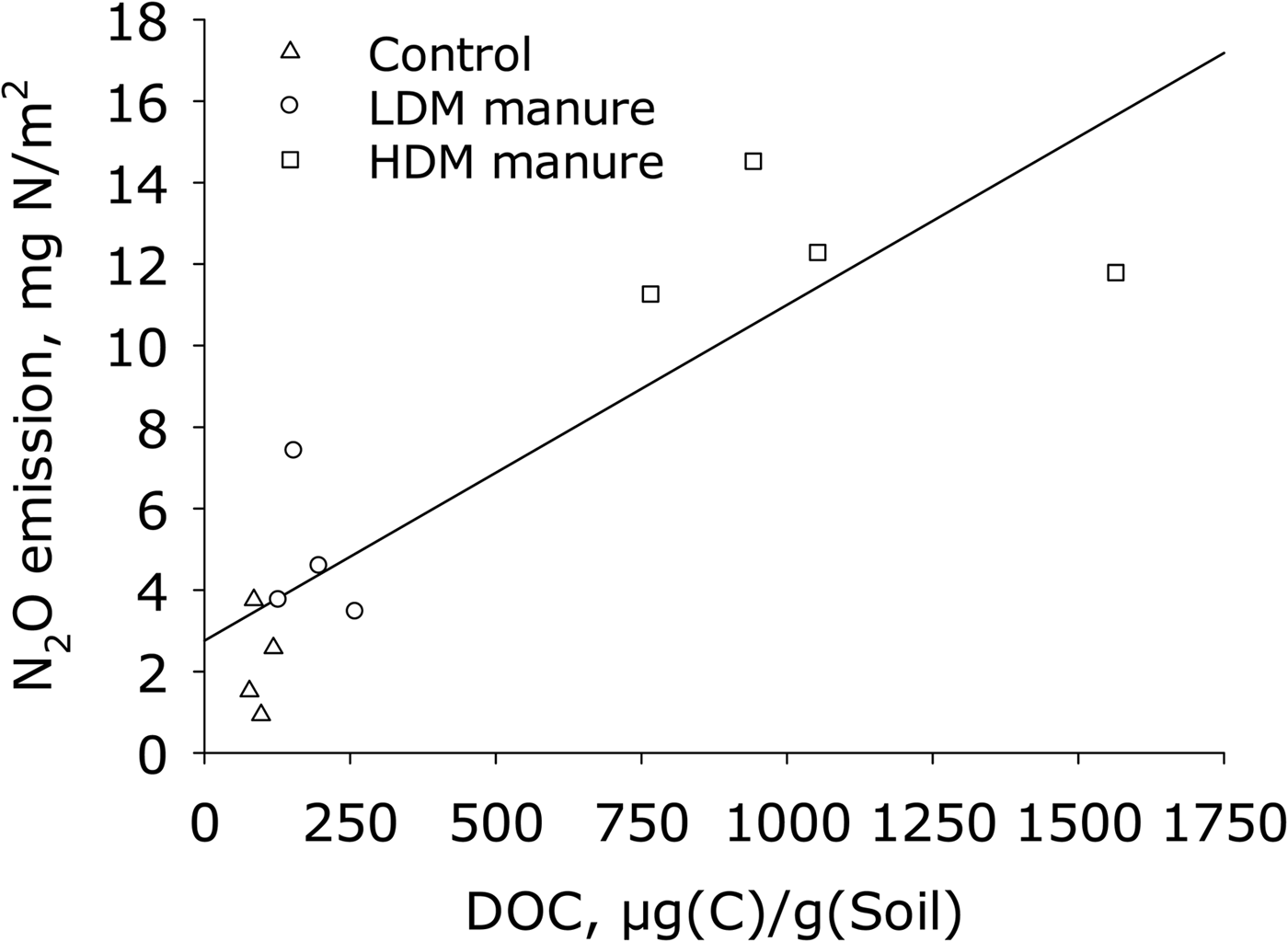
Fig. 6. Cumulative N2O emissions v. DOC at the surface 10 days after application of manure (A) and v. mean Dp/Do (n = 3), measured 7 days after application of manure. Regression equation: y = 0.0041x + 1.4; R 2 = 0.75.
Discussion
The DM concentration in the LDM manure was comparable with previous studies (Bourdin et al., Reference Bourdin, Sakrabani, Kibblewhite and Lanigan2014; Fangueiro et al., Reference Fangueiro, Surgy, Fraga, Cabral and Coutinho2015), while for HDM manure the level was relatively high and may be compared with that of the fibre fraction of slurry separated with a filter press (Møller et al., Reference Møller, Lund and Sommer2000); however, it was lower than the 0.25–0.40 DM typically achieved by screw presses (DairyNZ, 2013).
Concentrations of TAN (15.8–19.9 mg/l) in the manure were low when compared with an earlier study using pig slurry applied to the same pasture (Sherlock et al., Reference Sherlock, Sommer, Khan, Wood, Guertal, Freney, Dawson and Cameron2002) and when compared with the study that derived Eqn (2) following ruminant urine application to pasture (Sherlock et al., Reference Sherlock, Freney, Bacon and van der Weerden1994). A meta-analysis showed that concentrations of TAN in New Zealand dairy shed manure vary markedly from 36 to 1400 mg/l, potentially reflecting seasonal changes in pasture quality and farm management practices and thus the manures applied in the current study are at the lower end of this range.
The initial decline in soil TAN concentration after application of manure (day 0 to 10) is a common observation and is attributed to volatilization of NH3 and microbial immobilization, probably using volatile fatty acids as an easily digestible carbon source (Kirchmann and Lundvall, Reference Kirchmann and Lundvall1993). Given the relatively low NH3 losses it is possible immobilization was also responsible for the initial decline in TAN. However, the changes in soil pH and NH4+ concentration were also consistent with occurrence of NH3 volatilization and the initial decline in TAN. The increase in surface layer TAN after day 10 probably resulted from mineralization of manure N but, due to the low pH of the surface soil (<7.0) by this time, emissions of NH3 would have been relatively low.
There was a notable lack of NO3− accumulation in the surface layer of the HDM manure treatment. Since the temporal dynamics of TAN accumulation and removal were comparable with the treatment with LDM manure, it suggests that nitrification took place in both treatments at the soil surface but that there was a denitrification sink for NO3− at the soil surface in the HDM manure treatment. Past studies have documented that O2 disappears rapidly (Petersen et al., Reference Petersen, Nielsen, Frostegård and Olesen1996; Markfoged et al., Reference Markfoged, Nielsen, Nyord, Ottosen and Revsbech2011) and coupled nitrification–denitrification develops around manure–soil interfaces (Petersen et al., Reference Petersen, Henriksen and Blackburn1991, Reference Petersen, Nielsen, Haarder and Henriksen1992), which may also result in release of N2O (Nielsen and Revsbech, Reference Nielsen and Revsbech1998).
Ammonia emissions as a proportion of N applied were low when compared with previous studies. For example, losses of NH3 from slurry have been recorded to range from 0.04 to over 0.60 (Sintermann et al., Reference Sintermann, Neftel, Ammann, Häni, Hensen, Loubet and Flechard2012), while losses from urine and dung reportedly accounted for 0.26 (±0.020) and 0.12 (±0.027) of the deposited urine-N and dung-N deposited, respectively (Laubach et al., Reference Laubach, Taghizadeh-Toosi, Gibbs, Sherlock, Kelliher and Grover2013). These relatively low emissions can be attributed to differences in substrate supply, the relatively low soil pH, relatively low soil temperatures (10–15 °C) and low-wind speeds (0.8–3.8 m/s) that occurred (Søgaard et al., Reference Søgaard, Sommer, Hutchings, Huijsmans, Bussink and Nicholson2002) and because the dry soil would have enhanced the infiltration of liquid from the manure (Sommer and Jacobsen, Reference Sommer and Jacobsen1999). Cumulative emissions of NH3 from plots amended with LDM manure were lower than in HDM plots due to either the rate of TAN applied in the LDM treatment tending to be lower, the lower manure pH of the LDM treatment, or possibly because liquid from the LDM manure better infiltrated into the soil as shown by the lower initial TAN concentration in the surface layer (Braschkat et al., Reference Braschkat, Mannheim and Marschner1997). Conversely, the HDM manure contained a high amount of water in the surface following the first 2–3 days due to the capacity of organic matter in manure to retain water (Petersen et al., Reference Petersen, Nissen, Lund and Ambus2003). The initial decline in NH3 emission rates from manure-amended plots coincided with declining TAN concentrations and a decline in pH at the surface.
The NH3 flux method used is an indirect method based on soil measurements and facilitates a clear understanding of the dynamics, key variables and their interactions driving NH3 emissions (e.g. TAN, soil pH and depth). It is a simply applied protocol that can be followed easily. However, limitations and bias may arise if data required for the indirect method are not collected with sufficient frequency to account for potential nocturnal (e.g. wind) or diel (e.g. soil temperature) trends. With adequate data sets, however, the indirect method used in the current study is capable of determining low NH3 fluxes as derived here. Ideally, field experiments could also be performed for verification of indirect flux measurement and to further compare the NH3 flux method with measured emissions under differing N substrates.
The cumulative emissions of N2O from the manure-amended plots, as a proportion of total N applied, were also low when compared with results of a meta-analysis examining N2O emissions from dairy manure applied to grassland (van der Weerden et al., Reference van der Weerden, Cox, Luo, Di, Podolyan, Phillips, Saggar, de Klein, Ettema and Rys2016). These relatively low cumulative emissions are probably due to the manure being applied to a relatively dry soil (Luo et al., Reference Luo, Saggar, Bhandral, Bolan, Ledgard, Lindsey and Sun2008); however, they will also be a function of the shorter duration of the current study. Initial N2O emissions were higher from manure-amended compared with control plots. This is in accordance with the higher inorganic-N and DOC availability in manure-amended soil, and in particular the higher DOC availability in HDM manure represented a sink for O2 that could support O2 limited conditions even close to the soil surface. Soil gas diffusivity also changed for a period, as indicated by Dp/Do, and this would also restrict supply of O2 to manure–soil interfaces (Balaine et al., Reference Balaine, Clough, Beare, Thomas, Meenken and Ross2013; Baral et al., Reference Baral, Arthur, Olesen and Petersen2016). The higher daily N2O emissions, after day 5, from the HDM plots, were thus due to total TAN and DOC concentrations being higher than in the control and LDM plots.
There was a strong relationship between DOC and accumulated N2O across all treatments, suggesting that under the conditions of the current study, where manure was applied to grassland during a dry autumn, the O2 demand associated with heterotrophic processes was a driver for N2O production. Petersen et al. (Reference Petersen, Nielsen, Frostegård and Olesen1996) found that around 0.90 of degradable organic matter in manure was metabolized with O2 as the electron acceptor, and only 0.10 via denitrification when cattle manure was applied to a sandy loam soil at field capacity. This ratio could be shifted even more towards aerobic decomposition in drier soil (Baral et al., Reference Baral, Arthur, Olesen and Petersen2016). Relative gas diffusivity, Dp/Do, on days 7 or 24 did not explain the variability in the N2O emissions from the LDM and HDM manures, which was in contrast to previous studies with either NO3−, urea, or ruminant urine application (Balaine et al., Reference Balaine, Clough, Beare, Thomas, Meenken and Ross2013, Reference Balaine, Clough, Beare, Thomas and Meenken2016; Owens et al., Reference Owens, Clough, Laubach, Hunt, Venterea and Phillips2016, Reference Owens, Clough, Laubach, Hunt and Venterea2017). Baral et al. (Reference Baral, Arthur, Olesen and Petersen2016) also found a relationship between Dp/Do and N2O emissions, but showed there was a dynamic interaction between O2 consuming processes and O2 supply. In accordance with this, Petersen et al. (Reference Petersen, Ambus, Elsgaard, Schjønning and Olesen2013) found different relationships between N2O emission and Dp/Do depending on C input in four crop rotations, and proposed that this was due to a higher O2 demand associated with higher C inputs. It is possible that the measurements of Dp/Do by day 7 did not represent the potential dynamic range in Dp/Do during the initial phase of the experiment – for example if highly labile C had been consuming O2 in the first days of the experiment, it would mean that O2 limitation supporting N2O emissions could occur at a higher Dp/Do value.
Identifying pathways of N2O production was beyond the scope of the current study; however, the lower soil NO3− concentration under the HDM treatment, together with the positive relationship between DOC and accumulated N2O emissions and the lower Dp/Do in both LDM and HDM treatments after 7 days, indicate that denitrification was a dominant source of N2O. This may not have been the case by day 24, when there was no treatment effect on Dp/Do. However, it is well-known that organic matter applied with manure can enhance N2O production (Chadwick et al., Reference Chadwick, Sommer, Thorman, Fangueiro, Cardenas, Amon and Misselbrook2011). Baral et al. (Reference Baral, Arthur, Olesen and Petersen2016), using 15N-labelling, also found that denitrification was the main source of N2O from surface-applied cattle manure independent of soil water content. A recent study under wet conditions concluded that denitrification was the main N2O source in grassland soil following surface application of cattle manure (Van Nguyen et al., Reference Van Nguyen, Wu, Kong, Bol, Petersen, Jensen, Liu, Brüggemann, Glud, Larsen and Bruun2017).
It is recognized that NH3 emissions are affected by TAN (Huijsmans et al., Reference Huijsmans, Hol and Vermeulen2003) and by the manure DM content (Sommer and Olesen, Reference Sommer and Olesen1991). While manipulating TAN can alter NH3 emissions, so too can manipulation of the manure DM content, due to changes in the water retention capacity of manure at varying DM contents altering infiltration of TAN into soil. When manipulating manure to mitigate NH3 emissions, pollution swapping must also be considered. The lower NH3 emissions associated with the reduced DM content in LDM manure did not result in higher N2O emissions in the current study, which is in contrast to an Irish study where DM was adjusted before application of manure to grassland (Bourdin et al., Reference Bourdin, Sakrabani, Kibblewhite and Lanigan2014). In the study by Bourdin et al. (Reference Bourdin, Sakrabani, Kibblewhite and Lanigan2014), higher NH3 emissions reduced significantly the residual N available for N2O production in the soil. However, pollution swapping did not occur if slurry was applied in spring when plant demand for N was higher. Soil moisture, driven by seasonal rainfall, also strongly influenced N2O emissions in the study by Bourdin et al. (Reference Bourdin, Sakrabani, Kibblewhite and Lanigan2014) and thus soil moisture may be a strong determinant of the potential for pollution swapping. This indicates that not only manure specifications but also the environmental conditions at the application site must be considered to prevent pollution swapping.
The current study was of a relatively short-term duration (37 days) and while the majority of NH3 emissions will have occurred over this period there may be trade-offs between removing DM from dairy manure that would otherwise be applied and improvements in both pasture nutrient availability and soil quality (organic C amendments). An answer to this is beyond the scope of the current study but the potential impacts of DM separation over the long term are unknown and need to be addressed in future work. The current study was performed at a plot scale and so in situ manure management, involving manure DM separation, needs to be assessed at the farm scale with respect to gaseous N losses. Furthermore, future manure management studies should assess the influence of DM separation across a wider range of embodied TAN.
In summary, cumulative N2O emissions from livestock manure applied to grassland on well-drained soils were lower with a lower DM content in the manure if the emissions are considered simply as a gross flux (g/ha). This was probably due to a lower DOC concentration in the soil amended with the lower DM animal manure. However, when expressed as a proportion of TAN or total-N applied cumulative N2O, emissions did not differ with treatment. In the current study, where the soil was relatively dry, the N2O emissions were not related to gas diffusivity of the soil, 7 days after manure application, however, soil gas diffusivity did vary with treatment, declining with manure application at 7 days. Applying dairy shed manure to a dry soil, with the DM reduced in order to reduce NH3 emissions, did not cause pollution swapping in the form of increased N2O emissions in this instance.
Acknowledgements
The authors would like to thank Neil Smith for his technical assistance and Sasha Hafner for support in the statistical data analysis.
Financial support
This work was made possible by a Global Research Alliance Senior Scientific (GRASS) fellowship to S. G. Sommer from the Livestock Emissions and Abatement Research Network, and the Cleanwaste project financially supported by the Innovation Fund Denmark.
Conflict of interest
None.
Ethical standards
Not applicable.