INTRODUCTION
Q fever, caused by Coxiella burnetii, is a worldwide zoonosis with goats, sheep, and cattle as primary sources for human infections [Reference Maurin and Raoult1]. Humans are usually infected by inhalation of contaminated aerosols originating from parturient animals and their birth products [Reference Maurin and Raoult1–Reference Gonder3]. Acute Q fever presents itself as a self-limiting febrile illness, pneumonia or hepatitis, with a small proportion developing chronic infections (mainly endocarditis and vascular infections) [Reference Raoult4, Reference van der Hoek5].
From 2007 until 2009, large Q fever outbreaks occurred in The Netherlands, with over 3500 human cases notified [Reference van der Hoek6]. Abortion waves at dairy goat farms were the primary source of these infections [Reference Karagiannis7–Reference Schimmer9]. Between 2006 and 2008, C. burnetii abortion waves occurred on two dairy sheep farms [Reference Schimmer9]. Infected non-dairy sheep farms were not associated with an increased number of human cases living near these farms [Reference van der Hoek10], although cases occurred in individuals living a small distance from or having direct contact with non-dairy sheep in The Netherlands [Reference Koene11, Reference Whelan12]. Internationally, several sheep-related Q fever outbreaks have been reported [Reference Gilsdorf13–Reference Manfredi19].
In The Netherlands, sheep farms can be distinguished from dairy farms and fat lamb-producing farms. There is a small dairy sheep industry with <50 farms, in which sheep are usually milked twice a day during several months each year. The number of sheep per farm differs from <50 to almost 1000 with most kept outdoors for part of the year. On the fat lamb-producing sheep farms the sheep are kept outside, except for a few weeks around lambing, which usually occurs inside. Except for meat production, non-dairy sheep are also kept for breeding purposes or nature management.
So far, no international studies have addressed the seroprevalence and risk factors for acquisition of C. burnetii infection in sheep farmers and their household members. Therefore, our aim was to determine the C. burnetii seroprevalence in both dairy and non-dairy sheep farmers and their household members, and for the large non-dairy sector, to identify individual and farm-related risk factors for seropositivity.
MATERIAL AND METHODS
All dairy sheep and non-dairy sheep farms in The Netherlands with at least 100 breeding ewes in November 2008, according to the national identification and registration database, were eligible. A minimum of 100 ewes, considered to be a professional farm, was chosen because in the early stage of the Dutch epidemic it was clear that only (relatively large) commercial (dairy goat) farms were incriminated as a potential source; no obvious role for small farms was observed [Reference Schimmer9]. Besides, smaller hobby farms have different management and farm residents of those farms are assumed to have a more limited exposure to sheep-related pathogens compared to commercial farms. Between September and December 2009, 32 dairy sheep farmers were approached for the study. In addition, in March and April 2010, 1344 non-dairy sheep farmers were approached for participation. At the time of inclusion in 2010, those farms with at least 60 unvaccinated breeding animals were kept in the study. Farms with vaccinated sheep were excluded because in this integrated human-veterinary study the sheep at these farms were likely to be seropositive due to vaccination; vaccine-induced and naturally induced seroresponses cannot be distinguished to assess the true seroprevalence from natural infection. Second, we assumed that the infection rate for farm residents could be different for farms with vaccinated sheep (leading to reduced exposure) compared to farms with unvaccinated sheep. About 3 weeks after the initial invitation, all non-responding farmers were sent a written reminder. Because of the small number, dairy sheep farmers who did not respond to this second invitation were contacted by telephone.
After written informed consent, a maximum of three persons were selected from each farm, i.e. the farmer and a maximum of two family members aged ⩾12 years residing at the farm; in some instances other persons working or living on the farm were selected. Each participant received a questionnaire addressing individual-based risk factors like age, gender, profession, ownership or contact with ruminants and pets, consumption of unpasteurized milk, medical history, and contact with agricultural products. In addition, the farm owner or farm manager completed a farm-based questionnaire addressing characteristics like farm hygiene and management, herd size, presence of other livestock and pets, stable environment, and lambing season characteristics. Separate farm-based questionnaires were developed for dairy farms and non-dairy farms because of clear differences in farm management. A professional laboratory assistant visited the farms to collect blood samples from all participating individuals for serology. All data of the dairy sheep farms were collected between September 2009 and September 2010, for the non-dairy sheep farms data were collected between April and September 2010. The Medical Ethical Commission of the University Medical Center Utrecht approved the study protocol (no. 09–189/K).
Serological analysis
Serum samples were tested for C. burnetii IgM and IgG antibodies, both phases I and II, using an indirect immunofluorescence assay (IFA) with a screening dilution of 1:32. Participants without any positive antibody result and participants with a solitary IgM phase I or solitary IgM phase II result were classified as seronegative. All other outcomes were classified as seropositive. Those with IgM phase II antibodies were designated as ‘relatively recent infections’ and included possible current infections. The term ‘relatively recent’ was chosen as IgM phase II is found to persist in the majority of cases for 1 year post-infection and may even persist up to 4 years post-infection [Reference Wegdam-Blans20, Reference Hussain-Yusuf21] (C. C. H. Wielders, personal communication). Seropositives without IgM phase II antibodies were designated as ‘past infections’. As the latter group also includes possible chronic infections, a further distinction was made between serological profiles that had IgG phase I ⩾1:1024 indicative for a chronic infection according to the new Dutch consensus guidelines [Reference Wegdam-Blans22].
Statistical analyses
Dairy sheep farms
All data were analysed with SAS, version 9.2 (SAS Institute Inc., USA). For the dairy sheep farms in The Netherlands, participation bias was investigated by comparing participating and non-participating farms with regard to herd size, urbanization degree and region. The seroprevalence of C. burnetii in residents and the corresponding 95% confidence interval (CI) were calculated. Descriptive statistics were performed by analysing frequency tables and studying distributions of continuous variables. No risk factor analysis was performed because of the small number of participants.
Non-dairy sheep farms
To study participation bias, participating and non-participating farms were compared with regard to herd size, cattle, sheep, and goat density in the surroundings, urbanization degree, region, situated inside or outside a compulsory Q fever vaccination area, number of bulk-milk-positive dairy goat or dairy sheep farms in a radius of 5 and 10 km, and distance in metres to the closest bulk-milk-positive small ruminant farm.
The seroprevalence of C. burnetii and the corresponding 95% CI were calculated. For descriptive statistics, frequency tables were analysed. In addition, distributions of continuous variables were studied, and if not linearly related to the outcome variable, continuous variables were recoded into classes.
Univariate logistic regression analysis was performed to assess the main factors associated with C. burnetii seropositivity at the individual level [P < 0·20 in the likelihood ratio test (−2LL)]. Variables with <20 participants in one risk category were excluded. Age was always kept in the model because of the frequent association with Q fever seropositivity in the literature. Proxy outcomes, such as sheep seropositivity, were not included in the multivariate analysis. If several variables, which were associated in the univariate analysis, were interrelated, a preferred variable was chosen and related variables were excluded. The preferred variable was chosen based on the most informative value, the strongest association or most relevant exposure (exposure at own farm instead of comparable exposure at other farms). All identified individual variables were analysed with a manual backwards elimination procedure until all variables were significant at the 10% significance level in the likelihood ratio test, starting with a full multivariate logistic regression model.
Subsequently, potential risk factors derived from the farm-based questionnaire were analysed by univariate multilevel analyses considering clustered farm-based data for all persons within the same farm, using a unique farm number as cluster variable. All farm variables which were significant in the univariate analysis (P < 0·20), were analysed with a manual backward elimination procedure starting with a full multilevel model.
Finally, both the individual and farm-based characteristics from the two final submodels were combined in a multivariate multilevel analysis to identify the independent risk determinants for C. burnetii seropositivity. The final model fit was assessed by the quasi-likelihood under the independence model criterion (QIC) goodness-of-fit statistic for generalized estimation equation (GEE) models.
RESULTS
Dairy sheep farms
Out of the 32 invited farms, 12 participated (response rate 37·5%). The participating farms were all situated in a rural area (<500 addresses/km2). Participating and non-participating farms were comparable with regard to urbanization degree and province distribution. However, participating farms had a median number of 529 sheep (range 143–1163) vs. the significantly lower median of 353 sheep (range 96–730) for non-participating farms (P = 0·03).
Twenty-seven study participants (mean age 38·7 years, range 14–61, 63% male), provided a blood sample. Overall, 18 (66·7%) participants were seropositive: 80·0% for the 15 farmers (12 males), and 50·0% for the 12 household members (five children, five female spouses, one male spouse, one seasonal worker). Three (11·1%) participants had a relatively recent C. burnetii infection (IgM phase II antibodies). None consulted their general practitioner or were hospitalized because of influenza-like illness or fever. One participant had an IgG phase I titre of ⩾1:1024, indicating a possible chronic case [Reference Wegdam-Blans22].
Non-dairy sheep farms
Non-response analyses
Out of the 1344 approached farms, at least 32 appeared to be no longer eligible because they had <60 animals at inclusion or had vaccinated all their sheep. Of the remaining 1312 farms, 119 participated in the study (response rate 9·1%).
A significant difference was found for sheep density in the 5-km radius of participating and non-participating farms, 34·5 (range 1·8–143·6) and 47·5 (range 1·0–162·9) sheep/km2 in the 5-km radius (excluding own sheep), respectively (P = 0·01). In addition, the number of sheep was borderline significantly higher at the participating farms (median 191 sheep, range 102–1310), compared to the non-participating farms (median 167 sheep, range 100–2857). For the other variables, no significant differences were found between participating and non-participating farms (Table 1).
Table 1. Non-response analyses of non-dairy sheep farms, comparison of participating and non-participating farms
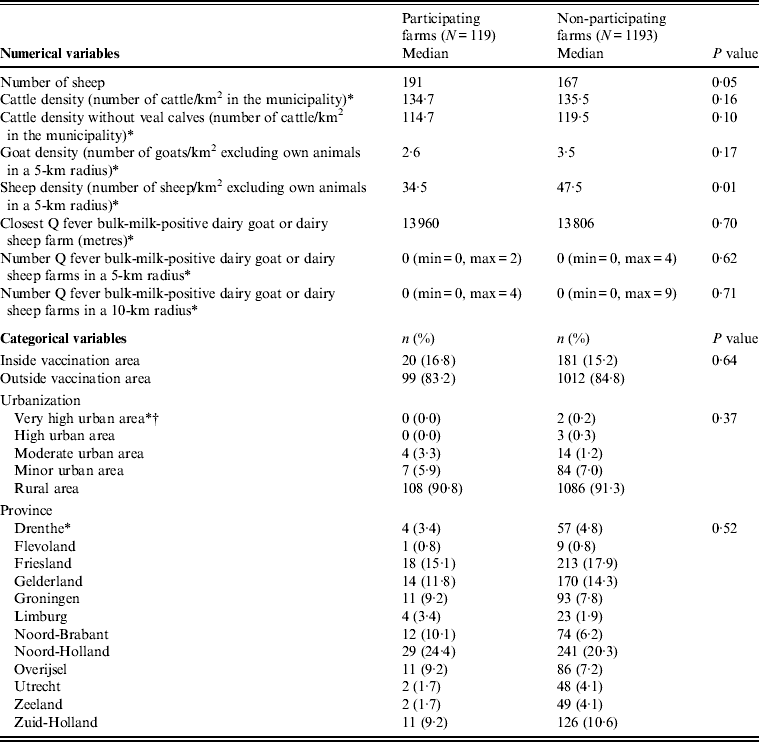
N, Total number of individuals.
* Four missing values at non-participating farms.
† Urbanization degree: very high urban area >2500 addresses/km2; high urban area = 1500–2500 addresses/km2; moderate urban area = 1000–1500 addresses/km2; minor urban area = 500–1000 addresses/km2; rural area <500 addresses/km2.
Descriptive characteristics
The 119 participating farms were mainly situated in the provinces of Noord-Holland and Friesland, commonly (90·8%) situated in rural areas (<500 addresses/km2) and the most common breeds at the farms were Texel (57·0%) and Swifter (46·5%). The farms were mainly started after 1950 (9·6% 1875–1950, 39·4% 1951–1980, 51·0% after 1980). Out of the 114 farms with a farm-based questionnaire, 23 (20·2%) kept one or more goats, 45 (39·5%) kept dairy cattle and/or beef cattle, and 13 (11·4%) other farms reported that cattle were present on their pastures. The farms could have one or more function; 95 (83·3%) farms kept sheep for meat production, 53 (46·5%) farms for rearing, and 20 (17·5%) farms for nature management. Of those 20 farms, 12 farms kept their sheep exclusively for nature management.
From the 119 farms, 271 persons provided a blood sample (mean age 47, range 12–93 years, 55% male). Of those, 266 completed the individual self-administered questionnaire and from 261 individuals information was available from the farm-based questionnaire.
C. burnetii seroprevalence was 51·3% (95% CI 45·5–57·4). In the univariate analysis, seroprevalence was significantly higher for farmers (58·8% vs. 36·3% for spouses) and for males (57·7% vs. 43·4% for females). Out of the 271 participants, seven (2·6%) had a relatively recent infection (IgM phase II antibodies). No participant had an IgG phase I titre suggestive for chronic infection.
Although the seroprevalence of the farm residents was higher for those living on a dairy sheep farm, the difference was not statistically significant [odds ratio (OR) 1·9, 95% CI 0·8–4·4] for dairy sheep farmers vs. non-dairy sheep farmers).
Univariate analyses at individual and farm level
All individual and farm-based variables, which were tested in the univariate analysis for relationship with human C. burnetii seropositivity, are displayed in Tables 2 and 3.
Table 2. Univariate logistic model of individual factors related to C. burnetii seropositivity in non-dairy sheep farm residents (P < 0·20, −2LL)
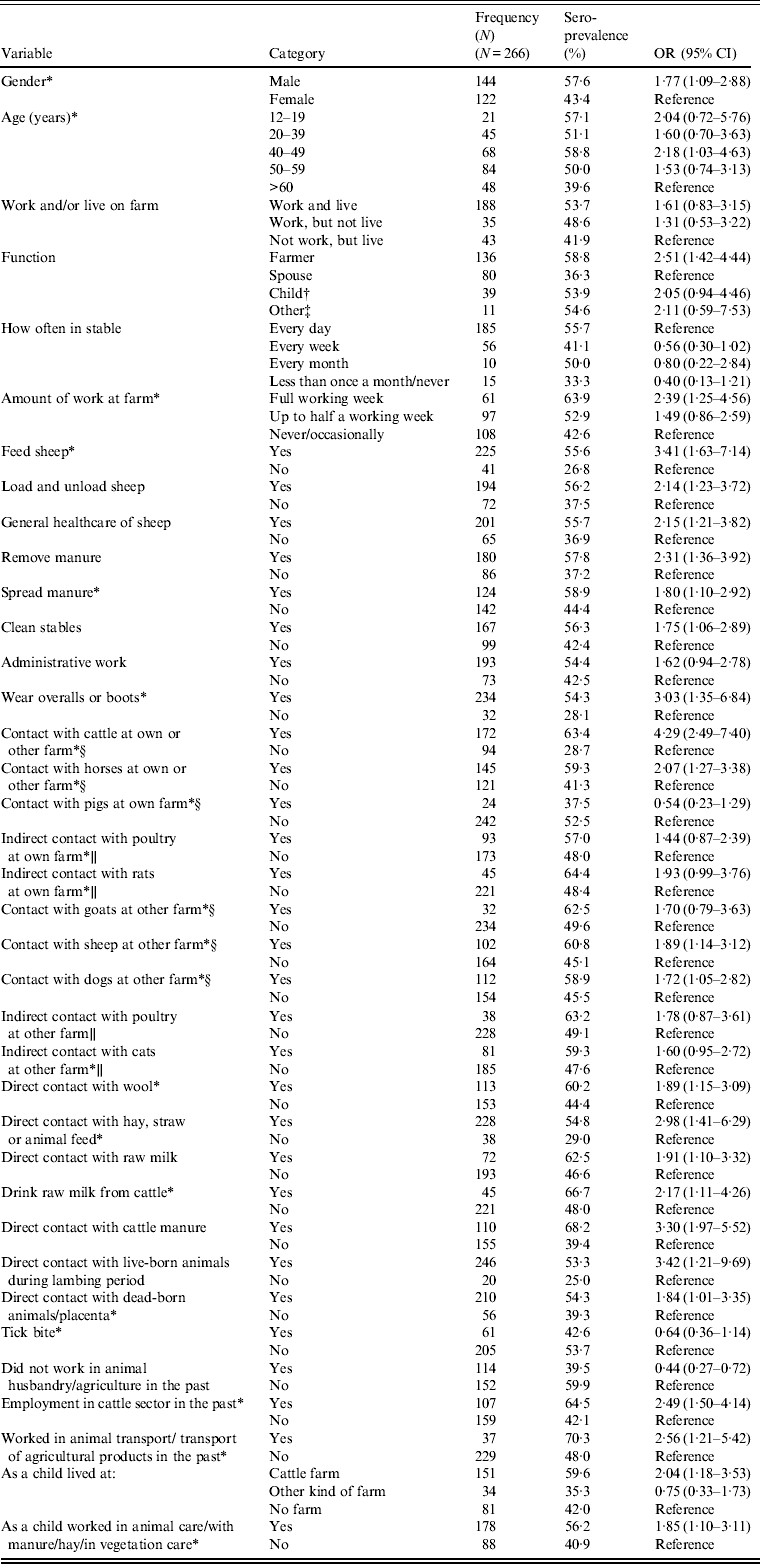
N, Total number of individuals; OR, odds ratio; CI, confidence interval, −2LL, likelihood ratio test.
* Variables included in subsequent multivariate individual analyses before manual backward elimination.
† Children aged <18 years (n = 17) and older children (n = 22) of the farmer.
‡ Employees, shepherds, other family members.
§ See animals at <5 m or touch animals.
∥ See animals at <5 m.
Table 3. Univariate multilevel analysis of farm-based factors related to C. burnetii seropositivity in non-dairy sheep farm residents (P < 0·20)
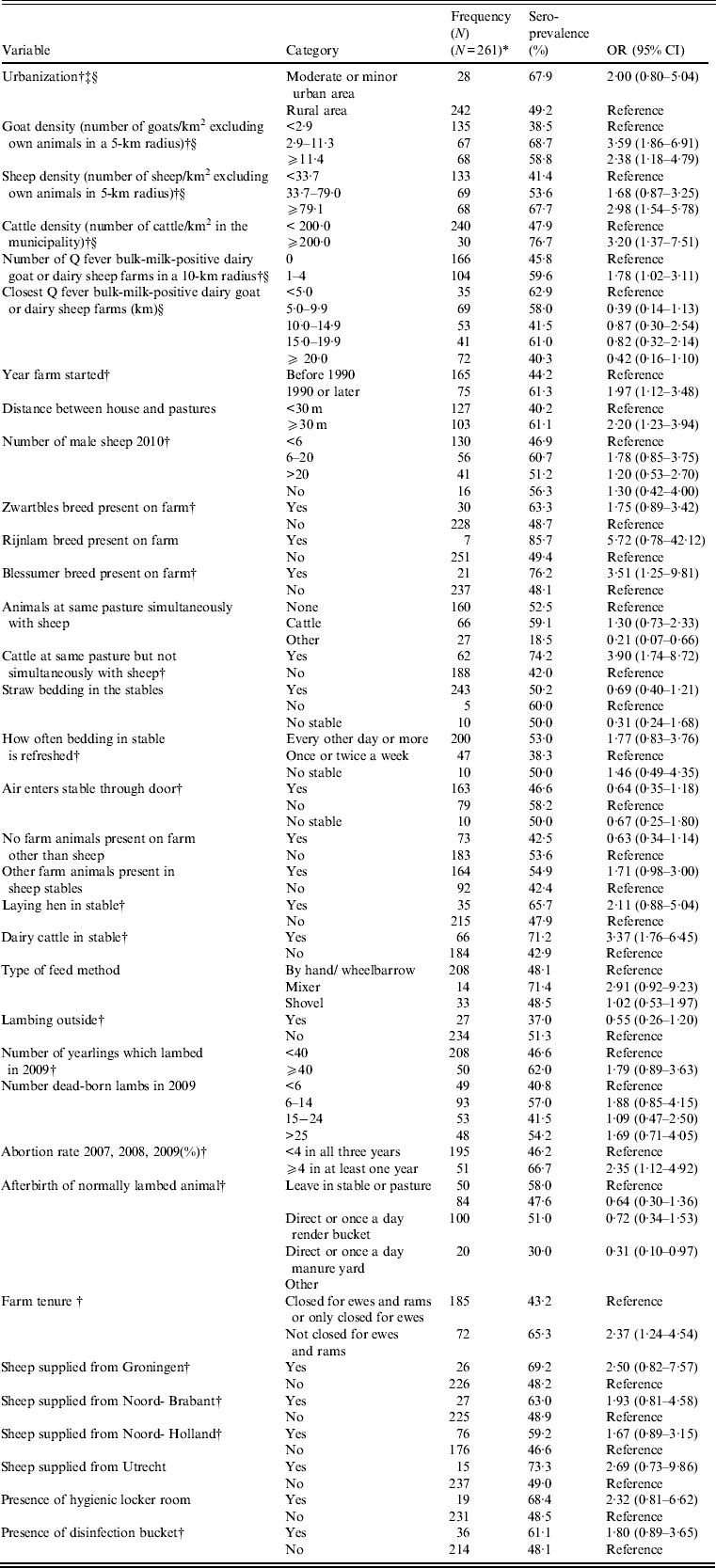
N, Total number of individuals; OR, odds ratio; CI, confidence interval.
* Not all numbers add up to the total due to missing values.
† Variable included in later multivariate farm-based analyses before manual backward elimination.
‡ Urbanization degree: moderate urban area = 1000–1500 addresses/km2; minor urban area = 500–1000 addresses/km2; rural area <500 addresses/km2.
§ For the geographical data, information was available for all 270 individuals, including the nine people without a farm-based questionnaire.
Multivariate and multilevel analyses
In the multivariate analyses, from 23 individual variables which were associated in the univariate analysis, four were independently associated with C. burnetii seropositivity (Table 4). In addition, 10/23 farm-based variables included in the multilevel analyses were significantly independent risk or protective factors and together were used as the full multilevel start model (Table 5).
Table 4. Results of the multivariate logistic regression analysis for the individual characteristics (P < 0·10, −2LL) in relation to non-dairy sheep farm residents C. burnetii seropositivity
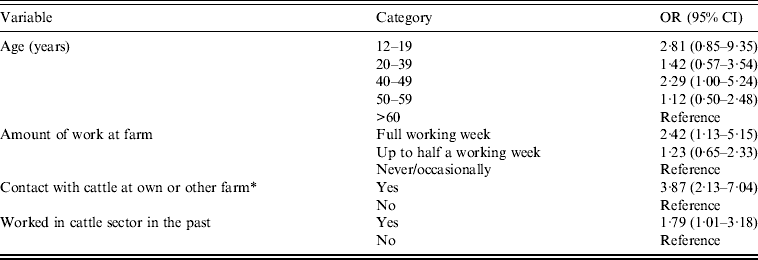
OR, Odds ratio; CI, confidence interval; −2LL, likelihood ratio test; AIC, Akaike's Information Criterion.
Number of observations used: 266 (AIC = 340·38).
* See animals at <5 m or touch animals.
Table 5. Results of the multilevel analysis with farm-based characteristics (P < 0·10) as independent factors in relation to non-dairy sheep farm residents C. burnetii seroprevalence
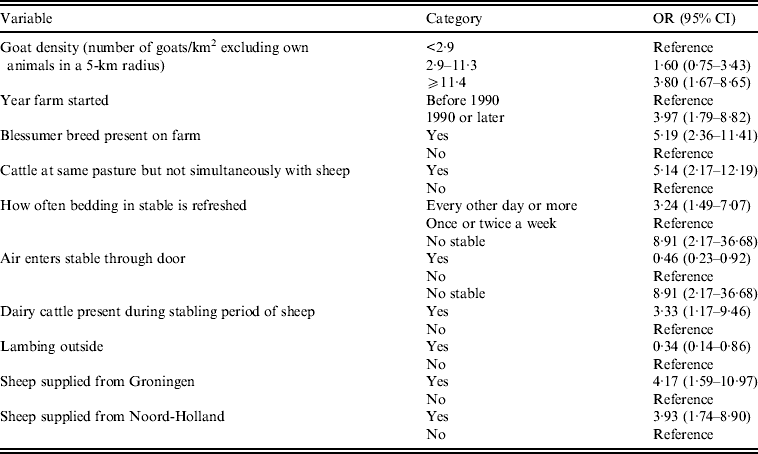
OR, Odds ratio; CI, confidence interval; QIC, quasi-likelihood under the independence model criterion.
Number of observations used: 212. Number of levels used: 107 (QIC = 232·9560).
Combined multilevel analyses of individual and farm-based factors
In the final combined multilevel model, significant risk factors were contact with cattle at own or other farm, past employment in the cattle sector, high goat density in the vicinity of the farm, living or working at a farm that was started in 1990 or later, the presence of Blessumer breed on the farm, cattle on the same pastures used by sheep, although not simultaneously with the sheep, high frequency of refreshing the bedding in the sheep stables, and sheep supplied from the provinces of Groningen or Noord-Holland (Table 6). Borderline significant risk factors were age 40–49 years, and presence of dairy cattle during the stabling period of the sheep. In addition, sheep lambing outside was a significant protective factor, and air entering the stable through the door was a borderline significant protective factor.
Table 6. Results of the multilevel analysis with individual and farm-based characteristics (P < 0·10) as independent factors in relation to non-dairy sheep farm residents C. burnetii seroprevalence
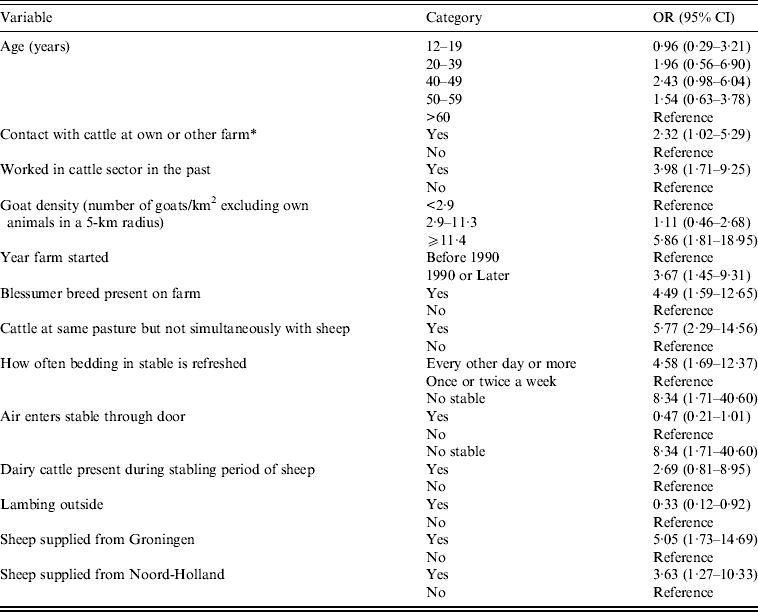
OR, Odds ratio; CI, confidence interval; QIC, quasi-likelihood under the independence model criterion.
Number of observations used: 208. Number of levels used: 105 (QIC = 219·1157).
* See animals at <5 m or touch animals.
DISCUSSION
Seroprevalence
The seroprevalence of non-dairy (51·3%) and dairy sheep farm residents (66·7%) is clearly higher compared to the seroprevalence estimate of 2·4% in the general population before the outbreak occurred in The Netherlands in 2006–2007. It is even higher compared to the seroprevalence found in a small community in the epicentre of the Q fever outbreak in 2007 (25·1%), and in blood donors in the most Q fever-affected areas in 2009 (12·2%), indicating that sheep farm residents have an increased life-time risk of acquiring a C. burnetii infection compared to the general Dutch population [Reference Karagiannis7, Reference Schimmer23, Reference Hogema24].
The observed seroprevalence in Dutch sheep farm households is also high compared to a study of sheep farmers in Sweden (28·5%) [Reference Macellaro, Akesson and Norlander25], and of farmers from all types of farms: 17·8% in Poland, and 27·3% in the UK [Reference Cisak26, Reference Thomas27]. Generally, it is difficult to compare international seroprevalence studies, because most studies use different tests or cut-off values. The cut-off value of the test in our study (⩾1:32) was chosen because it allowed comparison with other population surveys conducted in The Netherlands [Reference Schimmer23, Reference Schimmer28].
Dairy sheep farm residents had a higher seroprevalence compared to non-dairy sheep farm residents. Although no statistically significant difference in seroprevalence was found between the residents of both farm types, this might be due to lack of power because of the small number of participants from dairy sheep farms. In this study it was impossible to assess which risk factors were responsible for the higher seroprevalence in dairy sheep farm residents, due to the low number of participating dairy sheep farm residents. In addition, because of the differences in farm management, the farm-based questionnaires of both farm types were not the same, therefore pooling the analysis with the other sheep farm residents to increase power was not an option. Specific research, targeting all current dairy sheep farms in The Netherlands (n∼40), might elucidate further risk factors next to the higher sheep seroprevalence, explaining the higher seroprevalence in dairy sheep farm residents. Nevertheless, it might well be that dairy farm residents were more exposed to Coxiella, as the seroprevalence in dairy sheep at these same farms was significantly higher compared to that of non-dairy sheep (data not shown). A higher vulnerability for infection of breeds selected for milk production rather than for disease resistance has previously been observed for dairy cattle, dairy sheep, and dairy goats [Reference Rauw29, Reference van den Brom30]. In addition, dairy sheep are more often housed in stables compared to non-dairy sheep which spend most of the year outside. Indoor housing might facilitate the spread of C. burnetii in dairy sheep and to humans. Moreover, the higher seroprevalence in dairy farm residents might be explained by more intense contact with dairy sheep.
The seroprevalence of the dairy sheep farm residents (66·7%) was comparable to the seroprevalence of dairy goat farm residents (68·7%) in The Netherlands [Reference Schimmer28]. Furthermore, the percentage of relatively recent infections (clinical status unknown as no questions addressed current Q fever compatible symptoms) in the dairy sheep farm residents (11·1%) is comparable to that of the dairy goat farm residents (11·2%) [Reference Schimmer28]. Additionally, the percentage of participants with an indication for a possible chronic infection is also similarly high for dairy sheep and dairy goat farm residents (3·7% and 4·1%, respectively) [Reference Schimmer28]. In contrast, the percentage of relatively recent infections and possible chronic infections are lower for non-dairy sheep farm residents (2·6% and 0%, respectively). Therefore, currently C. burnetii infection seems to be a more serious and on-going health problem in dairy goat and dairy sheep farm residents compared to non-dairy sheep farm residents, although the numbers are relatively small.
Although numbers are too low to draw any conclusion and do not allow for valid statistical testing, the 10 (three from dairy and seven from non-dairy farms) relatively recent (IgM phase II positive) cases were generally younger (median 37 years vs. median 50 years for the seronegatives), were more often male (80% vs. 48%) and more often lived on a dairy sheep farm (30% of the recently infected vs. 6% of the seronegatives). This may point to ongoing infections especially in male dairy sheep farm residents, in the relatively early days of their contact with sheep.
Risk and protective factors for non-dairy sheep farm residents
One of the protective factors for C. burnetii seropositivty was sheep lambing outside. Farm residents might be less exposed to contaminated aerosols in that situation, compared to lambing inside stables.
In addition, several risk factors for C. burnetii seropositivity were identified in this study. McCaughey et al. [Reference McCaughey31] suggested in his study in the general population (age 12–64 years) that most people acquired C. burnetii infection between ages 25 and 34 years and after that age seroprevalence remained stable. This age trend was not seen in our study; sheep farm residents had already a high seroprevalence at young age (12–19 years). This might be explained by exposure to infected animals at a young age. The highest seroprevalence found in humans (age 40–49 years), matches the most common age group of notified clinical Q fever cases in The Netherlands [Reference Schimmer9]. The increased risk at this age seems not to be explained by differences in specific work activities, frequency of cattle contact, or hours worked. Perhaps host factors play a role in the increased risk, or it generally reflects regular exposure to the bacterium and repeated development of antibodies (booster effect), not adequately measured by the questions in the questionnaire.
Animal movement is a known risk factor for the transfer of microorganisms and should be discouraged [Reference Bölske32, Reference Mansley33]. Why specifically supply of sheep from the northern provinces of Noord-Holland and Groningen showed an independent increased risk for infection of the farm residents is not clear. The seroprevalence in sheep in these two provinces was not significantly different from prevalences in other provinces, both in the current study (B. Schimmer et al., unpublished data) and in a previous study in 2008 using convenience serum samples from sheep [Reference van den Brom30].
It is also unknown why the fact that a farm started before 1990 was a risk factor. No change in farm management is known around that year that could influence the risk of a C. burnetii infection.
Having the Blessumer sheep breed on the farm was the next significant risk factor. This breed is a crossing of the breeds of Texel (non-dairy sheep) and Flemish sheep (dairy sheep); therefore, the Blessummer breed might have a lower disease resistance [Reference Rauw29, Reference van den Brom30]. Differences in infection rates between sheep breeds have not yet been studied to investigate whether Blessumer sheep are more often infected.
In the environment of dairy goat farms with a history of abortion waves and of farms having PCR-positive bulk milk, relatively high levels of C. burnetii DNA were found [Reference de Bruin34]. A high goat density in the surrounding area of a participating farm is therefore considered a plausible risk factor for people living in the vicinity at the time of data collection. This was also demonstrated in several local outbreak investigations in The Netherlands in 2008–2009 [Reference Karagiannis7, Reference Schimmer8].
Maredly, several risk factors for C. burnetii seropositivity in non-dairy sheep farm residents point to cattle exposure at present or in the past. This might suggest that cattle were partially responsible for the infections observed in the sheep farm residents. In a previous study in farmers (all farm types) contact with cattle was also described as a risk [Reference Thomas27]. A recent published review including worldwide studies, suggested a higher seroprevalence of C. burnetii in cattle compared to goat and sheep [Reference Guatteo35]. In The Netherlands, a prevalence of 78·6% for antibodies in cattle bulk tank milk was found, confirming widespread circulation of the bacterium in cattle [Reference Muskens36]. To further assess the risk for human infection from cattle, a similar study addressing the seroprevalence and risk factors in dairy cattle farm residents is being finalized in The Netherlands. A role for cattle in the human infections observed in the current sheep farm study, is also supported by the fact that the high seroprevalence in sheep farm residents does not seem to correspond with the low sheep seroprevalence at the participating farms (<2%). The role of specific activities with sheep for the infection risk was presumably relatively small, although not absent taking into account the significant association between human and sheep seroprevalence at the participating non-dairy farms. Whether sheep themselves are at increased risk for infection because of contact with cattle or nearby goat populations is currently under investigation. In The Netherlands, a dominant C. burnetii genotype was identified in humans, goats, and sheep throughout the entire affected area; the genotype found in cattle appeared to be different [Reference Roest37, Reference Tilburg38].
Based on the results of the present study, some recommendations can be made. First, we want to elucidate the transmission cycle between different species of ruminants and farm residents; strains from goat, sheep, cattle, and sheep farm residents could be subtyped and compared. Second, more research is needed to investigate whether the Blessumer breed is more often infected compared to other breeds. Third, in this study a high seroprevalence in spouses was found (36·3% non-dairy farm spouses, 50·0% dairy farm spouses). Therefore, we emphasize the importance of the advice that pregnant women should avoid contact with sheep during the lambing season, and that they should avoid contact with birth products of sheep. Currently, the Dutch Health Council is preparing an advice about vaccination of high-risk professionals, including several farm populations. For this advice, they also will take into account the results of this study.
Limitations
The study of non-dairy sheep farms had a low response rate of 9·1%. As reported by several farmers not willing to participate, sheep were outside when the request to participate was made, and it would be too labour-intensive to collect about 60 sheep for blood sampling. In addition, this part of the sheep industry was not affected by the implemented control measures, mainly targeted at farms with dairy sheep and dairy goats. Therefore, non-dairy sheep farmers might be less motivated to participate compared to the small dairy sheep sector, which had a response rate of 38%.
Except for differences in sheep density in the surroundings and the number of sheep on their farms, participating and non-participating non-dairy sheep farms appeared to be comparable. As both factors were not related to seropositivity, this selective response is not thought to be of influence on the study results, which are therefore considered representative for the Dutch professional non-dairy sheep sector.
At 79% of the 119 participating non-dairy farms both the farmer and partner participated in the study. Therefore, results for the farmers and partners are considered representative of the group of farmers/partners at the participating farms. It was not registered how many children aged ⩾12 years lived at the participating non-dairy farms, and we cannot be absolutely sure that the participating children were representative of all children in this age category.
CONCLUSION
This study demonstrates that C. burnetii infection is common in individuals living and/or working at a sheep farm in The Netherlands. Except for their sheep, the risk also seems dictated by contact with cattle at present or in the past and by nearby goat populations.
ACKNOWLEDGEMENTS
We are grateful to all participants without whom the study could not have been conducted. We thank all laboratory assistants for collection of serum samples, Jamie Meekelenkamp for laboratory analyses, Noel Peters for sending test results to participants, Sanne Kelderman for invitation mailings and providing reference data, Helen Aangenend for organizing logistics of human data collection, and Ben Bom for generating geographical information. Finally, we thank all expert members of the Q-VIVE project group and affiliated organizations for their support and advice during the study: Jan van den Bergh, Olaf Stenvers, Rob van Oosterom, Mark Paauw, Harry Stinis, Ad de Rooij, Margo Vonk, Clementine Wijkmans and Wim van der Hoek.
The study was funded by The Netherlands Organization for Health Research and Development [grant no. 50-50800-98-100: An integrated study on Q fever in livestock farmers and their (small) ruminants in The Netherlands]; and co-financed by the Ministry of Public Health and the Ministry of Agriculture. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DECLARATION OF INTEREST
None.








