Approximately 4 billion people in the world depend primarily on a plant-based diet to obtain essential nutrients( Reference Pimentel and Pimentel1 ). Since the 1990s, a growing body of evidence has emerged regarding the health benefits of plant phenolic compounds consumed on a regular basis( Reference Rodriguez-Mateos, Vauzour and Krueger2 ). Legumes, such as common beans (Phaseolus vulgaris L.), have a long shelf life and are a cheap, rich source of macro- (e.g. protein), micronutrients (e.g. folate, Fe, Zn, B vitamin complex) and phytochemical compounds (e.g. phenolic compounds)( Reference Broughton, Hernández and Blair3 ). The phenolic composition of common beans depends greatly on the genotype, as well as on the environmental conditions and processing techniques applied before consumption( Reference Yang, Gan and Ge4 ).
Several epidemiological studies( Reference Blekkenhorst, Sim and Bondonno5 ), such as the Japanese Collaborative Cohort study( Reference Nagura, Iso and Watanabe6 ) and the First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study( Reference Bazzano, He and Ogden7 ), have reported an inverse association between legume intake and CVD, particularly when consumed more than four times per week. Extracts obtained from white kidney beans have also been proposed as promoters of body weight reduction, and a health claim has been submitted to the European Food Safety Authority panel for consideration. The lack of in vivo and human intervention studies, supporting an explanation for the inhibition of α-amylase activity, led to the rejection of the claimed effect in 2014( 8 ).
Studies on the bioavailability provide useful information to understand which metabolites from phenolic compounds can be responsible for the bioactivity in the human body.
Briefly, after ingestion, phenolic compounds are highly modified, by phase I and II metabolising enzymes, to increase water solubility( Reference Wu, Basu and Meng9 ) and only a minor amount of the native compounds appear on systemic circulation. Phase I reactions include oxidation, reduction, hydroxylation, decarboxylation, hydrolysis or a combination of such reactions, catabolised by cytochrome P450 superfamily, as well as by intestinal esterases( Reference Jančová, Šiller and Paxton10 ). Phase II reactions include conjugation reactions with sulphate, glucuronide, methyl groups and amino acids through the action of enzymes such as sulfotransferase, uridine diphosphate-glucosyltransferase, β-glucuronidase, catechol-O-methyltransferase, cholyl-CoA synthetase and N-acetyltransferase( Reference Jančová, Šiller and Paxton10 ). The unmodified compounds that reach colon, mostly those attached to fibre, are metabolised by the gut microbiota, remaining on bloodstream during a longer period of time, 48 h( Reference Bresciani, Scazzina and Leonardi11 , Reference Gómez-Juaristi, Martínez-López and Sarria12 ).
Human bioavailability studies, related with phenolic compounds in food, have been performed so far with food items such as olive oil( Reference Weinbrenner, Fitó and Farré13 , Reference Silva, Garcia-Aloy and Figueira14 ), coffee( Reference Del Rio, Stalmach and Calani15 , Reference Mena, Ludwig and Tomatis16 ), tea( Reference Del Rio, Stalmach and Calani15 , Reference Mena, Ludwig and Tomatis16 ), chocolate( Reference Wollgast17 ), wine( Reference Teissedre and Landrault18 ), berries( Reference Feliciano, Boeres and Massacessi19 , Reference Pimpão, Ventura and Ferreira20 ), apple( Reference Wruss, Lanzerstorfer and Huemer21 ), orange( Reference Pereira-Caro, Borges and Hooft22 ), almonds( Reference Urpi-Sarda, Garrido and Monagas23 ), wholegrains( Reference Bresciani, Scazzina and Leonardi11 , Reference Kern, Bennett and Mellon24 ), potato( Reference Tsang, Smail and McDougall25 ), soyabeans( Reference Zubik and Meydani26 ) among others, but for common beans, which represent one of the most important food items, on a daily basis diet, especially in developing countries, there is a scarcity of studies about phenolic compounds and their metabolites in human intervention studies. To date, only one study has investigated the bioavailability of phenolic compounds derived from common beans in humans, focusing exclusively in the metabolic fate of the flavonol, kaempferol( Reference Bonetti, Marotti and Dinelli27 ).
The human intervention study described herein aimed to report the metabolism of phenolic compounds after cooked common beans’ consumption, in plasma and urine samples, using a targeted metabolomics approach with authentic standards. An assessment of the inter-individual variability in bioavailability is also presented.
Material and methods
Chemicals
Folin-Ciocalteu’s phenol reagent and sodium carbonate (99 %) were purchased from Sigma-Aldrich. Methanol (99·9 %) was purchased from Carlo Erba Reagents. Acetonitrile for LC-MS Ultra Chromasolv was purchased from Honeywell Riedel-de HaënTM. Milli-Q® water (18·2 MΩ.cm) was obtained in a Millipore – Direct Q3 UV System equipment. l-(+) Ascorbic acid pro analysis was purchased from Merck. Pyrogallol-1-O-sulphate, pyrogallol-2-O-sulphate, 1-methylpyrogallol-O-sulphate, 2-methylpyrogallol-O-sulphate, 4-methylcatechol-O-sulphate, 4-methylgallic-3-O-sulphate, catechol-O-sulphate and vanillic acid-4-O-sulphate were kindly provided, and their synthesis has been described elsewhere( Reference Gómez-Juaristi, Martínez-López and Sarria12 ). Caffeic acid-4-O-β-d-glucuronide, dihydrocaffeic acid-3-O-sulphate, dihydrocaffeic acid-3-O-β-d-glucuronide, caffeic acid-3-O-β-d-glucuronide, dihydroferulic acid-4-O-sulphate, dihydroferulic acid-4-O-β-d-glucuronide, ferulic acid-4-O-sulphate, ferulic acid-4-O-glucuronide, isoferulic acid-3-O-β-d-glucuronide, dihydroisoferulic acid-3-O-sulphate and dihydroisoferulic acid-3-O-β-d-glucuronide were obtained from Toronto Research Chemicals. Kaempferol-3-O-glucuronide and quercetin-3-O-glucuronide were obtained from Extrasynthese. 3-Hydroxyhippuric acid and 4-hydroxyhippuric acid were purchased from Enamine. Gallic acid, protocatechuic acid, p-hydroxybenzoic acid, sinapic acid, catechin, epicatechin, hippuric acid, o-hydroxybenzoic acid, m-hydroxybenzoic acid, caffeic acid, p-coumaric acid, o-coumaric acid, m-coumaric acid, t-ferulic acid, kaempferol, quercetin and p-hydroxybenzaldehyde were obtained from Sigma-Aldrich Co. Formic acid (98 %), ortho-phosphoric acid (≥85 %) and acetic acid (100 %) were obtained from Carl Roth, and OASIS HLB Elution plates (2 mg sorbent per well, 30 µm particle size) were from Waters.
Plant material
Three Portuguese common bean traditional varieties, Patalar, Tarrestre and Moleiro, were compared in terms of phenolic content. These varieties were collected directly from local farmers in the centre-northern region of Portugal: Tarrestre from Arcos de Valdevez, Viana do Castelo; Moleiro from Celorico de Basto, Braga, and Patalar from Sintra, Lisboa, and kept in cold storage at the Germplasm bank located in the Research Unit of Biotechnology and Genetic Resources, INIAV, Oeiras, Portugal (PRT 005). The three varieties were multiplied before analysis at ESAC, using traditional farming techniques. Morphologically, Patalar and Tarrestre dry seeds have a kidney shape with white and brown coat colours, respectively. Moleiro dry seeds are characterised by a cuboid shape and coats with a light brown colour without pattern.
Based on the total phenolic content (TPC) determined in the extracts of raw common beans from these three different varieties, it was possible to select the one with the highest phenolic content.
Preparation of raw and cooked common beans extracts
In order to prepare extracts of raw beans, part of the raw seeds was grounded in a Falling nº 3100 miller (Perten) to a particle size of 0·8 mm. The other part of the raw seeds was cooked in a pressure cooker, only with water and salt, for 50 min (1 g of dried beans:4 ml of water:12 mg of salt). Extracts of raw and cooked beans were prepared according to Lin et al.( Reference Lin, Harnly and Pastor-Corrales28 ), with slight modifications. Briefly, 1 g of dry whole seed flour and 2·5 g of cooked beans were extracted with 20 ml of methanol–water (60:40, v/v) solution, followed by sonication for 60 min. The mixture was centrifuged at 420 g for 15 min. The final volume was adjusted to 20 ml, using volumetric flasks. Final extracts were filtered through a 0·22 µm 13 mm CA syringe filter (GE WhatmanTM). The extracts prepared in triplicate were kept on glass flasks at –20°C, until analysis.
Total phenolic content of raw and cooked common beans extracts
For TPC measurement, the method described by Stamatakis et al.( Reference Stamatakis, Tsantila and Samiotaki29 ) was applied, with some modifications. Briefly, the diluted sample extract (3·5 ml) was mixed to Folin–Ciocalteu’s reagent (0·100 ml) and, after 3 min, 0·400 ml of sodium carbonate solution (35 %, w/v) was added to the mixture. Absorbance was measured at 725 nm, after 1 h, against water, in a Spectrophotometer DU-70 (Beckman®). Gallic acid was used as the external standard. A blank of water was also prepared in the same conditions. All the measurements were performed in a Spectrophotometer DU-70 (Beckman®), and the final results were expressed as mg gallic acid equivalents (GAE) per g of seed’s dry weight (DW), considering the moisture content of the raw seeds. Moisture content (%) was determined by a Near-IR analyser (MPA; Bruker) with flour calibrations for grain legumes( Reference Serrano, Carbas and Castanho30 ).
Human study design
A human intervention study was designed in order to evaluate the metabolism of phenolic compounds derived from common beans in plasma and urine, after cooked common beans’ intake.
Seven healthy volunteers (six female and one male), with age between 24 and 40 years old and BMI of 19·9–34·4 kg/m2 were recruited.
The sample size was established based on previous bioavailability studies( Reference Stalmach, Mullen and Barron31 , Reference Anson, Aura and Selinheimo32 ) and justified by the nature of the study (accomplishment of a restrictive diet, free of phenolic compounds during 48 h, blood and urine collection).
The exclusion criteria for volunteers included the presence of diagnosed disease, use of medication and/or dietary supplements. The procedures were conducted according to the Declaration of Helsinki and the study protocol approved by the Ethics Committee for Clinical Experimentation of the Pharmacy Faculty, University of Lisbon, identification number 03/CEECFFUL/2016. All the volunteers were informed about the aim of the study, and a written informed consent was signed before the study. Volunteers followed a low phenolic diet, 48 h before the intervention day and during the 24 h post-bean consumption, online Supplementary Tables S1A and S1B. A list of allowed and not allowed food items, online Supplementary Table S1C, was supplied to promote volunteers compliance with dietary restrictions( Reference Kern, Bennett and Mellon24 ), particularly on food items such as coffee, chocolate, fruits, vegetables, wine, beer, juice, olive oil, nuts, tea and whole-grain products, in order to reduce the presence of phenolic compounds in blood and urine and to ensure the provenance of the phenolic compounds from cooked common beans. After an overnight fasting period, venous blood and urine were collected, at baseline level, before ingestion of cooked common beans, and volunteers were asked to report their food intake in the previous period of 48 h in order to check their compliance with the recommended diet. Venous blood was collected in EDTA containing vacutainers, 1, 2, 4, 6 and 8 h after consumption of a single meal of cooked common beans (404·7 (sd 2·7) g corresponding to 166·1 (sd 1·1) g of raw beans), and urine was collected in different time points (0–2, 2–4, 4–6, 6–8 and 8–24 h). The amount of cooked beans represented a full plate of beans, and volunteers were allowed to add salt according to their taste. A summary of the study design is shown in Fig. 1.
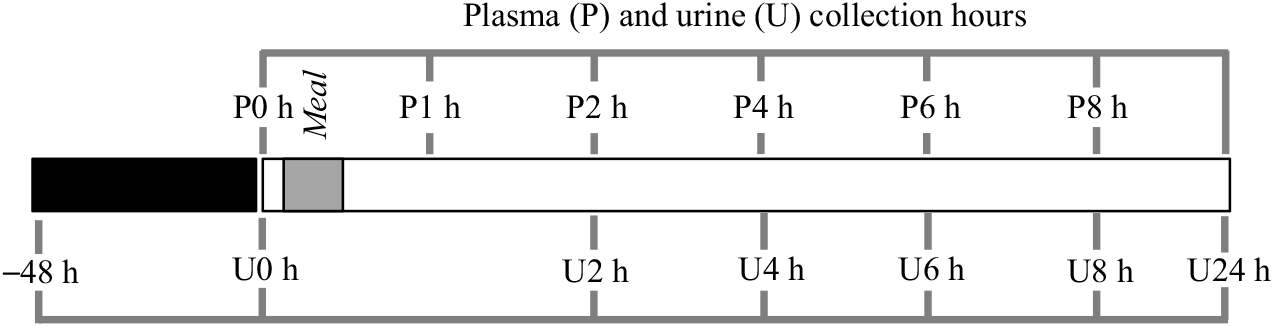
Fig. 1. Study design scheme. After a diet free of phenolic compounds for 48 h, plasma (P) and urine (U) were collected at different time points, after a single meal of cooked common beans (404·7 (sd 2·7) g). ![]() , Controlled diet, free of phenolic compounds;
, Controlled diet, free of phenolic compounds; ![]() , meal;
, meal; ![]() , plasma (P)/urine (U) collection.
, plasma (P)/urine (U) collection.
In order to quantify the amount of excreted phenolic compounds and corresponding metabolites in urine, the total volume of excreted urine was measured, for each volunteer, at different time points. For one of the seven volunteers, there was no urine collection, in time points 0–2 and 6–8 h.
l-(+) Ascorbic acid was added to the urine samples (1 g/2 litres of urine)( Reference Ito, Gonthier and Manach33 ). The venous blood was centrifuged, immediately after collection, at 657 g for 20 min at 4°C. The supernatant (plasma) was collected, and both plasma and urine samples were stored at –20°C and analysed according to Feliciano et al.( Reference Feliciano, Boeres and Massacessi19 ).
UPLC-Q-TOF-MS analysis
The common beans’ extracts of the selected variety with the highest phenolic content, as well as the human plasma and urine, were analysed by ultra-high performance liquid chromatography-quadrupole-time of flight-MS (UPLC-Q-TOF-MS), using an Agilent 6550 iFunnel Accurate-Mass Q-TOF MS (Agilent), in order to identify and quantify phenolic compounds and their metabolites, through an electrospray interface with Jet Stream technology, after separation on a 1290 Infinity UPLC system (Agilent) in the same conditions as the ones described by Feliciano et al.( Reference Feliciano, Boeres and Massacessi19 ).
A Zorbax Eclipse Plus RRHD column 2·1 × 50 mm, 1·8 mm with a compatible Eclipse Plus guard column 2·1 × 5 mm, 1·8 mm (Agilent) was used for sample analysis. The elution programme included 0·1 % HCOOH (eluent A) and acetonitrile with 0·1 % HCOOH (eluent B), during 10 min, at a flow rate of 0·4 ml/min. Eluent B increased from 1 to 10 % during the first 5 min, to 25 % at 8 min and to 99 % at 9·1 min. This percentage of eluent B stayed constant at 99 % during the remaining 0·9 min of analysis. The gradient returned to 1 % for 2 min to equilibrate the column. Samples were analysed in the negative mode as described by Feliciano et al.( Reference Feliciano, Boeres and Massacessi19 ). For data processing, the MassHunter Workstation Quantitative Analysis software, version B.06.00 (Agilent), was used.
Identification and quantification of metabolites in common beans’ extracts
Identification of compounds was performed by comparison with the retention time and m/z of the authentic standards analysed on the same conditions (gallic acid, protocatechuic acid, p-hydroxybenzoic acid, catechin, epicatechin, p-coumaric acid, t-ferulic acid, sinapic acid, quercetin and kaempferol). Variation of the retention time was calculated as the difference between the average value determined in the standard compound and the average value determined in the sample. The mass measurement error was calculated, to determine the accuracy of the detected m/z, following the equation:
![]() ${\rm Error} = {{{\rm Predicted}\ m/z - {\rm Observed}\ m/z} \over {{\rm Predicted}\ m/z}} \times \rm 1000\,000$
and the results expressed as ppm (parts per million) (online Supplementary Table S2). A retention time variation of 0·2 min and/or a mass error measurement lower than 5 ppm were adopted as quality criteria to identify the metabolites(
34
).
${\rm Error} = {{{\rm Predicted}\ m/z - {\rm Observed}\ m/z} \over {{\rm Predicted}\ m/z}} \times \rm 1000\,000$
and the results expressed as ppm (parts per million) (online Supplementary Table S2). A retention time variation of 0·2 min and/or a mass error measurement lower than 5 ppm were adopted as quality criteria to identify the metabolites(
34
).
In order to quantify the phenolic compounds in the extract of the Portuguese common bean variety, calibration curves of the authentic standards were prepared. With exception of the flavonoids, quercetin and kaempferol, prepared in aqueous methanol (50 %, v/v), all the standards were prepared in Milli-Q® water. Final results were expressed as μg/g of raw beans in DW. Procyanidins B1 and B2 were quantified using the catechin standard and expressing the final results as µg of catechin equivalents per g of raw beans in DW. The lowest concentration of the selected range (8–9916 nm), in the different calibration curves, was higher than the limit of quantification defined as a S:N ratio of 10.
Identification and quantification of metabolites in plasma and urine
Plasma and urine were prepared for analysis as described by Feliciano et al.( Reference Feliciano, Boeres and Massacessi19 ) with slight modifications. Briefly, plasma and urine (1000 μl) were thawed in an ice bath and centrifuged at 15 000 g for 15 min at 4°C. The supernatant (353 μl) of plasma or urine was diluted with 4 % phosphoric acid (353 μl). Each sample was loaded (600 μl) on a 96-well microelution solid phase extraction plate, washed with water (200 μl) and 0·2 % acetic acid (200 μl). After eluting with methanol (60 μl), the ninety-six-well collection plate was immediately covered with a polyolefin tape to avoid evaporation and put in the UPLC autosampler, using the UPLC-Q-TOF-MS equipment for the analysis, as described in the UPLC-Q-TOF-MS analysis section.
A total of forty compounds (pyrogallol-1-O-sulphate, pyrogallol-2-O-sulphate, 2-methylpyrogallol-O-sulphate, 1-methylpyrogallol-O-sulphate, gallic acid, protocatechuic acid, 4-methylgallic acid-3-O-sulphate, vanillic acid-4-O-sulphate, p-hydroxybenzoic acid, m-hydroxybenzoic acid, o-hydroxybenzoic acid, catechol-O-sulphate, 4-methylcatechol-O-sulphate, 4-hydroxyhippuric acid, 3-hydroxyhippuric acid, hippuric acid, caffeic acid-4-O-β-d-glucuronide, ferulic acid-4-O-glucuronide, dihydrocaffeic acid-3-O-sulphate, dihydrocaffeic acid-3-O-β-d-glucuronide, caffeic acid-3-O-β-d-glucuronide, caffeic acid, dihydroferulic acid-4-O-β-d-glucuronide, dihydroferulic acid-4-O-sulphate, ferulic acid-4-O-sulphate, isoferulic acid-3-O-β-d-glucuronide, dihydroisoferulic acid-3-O-sulphate, dihydroisoferulic acid-3-O-β-d-glucuronide, p-coumaric acid, ferulic acid, sinapic acid, m-coumaric acid, o-coumaric acid, p-hydroxybenzaldehyde, catechin, epicatechin, quercetin-3-O-glucuronide, kaempferol-3-O-glucuronide, quercetin, kaempferol) were investigated. For quantification purposes, in plasma and urine, authentic standards prepared in Milli-Q® water were used for the calibration curves. The considered concentration range was above the validated method quantification limit in plasma and urine determined by Feliciano et al.( Reference Feliciano, Mecha and Bronze35 ). The phenolic compounds or their corresponding metabolites with a concentration below the validated method quantification limit, described by Feliciano et al.( Reference Feliciano, Mecha and Bronze35 ), in at least one volunteer, were not considered for data analysis.
Data analysis
In plasma samples, the AUC, of phenolic compounds and their metabolites, was calculated using the PK Solver tool of Microsoft Excel (Microsoft). To determine the amount, in µg, of excreted metabolites, the volume of urine excreted, at each time point, was measured. The urinary recovery (%) was determined as described by Feliciano et al.( Reference Feliciano, Istas and Heiss36 ). Briefly, it was calculated as the ratio between the total amount of excreted metabolites and the TPC consumed in the intervention study. The human inter-individual variability, described as the CV in percentage, was determined using the AUC of each metabolite studied in the plasma and also for the total excreted amount of each metabolite, in urine( Reference Feliciano, Mills and Istas37 ).
Multivariate analysis was applied to describe inter-individual variability and select the most relevant metabolites associated with sample grouping. Using IBM® SPSS® Statistics, version 22, software, the normality of variables distribution was assessed by the Shapiro–Wilk test (n < 50) at a significance level of 1 %. To achieve normality, in some variables, different transformation approaches, such as logarithmic, inverse, squared root or two-step transformation( Reference Templeton38 ), were tested and for principal component analysis (PCA), only the metabolites with communalities higher than 0·5 were considered. PCA in articulation to cluster analysis was used for exploratory analysis of the inter-individual variability. After retaining the PCA scores of the PCA components with higher contribution (>50 % of the total variance), different cluster solutions (K = 2, K = 3 and K = 4), obtained by K-means cluster analysis, were applied as Y responses in partial least square regression-discriminant analysis (PLS-DA). PLS-DA amplified group separation allowing the prediction of the clusters’ membership. The Unscrambler® X 10.4.1, Camo Analytics Software, was used to select the best model of PLS-DA after full cross-validation. The statistical parameters, correlation coefficient of multiple determination for Y–R 2(Y), correlation coefficient of multiple determination for X–R 2(X), root-mean-square error of calibration and validation – RMSEC and RMSECV, respectively, and cross-validated correlation coefficient – Q 2, were assessed to control the quality of the model. The last parameter, Q 2, was extrapolated from the equations proposed elsewhere( Reference Kiralj and Ferreira39 ), using the following equation:
where RMSECV is the root-mean-square error of cross-validation; RMSEC is the root-mean-square error of calibration; M is the number of samples; K is the number of descriptors; R 2(Y) is the correlation coefficient for Y.
The PLS-DA model was built with all the samples, and the most relevant metabolites were selected based on the correlation loadings and weighted regression coefficients.
To compare the different clusters, the differences between plasma concentrations, as well as between the excreted amounts of metabolites, were defined by one-way ANOVA and the significantly different clusters identified by post hoc Scheffé or Games–Howell tests (depending on the acceptance or rejection of homoscedasticity between clusters), at a significance level of 5 %, using IBM® SPSS® Statistics, version 22, software.
Results
Selection and characterisation of the Portuguese common bean traditional variety used in the human intervention study
Based on the TPC analysis (Table 1), the three Portuguese traditional varieties (Patalar, Tarrestre and Moleiro) were compared. Moleiro stood out as the variety with the highest TPC value, 3·36 (sd 0·11) mg GAE/g DW, P < 0·05; therefore, it was the one selected to be used in the human intervention study.
Table 1. Comparison of the moisture content and the total phenolic content (TPC) determined in traditional Portuguese common bean varieties (Averages and standard deviations)

GAE, gallic acid equivalents.
a,b,c Average values in a column with unlike superscript letters are significantly different (P < 0·05).
Both extracts obtained from raw and cooked Moleiro beans were analysed by UPLC-Q-TOF-MS, and using commercial standards, it was possible to identify and accurately quantify thirteen compounds (Table 2 and online Supplementary Table S2) belonging to different classes: benzoic acids (gallic acid, protocatechuic acid and p-hydroxybenzoic acid), cinnamic acids (caffeic acid, p-coumaric acid, t-ferulic acid and sinapic acid) and flavonoids such as proanthocyanidins (procyanidins B1 and B2), flavan-3-ols (catechin and epicatechin) and flavonols (quercetin and kaempferol).
Table 2. Phenolic composition of raw and corresponding cooked common beans by ultra-high performance liquid chromatography-quadrupole-time of flight-MS (UPLC-Q-TOF-MS): comparison with the published data
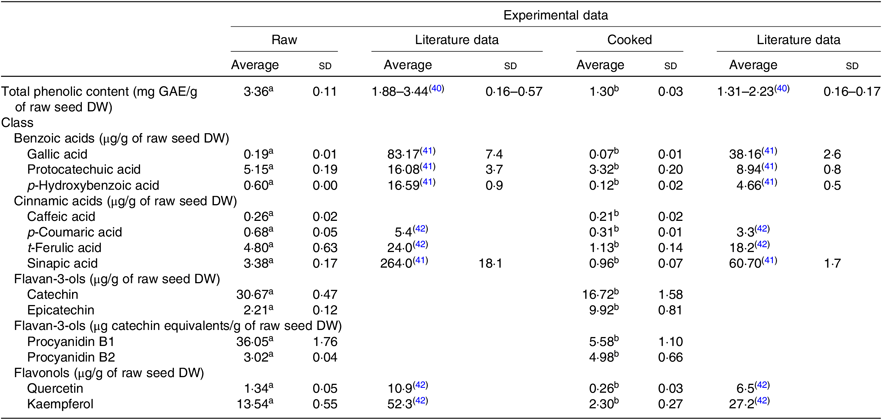
GAE, gallic acid equivalents; DW, dry weight.
a,b Average values in a row with unlike superscript letters are significantly different (P < 0·05).
The most abundant phenolic compounds, in raw seeds, were catechin, procyanidin B1 and kaempferol representing, respectively, 30, 35 and 13 % of the total quantified individual phenolic compounds. After the cooking process, the weight of common bean seeds increased to more than the double, due to the hydration process. In order to compare the phenolic composition of raw and cooked samples, results were expressed per g of raw seeds’ DW. As shown in Table 2, after the cooking process, the TPC decreased about 61 %. Results from quantification of individual phenolic compounds showed that compounds as gallic acid, protocatechuic acid, p-hydroxybenzoic acid, procyanidin B1, catechin, p-coumaric acid, caffeic acid, ferulic acid, sinapic acid, quercetin and kaempferol decreased significantly. In opposition, for epicatechin and procyanidin B2, there was a significant increase in the average contents. For the cooked beans, the most abundant phenolic compounds were catechin and epicatechin corresponding, respectively, to 36 and 22 % of the total quantified individual phenolic compounds.
Identification and quantification of metabolites in plasma and urine by UPLC-Q-TOF-MS
Compounds in plasma and in urine were identified, unequivocally in all volunteers, by comparison with the retention time and m/z values of the available standards. From the twenty-four metabolites identified in plasma (online Supplementary Table S3), seventeen were quantified (Table 3 and online Supplementary Table S4), corresponding to five sulphate conjugates, four glucuronide conjugates and three N-containing conjugates. Four compounds were detected as aglycones and one as an aldehyde.
Table 3. Concentration (nm) of phenolic compounds and their metabolites in plasma, before (0 h) and after (1, 2, 4, 6 and 8 h) beans’ consumption, considering n 7 (Average values with their standard errors; CV %)
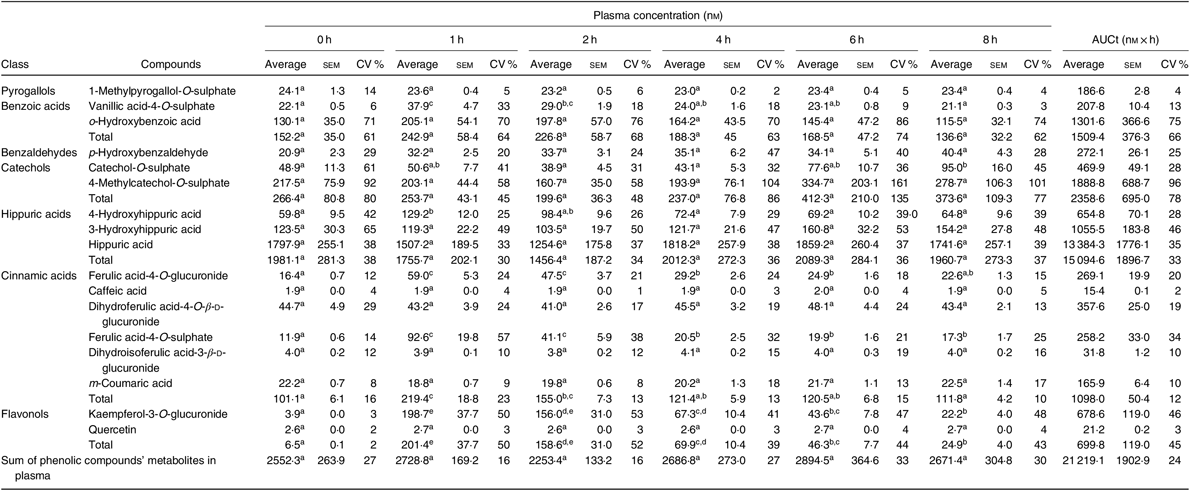
a,b,c,d,e Average values in a row with unlike superscript letters are significantly different (P < 0·05).
Based on the total AUC, Table 3, determined for the different quantified compounds in plasma, after 8 h of a single meal of cooked common beans, the most abundant class of phenolic compounds’ metabolites were hippuric acids (71 %) followed by catechols (11 %), benzoic acids (7 %), cinnamic acids (5 %), flavonols (3 %), benzaldehydes (1 %) and pyrogallols (1 %).
In plasma (Table 3), vanillic acid-4-O-sulphate, 4-hydroxyhippuric acid, ferulic acid-4-O-glucuronide, ferulic acid-4-O-sulphate and kaempferol-3-O-glucuronide showed a significant increase 1 h after common beans’ consumption and corresponded to 10 % of the quantified metabolites. For the other compounds, included in pyrogallols, benzoic acids, benzaldehydes, catechols and hippuric acids classes, the average plasma concentrations were not significantly different at the different collection time points.
From the twenty-eight metabolites identified in urine (online Supplementary Table S5), twenty-four were quantified (Table 4 and online Supplementary Table S6) in the urine of all volunteers. Nine of them were sulphate conjugates, six were glucuronide conjugates, six were detected in the aglycone form and three in the N-containing form.
Table 4. Urinary excretion (amount in µg) of phenolic compounds metabolites determined at different time points* (Average values with their standard errors; CV %)
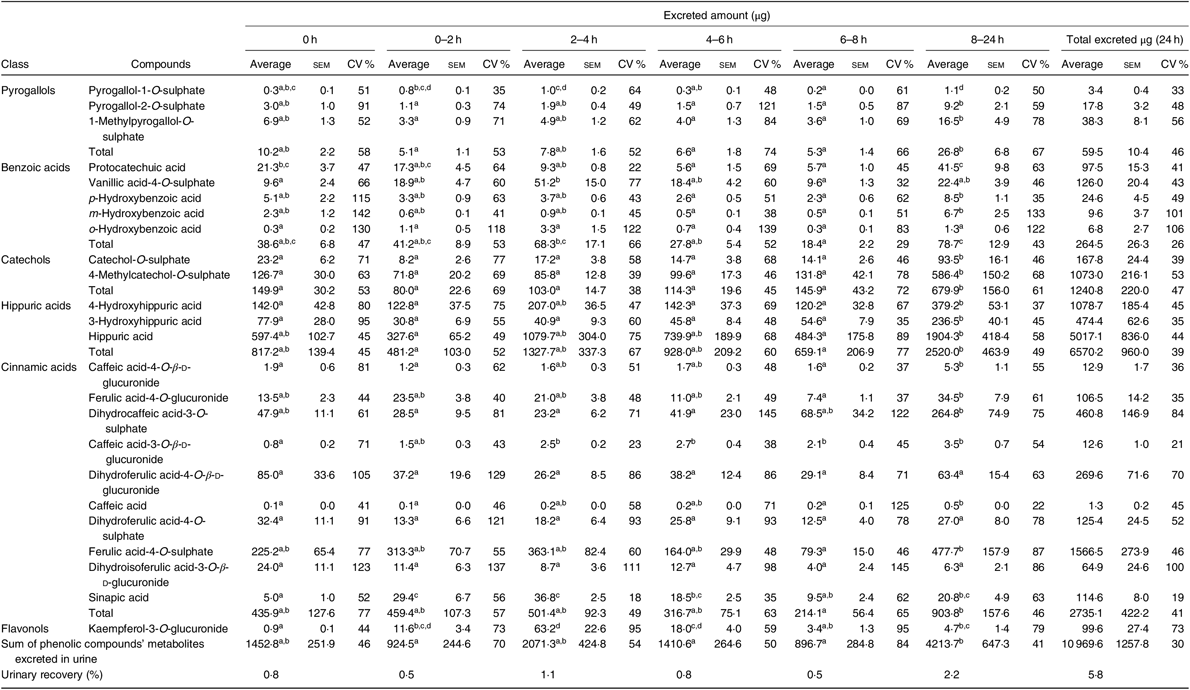
a,b,c,d Average values in a row with unlike superscript letters are significantly different (P < 0·05).
* The average, standard error of the mean and the CV (%) were determined for each compound, considering n 7, except at time points 0–2 h and 6–8 h, when it was considered n 6.
As shown in Table 4, the most abundant class of phenolic compounds’ metabolites in urine was hippuric acids (60 %) followed by cinnamic acids (25 %) and catechols (11 %).
Human inter-individual variability
There was inter-individual variability in plasma concentration and urinary excretion of phenolic compounds derived from common beans and their metabolites (Figs. 2 and 3). In plasma, Table 3, a variation of 24 % was obtained for the total AUC. Considering only the metabolites with a significant plasma increase, the variation of AUC ranged from 13 % in vanillic acid-4-O-sulphate to 46 % in kaempferol-3-O-glucuronide. In urine, Table 4, it was possible to determine a variation of 30 % in the volunteers’ excretion, ranging from 19 %, in sinapic acid, to 73 %, in kaempferol-3-O-glucuronide excretion.
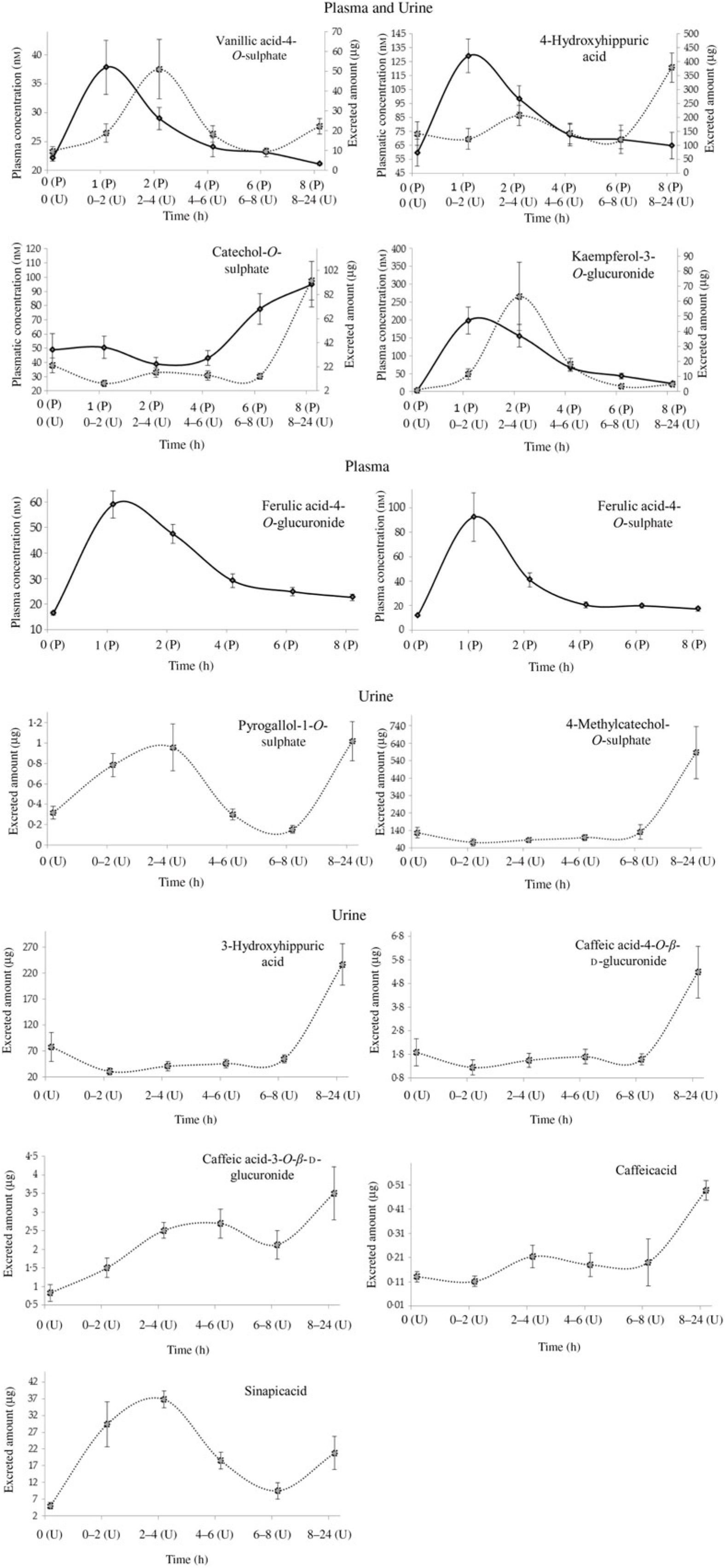
Fig. 2. Plasma pharmacokinetic and/or urinary excretion profiles of metabolites related to cooked common beans’ intake. Data are means (n 7), with standard errors represented by vertical bars. ![]() , Plasma (P);
, Plasma (P); ![]() , urine (U).
, urine (U).

Fig. 3. Variability of total urinary excretion (averages and standard deviations, in µg) of the phenolic compounds and their metabolites at different time points, before (0 h) and after common beans’ intake (0–2, 2–4, 4–6, 6–8 and 8–24 h), n 7.
Although not specifically related to common beans’ intake (without a significant increase in the urinary excretion during the study period), the compounds o-hydroxybenzoic acid, m-hydroxybenzoic acid and dihydroisoferulic acid-3-O-β-d-glucuronide showed the highest inter-individual variability with variations of 106, 101 and 100 %, in urine, respectively.
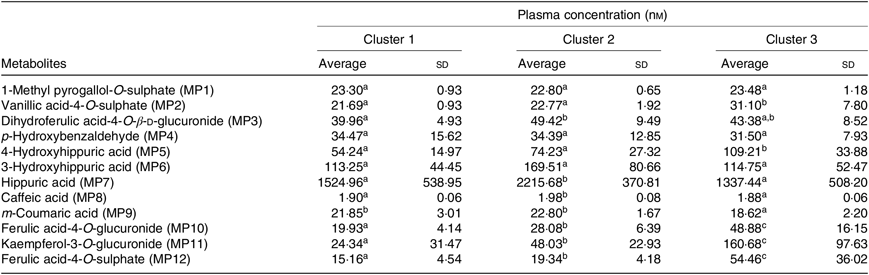
PCA was applied to explore the inter-individual variability among the seven volunteers (P1–P7) (Fig. 4). The plasma samples named P1_1, P6_0 and P7_0 were excluded from the analysis since those samples were out of the rank in the two-step transformation approach. In plasma samples, the three first principal components (PC) explained 71·7 % of the total variance with the first two accounting to more than 50 % of the variability (57·5 %). As suggested by the metabolites (MP) arrangement in the bi-dimensional space defined by the first two principal components (Fig. 4(a)), the PCA analysis indicated a clear separation between the plasma samples collected in early collection times (1 and 2 h after common beans intake), and the ones collected lately at 4, 6 and 8 h after common beans’ intake (Fig. 4(b)). The selected metabolites, in the PLS-DA analysis, explained 62 % of the clusters’ variability in the regression model and reinforced the PCA observations (Fig. 5). As shown in the correlation loadings plot, Fig. 5(a), for the first two factors, the variables positioned in the 50–100 % explained circle (defined as the space delimited by the outer and inner circumferences), vanillic acid-4-O-sulphate (MP2), located near the cluster 3, and ferulic acid-4-O-sulphate, (MP12), located near the cluster 1, were the main metabolites responsible for samples’ classification into two different groups, clusters 1 and 3. The metabolites ferulic acid-4-O-glucuronide (MP10), 4-hydroxyhippuric acid (MP5) and kaempferol-3-O-glucuronide (MP11) near cluster 3 were also responsible for samples’ separation, but with lower discrimination capacity. In the different volunteers, such metabolites were predominant at 1 and 2 h, after common beans’ intake (Fig. 5(b)). The remaining metabolites, hippuric acid (MP7), caffeic acid (MP8) and dihydroferulic acid-4-O-β-d-glucuronide (MP3) contributed mostly to the cluster 2, Table 5. m-Coumaric acid (MP9) explained the proximity of samples grouped in clusters 1 and 2. Although not responsible for clusters’ separation, 3-hydroxyhippuric acid (MP6) allowed samples’ dispersion along factor 2, contributing to the variance within clusters.

Fig. 4. Principal component analysis (PCA) of the plasma samples collected before (0 h) and after common beans’ intake. (a) Loading plot of plasma metabolites (PC1 v. PC2), MP1, two-step_1-methylpyrogallol-O-sulphate; MP2, two-step_vanillic acid-4-O-sulphate; MP3, two-step_dihydroferulic acid-4-O-β-d-glucuronide; MP4, p-hydroxybenzaldehyde; MP5, 4-hydroxyhippuric acid; MP6, 3-hydroxyhippuric acid; MP7, hippuric acid; MP8, caffeic acid; MP9, m-coumaric acid; MP10, log_ferulic acid-4-O-glucuronide; MP11, log_kaempferol-3-O-glucuronide; MP12, Inverse_ferulic acid-4-O-sulphate. (b) Score plot of the plasma samples distributed in a space defined by the first two principal components (PC1 v. PC2). The label attributed to the plasma (P) included a first number, which defined the anonymous identification of each volunteer and after the underscore character the collection time period, meaning, for example, in the label P1_0, the plasma sample of volunteer 1 collected in the fasting period (0 h).
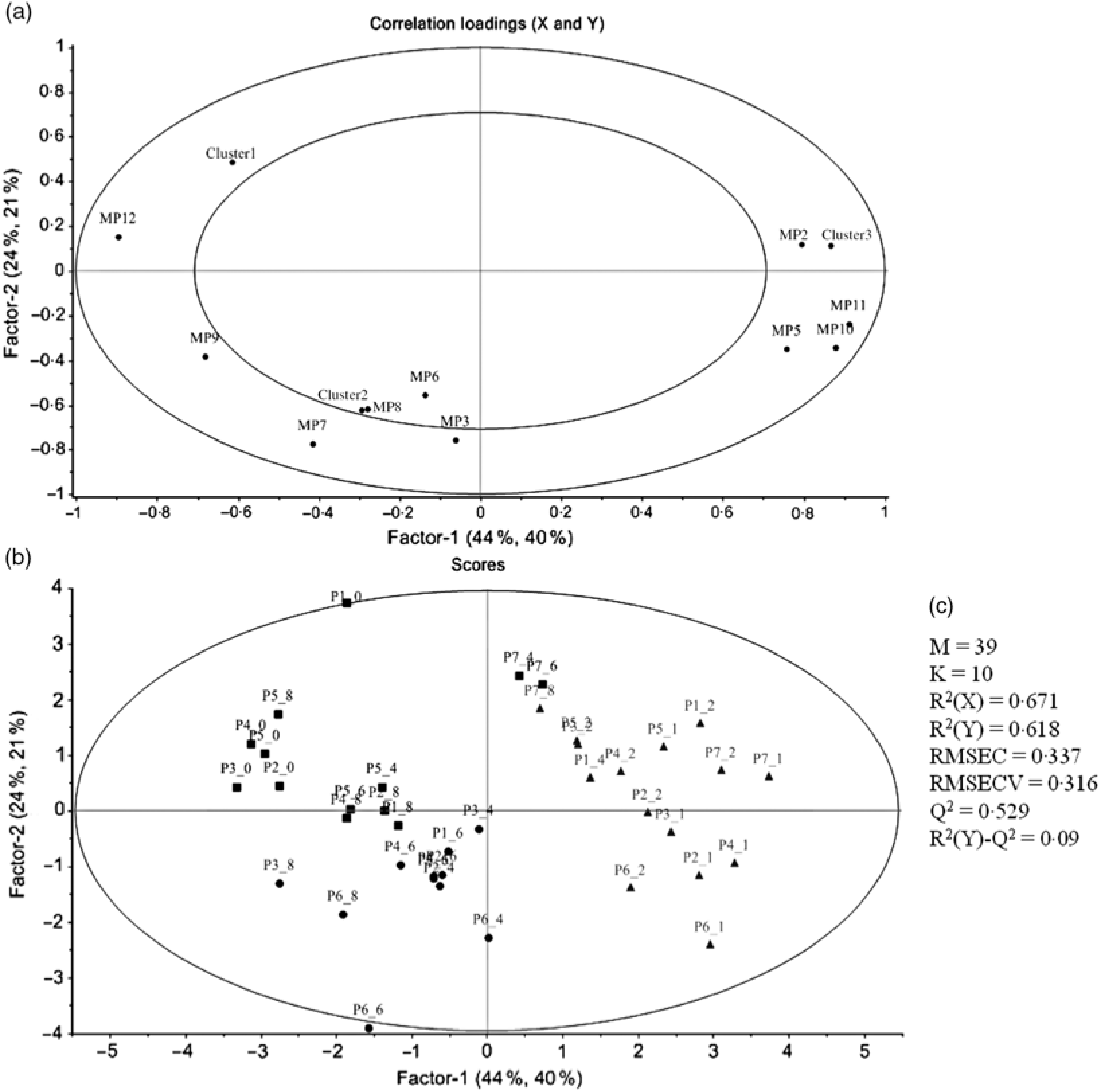
Fig. 5. Partial least square-discriminant analysis (PLS-DA) highlighting the plasma samples’ clustering. (a) Correlation loading plot of plasma metabolites, MP2, two step_vanillic acid-4-O-sulphate; MP5, 4-hydroxyhippuric acid; MP6, 3-hydroxyhippuric acid; MP8, caffeic acid; MP9, m-coumaric acid; MP10, log_ferulic acid-4-O-glucuronide; MP11, log_kaempferol-3-O-glucuronide; MP12, inverse_ferulic acid-4-O-sulphate. (b) Score plot of plasma samples distributed in the two first factors (▪, cluster 1; •, cluster 2; ▴, cluster 3). (c) Quality parameters of the PLS-DA model defined for the plasma samples. The samples’ identification was the same of Fig. 4.
Table 5. Plasma concentration of different metabolites (nm) in the described clusters (Averages and standard deviations)
a,b,c Average values in a row with unlike superscript letters are significantly different (P<0·05).
Regarding the urinary excretion of metabolites, the three first principal components retained 72·9 % of the variability and suggested samples’ separation into two distinct groups (Fig. 6). The PLS-DA model with some selected metabolites explained 66 % of the samples variability into three different clusters (Fig. 7). As shown in the correlation plot of the urinary excreted metabolites, Fig. 7(a), dihydrocaffeic acid-3-O-sulphate (MU16), 3-hydroxyhippuric acid (MU12), 4-methylcatechol-O-sulphate (MU10) and m-hydroxybenzoic acid (MU7) were responsible for samples’ separation into clusters 1 and 2. Cluster 2, highlighted as the one with the highest content on such metabolites, Table 6, included the urine samples collected lately, at time point 8–24 h, after common beans’ intake (Fig. 7(b)). By opposition, cluster 1 was the one with the lowest content on such metabolites (Table 6). In the 50–100 % explained circle, Fig. 7(a), the metabolites sinapic acid (MU23) and kaempferol-3-O-glucuronide (MU24) were mostly responsible for sample grouping in cluster 3, which included mainly the urine samples of different volunteers collected at time points 2–4 and 4–6 h after common beans’ intake (Fig. 7(b)). The metabolites vanillic acid-4-O-sulphate (MU5) and o-hydroxybenzoic acid (MU8) were also related to cluster 3 but with lower discrimination ability. Despite the high concentration of those metabolites in cluster 3, the average value obtained for such cluster was not significantly different from cluster 2 (P > 0·05, Table 6). For caffeic acid-3-O-β-d-glucuronide (MU17), located in an intermediate position between cluster 2 and cluster 3, the urinary excretion was prolonged on time, from 2–4 to 8–24 h after common beans’ intake, allowing clusters 2 and 3 approximation.
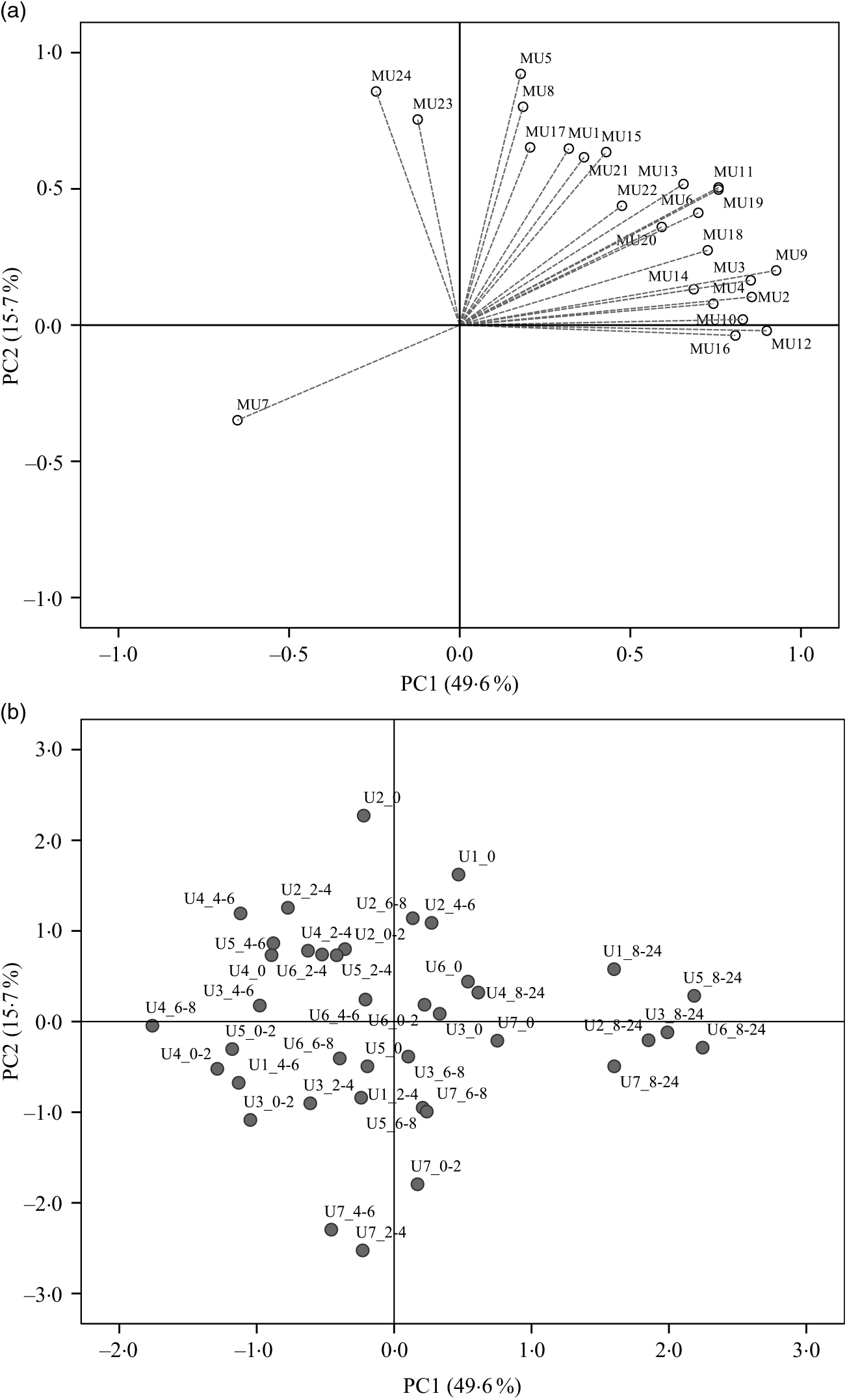
Fig. 6. Principal component analysis (PCA) of the urine samples collected before (0 h) and after common beans’ consumption. (a) Loading plot of the urinary excreted metabolites (PC1 v. PC2), MU1, log_pyrogallol-1-O-sulphate; MU2, log_pyrogallol-2-O-sulphate; MU3, log_1-methyl pyrogallol-O-sulphate; MU4, log_protocatechuic acid; MU5, log_vanillic acid-4-O-sulphate; MU6, log_p-hydroxybenzoic acid; MU7, Inverse_m-hydroxybenzoic acid; MU8, log_o-hydroxybenzoic acid; MU9, log_catechol-O-sulphate; MU10, log_4-methylcatechol-O-sulphate; MU11, log_4-hydroxyhippuric acid; MU12, log_3-hydroxyhippuric acid; MU13, hippuric acid; MU14, log_caffeic acid-4-O-β-d-glucuronide; MU15, log_ferulic acid-4-O-glucuronide; MU16, log_dihydrocaffeic acid 3-O-sulphate; MU17, log_caffeic acid-3-O-β-d-glucuronide; MU18, log_dihydroferulic acid-4-O-β-d-glucuronide; MU19, log_caffeic acid; MU20, squared root_dihydroferulic acid-4-O-sulphate; MU21, log_ferulic acid-4-O-sulphate; MU22, log_dihydroisoferulic acid-3-O-β-d-glucuronide; MU23, log_sinapic acid; MU24, log_kaempferol-3-O-glucuronide. (b) Score plot of the urine samples in the space defined by the two first principal components (PC1 v. PC2). For the volunteer 1, the urine samples were not provided at 0–2 h and 6–8 h. The label attributed to the urine (U) samples included a first number, which defined the anonymous identification of each volunteer and after the underscore character the collection time period, meaning, for example, in the label U1_8–24, the urine sample of volunteer 1 collected in the time period 8–24 h after common beans’ intake.
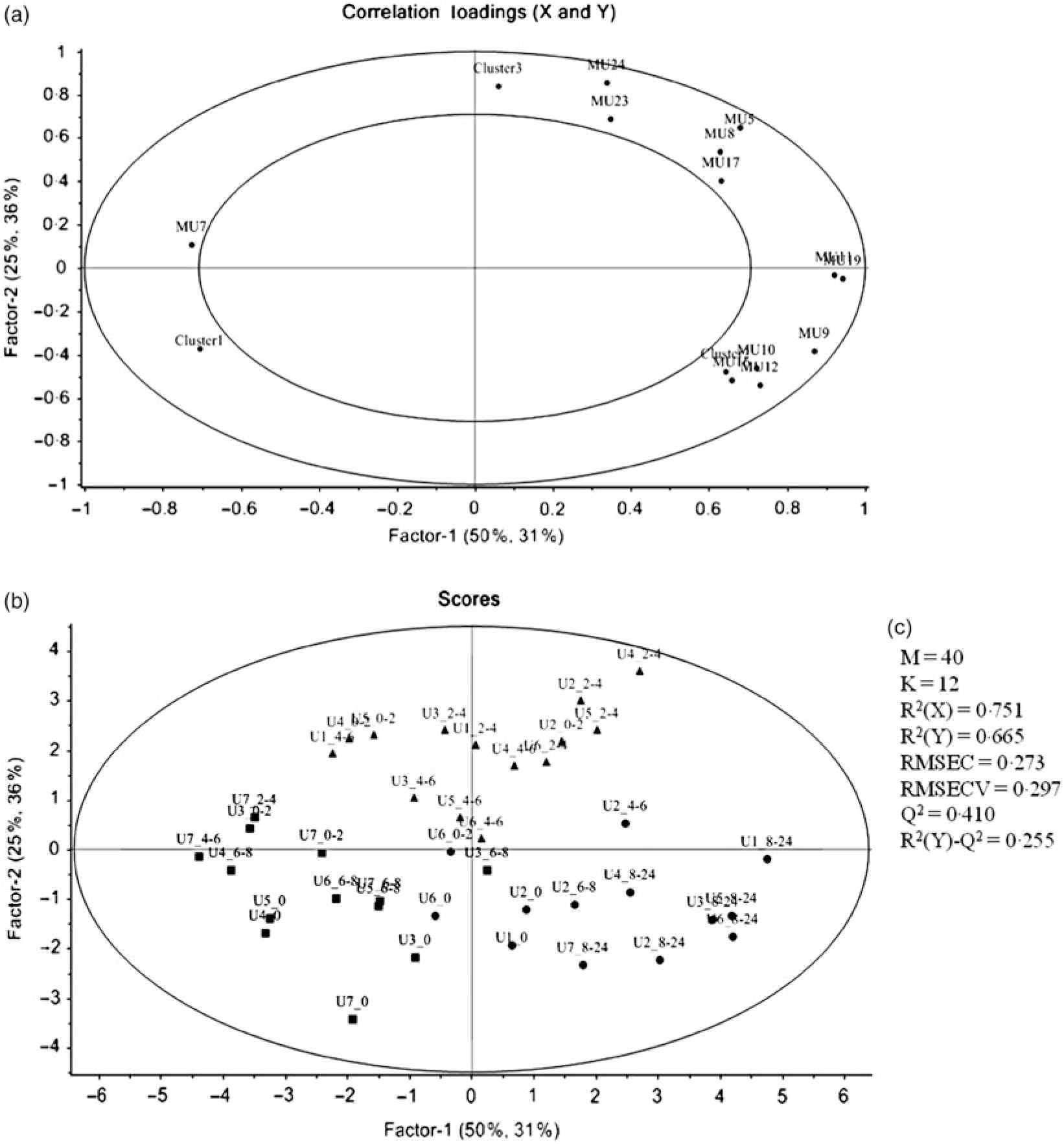
Fig. 7. Partial least square-discriminant analysis (PLS_DA) highlighting the urine samples’ clustering. (a) Correlation loading plot of some selected urinary metabolites excreted during the study period, MU5, log_vanillic-4-O-sulphate; MU7, inverse_m-hydroxybenzoic acid; MU9, log_catechol-O-sulphate; MU10, 4-methylcatechol-O-sulphate; MU11, log_4-hydroxyhippuric acid; MU12, log_3-hydroxyhippuric acid; MU16, log_dihydrocaffeic acid-3-O-sulphate; MU17, log_caffeic acid-3-O-β-d-glucuronide; MU19, log_caffeic acid; MU23, log_sinapic acid; MU24, log_kaempferol-3-O-glucuronide. (b) Score plot of urine samples distributed along the two first factors (▪, cluster 1; •, cluster 2; ▴, cluster 3). For the volunteer 1, the urine samples were not provided at 0–2 h and 6–8 h. (c) Quality parameters of the PLS-DA model defined for the urine samples. The samples’ identification was the same of Fig. 6.
Table 6. Excreted amount of different metabolites (μg) in the described clusters (Averages and standard deviations)

a,b,c Average values in a row with unlike superscript letters are significantly different (P<0·05).
Discussion
As far as we know, this work is the most complete study that has been performed to evaluate the bioavailability of phenolic compounds from cooked common beans, using UPLC-Q-TOF-MS.
Based on the phenolic content of the three Portuguese studied varieties, the Portuguese common bean variety Moleiro was chosen as the variety with the highest TPC (Table 1). Despite of the morphological differences in the seed colour, the TPC of Moleiro raw beans, 3·36 (sd 0·11) mg GAE/g of raw seed DW (2·91 (sd 0·09) mg GAE/g of raw seed fresh weight (FW)), characterised by light brown seeds, was within the range of values described by Heimler et al.( Reference Heimler, Vignolini and Dini43 ) for light green, white and yellow varieties (1·17–4·40 mg GAE/g of raw seed FW) and by Silva et al.( Reference Silva, Brigide and Toledo40 ) for pinto varieties, characterised by cream coloured seeds with speckles (Table 2).
The cooking process was responsible for changes in the beans’ accessible compounds. A reduction in the TPC (–61 %) was detected after cooking, and it was comparable to the TPC value described for the pinto cooked beans( Reference Silva, Brigide and Toledo40 ), representing 63–77 % of the TPC determined in the raw seeds of pinto beans( Reference Xu and Chang41 ).
Most of the studies on beans are still focused on the TPC and only a few ones( Reference Xu and Chang41 , Reference Díaz-Batalla, Widholm and Fahey42 , Reference Telles, Kupski and Furlong44 – Reference Romani, Vignolini and Galardi49 ) on the individual phenolic compounds. In our work, the data obtained using UPLC-Q-TOF-MS showed that Moleiro raw beans represented a source of catechin, 30·67 (sd 0·47) μg/g of raw seed DW (26·54 (sd 0·41) μg/g of raw seed FW), with higher content than the one described by Owino et al.( Reference Owino, Mukashyaka and Ndayisaba46 ) for a pink variety (13·50 (sd 0·50) μg/g of raw seed FW), and lower than the value described by de Pascual-Teresa et al.( Reference de Pascual-Teresa, Santos-Buelga and Rivas-Gonzalo47 ) for pinto beans (50·7 μg/g of raw seed FW). It also represented a source of procyanidin B1, 36·05 (sd 1·76) μg catechin equivalents/g of raw seed DW (31·20 (sd 1·52) μg catechin equivalents/g of raw seed FW), with a lower content than the pinto beans described by Aguilera et al.( Reference Aguilera, Estrella and Benitez45 ), 41·20 (sd 1·85) μg/g (without specification of DW or FW). Furthermore, Moleiro beans were a source of kaempferol, 13·54 (sd 0·55) μg/g of raw bean DW (11·72 (sd 0·47) μg/g of raw bean FW), with a similar content to the black beans described by Romani et al.( Reference Romani, Vignolini and Galardi49 ), 18 (sd 0·23) μg/g of raw bean FW, but with a considerably lower content than the average value determined by Diaz-Batalla et al.( Reference Díaz-Batalla, Widholm and Fahey42 ) for Mexican cream-red, black, grey, cream, brown and black-brown varieties (Table 2). Several factors such as the common bean variety, the maturity of seeds at harvest, the climatic conditions, the agronomic practices and the post-harvest storage conditions( Reference Owino, Mukashyaka and Ndayisaba46 ) contribute to explain the differences between the experimental data obtained in the present study and the described data in the literature (Table 2). Following the same trend noticed in raw beans, in cooked beans, the compounds, gallic acid, protocatechuic acid and sinapic acid, showed lower amounts than the ones reported by Xu et al.( Reference Xu and Chang41 ) and for the compounds, p-hydroxybenzoic acid, p-coumaric acid, t-ferulic acid, quercetin and kaempferol, there were also lower amounts than the average values described by Diaz-Batalla et al.( Reference Díaz-Batalla, Widholm and Fahey42 ) (Table 2). Despite the differences between the obtained and the described results, possibly explained by differences in the processing conditions, the loss of protocatechuic acid, reported in the present study (–36 %), was quite similar to the loss described in Xu et al.( Reference Xu and Chang41 ) study (–44 %).
During the cooking process, the instability of the phenolics’ chemical structure can contribute to explain the decrease of their content in cooked beans as already reported by Díaz-Batalla et al.( Reference Díaz-Batalla, Widholm and Fahey42 ) for quercetin, kaempferol, p-hydroxybenzoic acid and t-ferulic acid. The high temperature during cooking may cause evaporation of intracellular water, which triggers chemical reactions such as depolymerisation of phenolic compounds attached to polysaccharides and denaturation of proteins linked to phenolic compounds on the cell walls of cotyledons( Reference Xu and Chang50 ). Those reactions responsible for changes in the cell wall structure may increase the accessibility of some phenolic compounds( Reference Shiga, Lajolo and Filisetti51 ), such as procyanidin B2. As reported for cocoa beans, there are content variations in monomeric and dimeric forms of flavanols at high temperatures (100–140°C). Such variations can be attributed to epimerisation reactions that may induce losses and increments in flavanol contents( Reference Kothe, Zimmermann and Galensa52 ). Unlike Kothe et al.( Reference Kothe, Zimmermann and Galensa52 ), who reported for cocoa beans a significant increase of catechin (+240 %) after the roasting process, in the present study, in cooked common beans, there was a significant increase of epicatechin (+350 %) probably at the expense of procyanidins degradation and catechin epimerisation.
In the present study, the volunteers had straight nutritional recommendations regarding a controlled diet, free of phenolic compounds during 48 h. After such period, in plasma, the concentration of vanillic acid-4-O-sulphate, 4-hydroxyhippuric acid, ferulic acid-4-O-glucuronide, ferulic acid-4-O-sulphate and kaempferol-3-O-glucuronide increased significantly (P < 0·05), 1 h after common beans intake (Table 3, Fig. 2). This pattern was common to all volunteers and allowed to separate the plasma samples in different clusters (Fig. 5(b)). 4-Hydroxyhippuric acid (derived from conjugation reactions of 4-hydroxybenzoic acid and glycine( Reference Abbas, Greige-Gerges and Karam53 ) and/or produced endogenously, from catecholamine’s metabolism( Reference Eccleston and Ritchie54 , Reference van Duynhoven, van der Hooft and van Dorsten55 )) has been described in association with different dietary sources (e.g. berries( Reference Feliciano, Boeres and Massacessi19 ), green and black tea( Reference Henning, Wang and Abgaryan56 )). Herein, 4-hydroxyhippuric acid was associated with common beans’ intake, considering that a diet, free of phenolic compounds, was performed previously, during 48 h. Additionally, to the 4-hydroxyhippuric acid plasma concentration increase, there was a concomitant increase of kaempferol-3-O-glucuronide (the main quantified flavonol, in plasma, after Moleiro common beans intake), Fig. 2, which is in accordance with Penczynski et al.( Reference Penczynski, Krupp and Bring57 ).
The phase II conjugation reactions with sulphate and glucuronide groups occurred with vanillic acid, ferulic acid and kaempferol in the upper part of the gastrointestinal tract, by sulfotransferases and uridine diphosphate-glucosyltransferase, as suggested by the time at which the maximum plasma concentration of vanillic acid-4-O-sulphate, ferulic acid-4-O-sulphate, ferulic acid-4-O-glucuronide and kaempferol-3-O-glucuronide was reached, 1 h post-consumption. Such results are in accordance with Feliciano et al.( Reference Feliciano, Boeres and Massacessi19 ) and Bresciani et al.( Reference Bresciani, Martini and Mena58 ). Since no data regarding such metabolites were found in literature after common beans intake, it was necessary to compare the obtained data with results described for different food matrices by other authors. The maximum plasma concentration of ferulic acid-4-O-sulphate was slightly higher than the value described for whole-grain bread( Reference Bresciani, Scazzina and Leonardi11 ) but considerably lower than the one described in berries purée( Reference Pimpão, Ventura and Ferreira20 ) and in cranberries( Reference Feliciano, Boeres and Massacessi19 ). Catechol-O-sulphate was also associated with common beans intake, but, contrarily to the previous compounds, its plasma concentration increased significantly only 8 h after common beans’ intake. Such late increase in catechol-O-sulphate plasma concentration was also reported 7 h after cranberries intake( Reference Feliciano, Boeres and Massacessi19 ). More similar to cereals than to berries, in beans, the presence of free accessible phenolic compounds available to be metabolised in phase I and II reactions is limited, as a consequence of the strong covalent interactions of phenolic compounds and cell wall glycosides( Reference Gutiérrez-Grijalva, Ambriz-Pérez and Leyva-López59 ).
In urine, a total of twenty-four different metabolites was identified and quantified after common beans’ intake, which represented a higher number of compounds than those determined in plasma (Table 4). Contrarily to plasma, in urine, it was possible to detect and quantify compounds such as pyrogallol-1-O-sulphate, pyrogallol-2-O-sulphate, protocatechuic acid, p-hydroxybenzoic acid, m-hydroxybenzoic acid, caffeic acid-4-O-β-d-glucuronide, dihydrocaffeic acid-3-O-sulphate, caffeic acid-3-O-β-d-glucuronide, dihydroferulic acid-4-O-sulphate and sinapic acid. Nevertheless, in urine, the compounds, p-hydroxybenzaldehyde, m-coumaric acid and quercetin, were not quantified (Table 7). The absorption of metabolites derived from gut microbiota catabolism( Reference Bresciani, Scazzina and Leonardi11 ), such as dihydrocaffeic acid-3-O-sulphate, dihydroferulic acid-4-O-sulphate, only detected after 8 h, could contribute to explain the higher number of compounds in urine.
Table 7. Summary of compounds quantified in raw and cooked Moleiro common bean extracts (CBE), plasma (P) and urine (U)
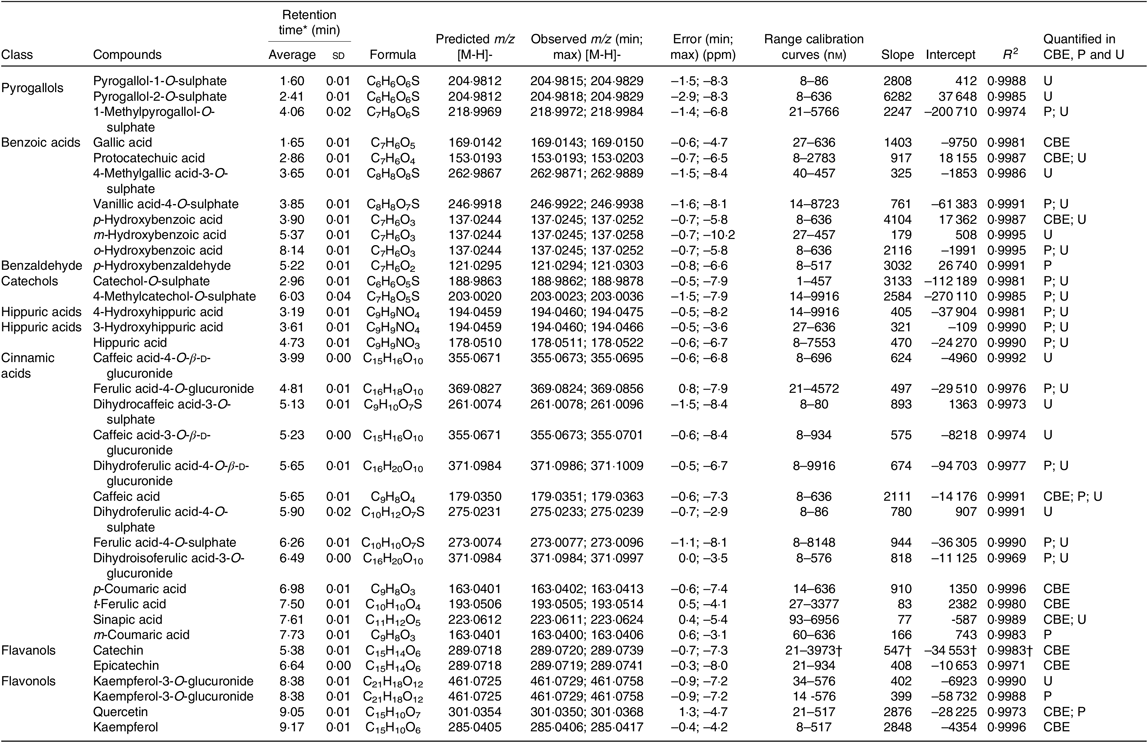
ppm, Parts per million.
* Average and standard deviation of the retention time determined for each standard at different concentration levels.
† The calibration curve applied for procyanidin B1 and procyanidin B2 was the one obtained for (+) catechin standard. The results are expressed in catechin equivalents.
With the exception of vanillic acid-4-O-sulphate, sinapic acid and kaempferol-3-O-glucuronide (which maximum amounts were excreted earlier than 8 h, at the time point 2–4 h post-consumption, cluster 3) (Table 4, Figs. 2 and 7(b)), for the majority of the metabolites (catechol-O-sulphate, 4-methylcatechol-O-sulphate, 4-hydroxyhippuric acid, 3-hydroxyhippuric acid, caffeic acid-4-O-β-d-glucuronide and caffeic acid), the excreted amount only increased significantly 8 h after common beans intake. For pyrogallol-1-O-sulphate and caffeic acid-3-O-β-d-glucuronide, the urinary excretion peaks were registered at different collection time points, following a multiphasic urinary excretion (Table 4). Despite the limited amount of bio accessible phenolic compounds in common beans, the excreted amounts of 4-methylcatechol-O-sulphate, 3-hydroxyhippuric acid, dihydrocaffeic acid-3-O-sulphate, vanillic acid-4-O-sulphate, sinapic acid and kaempferol-3-O-glucuronide were considerably higher after common beans’ intake, than after cranberries’ juice consumption( Reference Feliciano, Istas and Heiss36 ). Based on the metabolites quantified in urine, 8 h after common beans intake, Fig. 3, a colonic metabolism, by gut microbiota, is expectable and supported by the phenolic compounds’ entrapment in common beans’ fibre.
For 4-hydroxyhippuric acid, the urinary excretion earlier than 4 h, and at time points higher than 4 h, might be an indication of the metabolite’s enterohepatic recirculation or the additional synthesis of the metabolite at the colon.
Contrarily to the study conducted by Bonetti et al.( Reference Bonetti, Marotti and Dinelli27 ), which described the urinary excretion of kaempferol, after β-glucuronidase and sulfatase enzymatic hydrolysis, in a percentage of 5·4 (sd 5·4) % and 6·1 (sd 5·5) % of the kaempferol consumed in common beans, in the present study, the kaempferol-3-O-glucuronide was the flavonols’ metabolite detected and quantified, Fig. 2, in both plasma and urine, representing 30 % of the consumed kaempferol. In urine, the sinapic acid represented 83 % of the consumed sinapic acid. Part of this percentage should derive not only from the native compound present in cooked common beans but also from O-methylation reactions of the cinnamic acids, caffeic acid, p-coumaric and ferulic acids( Reference Kumar and Pruthi60 ) (Fig. 8).
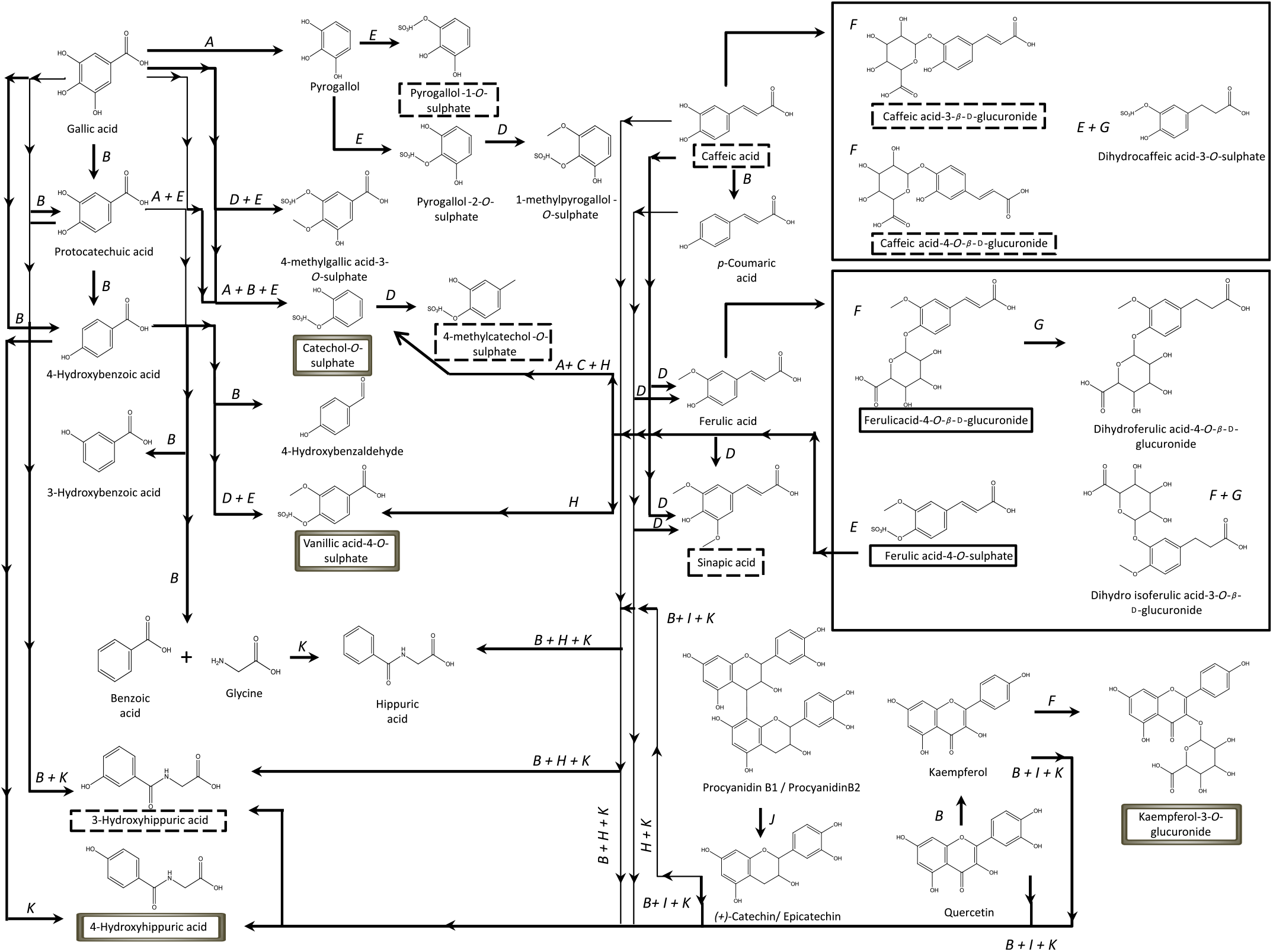
Fig. 8. Proposed metabolic pathways involved in human metabolism of phenolic compounds from common beans, based on previous literature(
Reference Del Rio, Stalmach and Calani15
,
Reference Feliciano, Boeres and Massacessi19
,
Reference Gutiérrez-Grijalva, Ambriz-Pérez and Leyva-López59
). ![]() , Metabolites excreted in urine at a significant level;
, Metabolites excreted in urine at a significant level; ![]() , metabolites with significant plasma concentrations;
, metabolites with significant plasma concentrations; ![]() , metabolites with significant plasma concentrations and excreted amounts in urine; A, decarboxylation (phase I); B, dehydroxylation (phase I); C, dealkylation (phase I); D, O-methylation (phase II); E, O-sulfation (phase II); F, O-glucuronidation (phase II); G, reduction (phase I); H, oxidation of the C3 chain (phase I); I, fission of the C-ring (phase I); J, dimer’s cleavage (phase I); K, conjugation with glycine (phase II).
, metabolites with significant plasma concentrations and excreted amounts in urine; A, decarboxylation (phase I); B, dehydroxylation (phase I); C, dealkylation (phase I); D, O-methylation (phase II); E, O-sulfation (phase II); F, O-glucuronidation (phase II); G, reduction (phase I); H, oxidation of the C3 chain (phase I); I, fission of the C-ring (phase I); J, dimer’s cleavage (phase I); K, conjugation with glycine (phase II).
Regarding the human inter-individual variability, the total excreted amount of metabolites (11·0 (sd 1·3) mg of excreted compounds) represented only 5·8 % of the total phenolic compounds consumed in the common beans portion (187·5 (sd 4·0) mg of GAE in 143·8 (sd 1·0) g of raw seeds’ DW), which was in accordance with the one described for cranberries juice, where 6·2 % of the total phenolic compounds consumed is reported, and in line with the low urinary recovery related to other food products rich in (poly)phenols( Reference Feliciano, Boeres and Massacessi19 ). The low urinary recovery of phenolic compounds could be attributed to the low bioaccessibility of the phenolic compounds derived from cooked common beans. These compounds are mostly entrapped in the dietary fibre (17 % of total seed weight)( Reference Kutoš, Golob and Kač61 ) which slows down their absorption and excretion( Reference Pérez-Jiménez, Serrano and Tabernero62 ), contributing possibly to faecal metabolites, not quantified in the present study.
In common beans, the inter-individual variability was evident not only on the plasma concentration but also on the urinary excretion of specific phenolic compounds’ metabolites at different time points. The PLS-DA models, defined herein by values of R 2(Y) > 0·6 and small differences (<0·3)( Reference Kiralj and Ferreira39 ) between R 2(Y) and Q 2, indicated a common metabotype in the different volunteers after common beans’ intake. Metabolites such as vanillic acid-4-O-sulphate, 4-hydroxyhippuric acid, ferulic acid-4-O-glucuronide, ferulic acid-4-O-sulphate and kaempferol-3-O-glucuronide were predominant in plasma at early times (1–2 h) after common beans’ intake. In urine, Kaempferol-3-O-glucuronide was also one of the metabolites early excreted (2–4 h) and others, such as 4-methylcatechol-O-sulphate, dihydrocaffeic acid-3-O-sulphate and 3-hydroxyhippuric acid, were only excreted after 8 h of common beans’ intake. As reported previously( Reference Feliciano, Mills and Istas37 ), the variability in specific metabolites, such as kaempferol-3-O-glucuronide, Table 4, higher than the variability obtained for the sum of compounds in urine could be an indication about the individual variation on the enzymatic activity and the complex interaction between the gut microbiota (with possible different bacteria compositions) and phenolic compounds from common beans. Several factors could contribute for such variability (e.g. enzyme activity, microbiota composition, gastro-intestinal transit time, age, sex and genetics)( Reference D’Archivio, Filesi and Varì63 , Reference Souza, Casanova and Costa64 ).
In conclusion, to our knowledge, the present work was the first human intervention study developed, through targeted metabolomics, to identify and quantify accurately phenolic compounds and their metabolites, in plasma and/or urine, after cooked common beans’ intake. It also explored the effect of the cooking process on phenolic composition of common beans, since it influences the bioaccessibility and consequently the bioavailability of the compounds present in the original raw beans. The metabolites associated with plasma concentration and/or urinary excretion increments, after a diet free of phenolic compounds during 48 h followed by a single meal of cooked beans, were vanillic acid-4-O-sulphate, 4-hydroxyhippuric acid, ferulic acid-4-O-sulphate, ferulic acid-4-O-glucuronide, kaempferol-3-O-glucuronide, pyrogallol-1-O-sulphate, caffeic acid, catechol-O-sulphate, 4-methylcatechol-O-sulphate, 3-hydroxyhippuric acid, caffeic acid-4-O-β-d-glucuronide, caffeic acid-3-O-β-d-glucuronide and sinapic acid. Even if not specific of common beans’ intake (it can also derived from other dietary sources), the plasma concentration and/or urinary excretion increase of these compounds, during the study period, made possible to define clusters of metabolites and associate them with cooked common beans’ intake. Most of the metabolites, such as the ones produced from the hydroxycinnamic acids, for example, caffeic acid-4-O-β-d-glucuronide, were excreted during the period of 8–24 h, indicating their persistence in the systemic circulation for a longer period of time.
To access the kinetic profile of the metabolites with a return to the baseline level, future studies should be extended to at least 48 h including, if possible, a higher number of volunteers. In order to understand the role of metabolites derived from phenolic compounds of common beans in human health, future in vitro and in vivo studies regarding the biological activity of the different metabolites (namely those whose concentration increased significantly in plasma and urine) should be performed. Additionally, studies regarding individual differences on microbiota composition and concerning the faecal metabolites obtained after common beans’ consumption could also contribute to understand the impact of common beans in human health, especially in gut health.
Acknowledgements
The authors acknowledge all the volunteers involved in the study, and also Dr Claudia Nunes dos Santos (iBET) and Dr Rita Ventura (ITQB) for providing some metabolites (pyrogallol-1-O-sulphate, pyrogallol-2-O-sulphate, 1-methylpyrogallol-O-sulphate, 2-methylpyrogallol-O-sulphate, 4-methylcatechol-O-sulphate, 4-methylgallic-3-O-sulphate, catechol-O-sulphate, and vanillic acid-4-O-sulphate) used in the study.
This study was financially supported by FCT Portugal through the BEGEQA project (PTDC/AGR-TEC/3555/2012), a PhD fellowship to E. M. (SFRH/BD/89287/2012) and a FCT Investigator Program Development Grant to M. C. V. P. (IF/01337/2014), R&D unit, UID/Multi/04551/2019 (Green-IT) and COST Action FA1403 (STSM-FA1403-290815-063873).
E. M., M. R. B., M. E. F., M. C. V. P., A. R.-M. and R. P. F. planned and designed the research. M. E. F. collected venous blood from volunteers. A. R.-M. and R. P. F. supervised the work with UPLC-Q-TOF-MS. E. M. developed the experimental work and analysed the data. E. M., M. R. B., S. D. S., R. P. F., M. C. V. P. and A. R.-M. wrote the paper. All the authors contributed to the final version.
The authors declare no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519002836


















