People with psychotic illnesses die prematurely, mostly from natural causes, with markedly raised standardised mortality ratios for cardiovascular disease (CVD, 3.6, 95% CI 3.5–3.6), with young adults (4.5, 95% CI 4.1–4.8), women (4.6, 95% CI, 4.5–4.7) and people of White ethnicity (4.9, 95% CI, 4.8–5.0) especially at risk.Reference Olfson, Gerhard, Huang, Crystal and Stroup1 Cardiometabolic dysregulation is evident even from the early stages of psychosisReference Pillinger, Beck, Gobjila, Donocik, Jauhar and Howes2 and increases in the months after first presentation, with olanzapine having the most marked effect.Reference Fleischhacker, Siu, Bodén, Pappadopulos, Karayal and Kahn3 Clozapine also affects metabolism, with 55% exhibiting glucose dysregulation within 3 months of starting.Reference Howes, Bhatnagar, Gaughran, Amiel, Pilowsky and Murray4 A recent meta-analysis reported that antipsychotics were associated with greater weight gain in Asian as opposed to Western studies, although very few studies looked directly at the effects of ethnicity.Reference Tek, Kucukgoncu, Guloksuz, Woods, Srihari and Annamalai5 Evidence is needed to determine whether or not interventions to modify cardiometabolic risk can be targeted in first-episode psychosis (FEP). Few studies have investigated whether health behaviours at presentation including diet, physical activity and substance use predict subsequent deterioration in cardiometabolic status. An exception is a recent prospective study of 101 people with FEPReference Nyboe, Vestergaard, Moeller, Lund and Videbech6 that found that low aerobic fitness was a significant risk factor for metabolic syndrome. Overall, however, insufficient prospective observational studies have attempted to disentangle the impact of lifestyle on the emergence of cardiometabolic risk to reliably inform the development and targeting of effective preventative strategies.
The main aims of this study were therefore to (a) to determine the prevalence of cardiometabolic risk factors in an ethnically diverse population at first presentation with psychosis; (b) to describe the emergence of additional cardiometabolic risk over the year following first presentation with psychosis and (c) to determine the relationship between lifestyle factors (diet, sedentary behaviour, smoking, substance use) and antipsychotic medication, in particular olanzapine and clozapine use, on cardiometabolic risk both at baseline and at 12-month follow-up. We hypothesised that there would be an association between baseline lifestyle choices and (a) cardiometabolic risk at baseline and (b) prospectively in change in cardiometabolic risk over a 1-year follow-up period. We also hypothesised that the emergence of glucose and lipid dysregulation would be greater in those prescribed the dibenzodiazepines, clozapine or olanzapine, compared with other antipsychotics.
Method
Setting and design
A prospective observational cohort of people was followed up for 12 months after their first presentation with psychosis. The study took place in in-patient and early intervention in psychosis community mental health teams in three English mental health National Health Service (NHS) services.
Eligibility criteria
Inclusion criteria were as follows: (a) aged between 16 and 65 years; (b) within 6 months of first presentation with psychosis (ICD-10 codes F20–29 and F30–33);7 (c) proficient in English with no requirement for an interpreter. Patients were excluded if they were pregnant or had a major medical illness or neurological disease; had been diagnosed with a severe intellectual disability; had an organic cause for their psychosis; or had previous contact with health services for the presence of psychosis. All participants were included in the study only after giving written, confirmed consent. The study protocol was approved by the Joint South London and Maudsley and the Institute of Psychiatry NHS Research Ethics Committee (08/H0807/53).
Assessments
Patients were assessed at baseline and after 12 months. Sociodemographic data were recorded at baseline, namely age, gender, ethnicity (self-report) and years of education. The operational criteria checklist for psychotic and affective illness (OPCRIT) was used to assess type of mental health state over the lifetime.Reference Craddock, Asherson, Owen, Williams, McGuffin and Farmer8 All other measures were recorded at both time points. Selected clinical measures unaffected by fasting state (weight, blood pressure, haemoglobin A1c (HbA1c), cholesterol, high-density lipoprotein (HDL)) were recorded where available and utilised in analyses at baseline if the relevant measure post-dated the onset of psychosis and preceded the research measures, being thus less likely to have been affected by the introduction of psychotropic medication. Follow-up clinical measures were utilised if research measures for that period were missing.
Psychopathology
Mental health status was measured using the Positive and Negative Symptom Scale,Reference Kay, Fiszbein and Opler9 Global Assessment of Functioning,Reference Richard and Hall10 Clinical Global Impression Scale,Reference Guy and Rush11 Calgary Depression score,Reference Addington, Addington and Schissel12 and the Young Mania Rating Scale.Reference Young, Biggs, Ziegler and Meyer13
Pharmacological history
Type(s) (British National Formulary definitions) of psychotropic medication and duration of antipsychotic (in days) were retrospectively extracted from electronic medical records.
Cardiometabolic measures
Metabolic measures comprised anthropometric measures of weight, height, body mass index (BMI), waist circumference and blood pressure, as well as fasting blood samples for glucose, glycated haemoglobin (HbA1c), lipids and C-reactive protein (CRP). Standardised techniques were used to measure height, blood pressure and weight. Where available, the blood pressure values are the second blood pressure measurements (first reading presented in 75/219 participants at baseline; and 39/147 at 12 months). Waist circumference was measured at the umbilicus with the patient standing.
Obesity was defined by the World Health Organization (WHO)14 reference standard (BMI ≥30 kg/m2) and overweight as BMI ≥25 kg/m2. Diabetes was diagnosed based on a HbA1c of ≥48 mmol/mol (≥6.5%), or a fasting glucose ≥7.0 mmol/L or a prior diagnosis of diabetes. In keeping with recent guidance from the American Diabetes Association, an HbA1c of 39–47 mmol/mol (5.7–6.4%) was taken to indicate glucose dysregulation or a high risk of diabetes.15 The International Diabetes Federation definitions of other cardiometabolic risk factors were used.Reference Alberti, Zimmet and Shaw16 Insulin resistance was presented using the homeostasis model assessment – insulin resistance (HOMA-IR), calculated from fasting glucose and insulin levels. Inflammation was estimated using high-sensitivity CRP as a marker.
Physical activity and dietary intake
Physical activity was assessed by the International Physical Activity Questionnaire,Reference Craig, Marshall, Sjöström, Bauman, Booth and Ainsworth17 which has been validated in psychosis.Reference Faulkner, Cohn and Remington18 The self-report Dietary Instrument for Nutrition Education (DINE)Reference Roe, Strong, Whiteside, Neil and Mant19 was used to assess dietary patterns over the previous week. Additional questions were included to account for sugar intake, the consumption of take-away food, carbonated drinks and adding either salt or sugar to food/drinks.
Substance use
Cigarette and tobacco consumption were measured using the Nicotine Dependence questionnaireReference Fagerstrom20 and alcohol use with the Alcohol Use Disorders Identification Test (AUDIT),Reference Saunders, Aasland, Babor, de la Fuente and Grant21 where scores of eight or more indicate hazardous and harmful alcohol use. A modified Cannabis Experience Questionnaire (CEQ-4) was used to enquire about lifetime and current use of substancesReference Di Forti, Morgan, Dazzan, Pariante, Mondelli and Marques22,Reference Barkus, Stirling, Hopkins and Lewis23 and a urine drug screen requested.
Statistical analysis
Descriptive data are presented in tabular form. Pairwise comparisons were done using t-tests for continuous and χ2 tests for categorical outcomes. Baseline associations between continuous scores for lifestyle and cardiometabolic factors were investigated using unadjusted and adjusted linear regression models. To account for different patterns of missingness, we used multiple imputation by chained equations prior to these analyses, using the lifestyle factors (DINE saturated fat score, AUDIT hazardous drinking score, number of h sitting per day and whether taking olanzapine), cardiometabolic factors (cholesterol, diastolic blood pressure, HbA1c, waist circumference, BMI, HDL and triglycerides), age, gender, ethnicity and pre-baseline number of days on antipsychotic medication in the multiple imputation model. We used the ‘mi impute chained’ command in Stata version 15.1, with 50 imputed data-sets for each multiple imputation model. Binary variables were imputed using logistic regression, using augmented logistic regression where perfect prediction was detected.Reference White, Daniel and Royston24 Continuous variables were imputed using linear regression unless they appeared non-normal, in which case the predictive mean matching imputation method, drawing from five nearest neighbours, was used.Reference Schencker and Taylor25
Imputed data-sets were then used to examine cross-sectional associations between each lifestyle factor and cardiometabolic factor separately using linear regression, unadjusted and adjusted for prespecified potential confounders of CVD risk, namely age, gender, ethnicity and pre-baseline number of days on antipsychotic medication. We further explored the cross-sectional associations between use of dibenzodiazepine medications (yes/no) prior to baseline and cardiometabolic factors at baseline using the same model and potential confounders.
For each outcome (change in cardiometabolic factor) at 12 months we employed separate multiple imputation models (using the same method as above) using the outcome and the same variables at baseline. We then explored for each baseline lifestyle factor, separately, whether they were associated with change in cardiometabolic factors over the 12-month follow-up period, unadjusted, and then adjusted for the above potential confounders, investigating associations between use of dibenzodiazepine medications between baseline and 12 months (yes/no) and cardiometabolic factors in the same way.
We separately assessed whether cardiometabolic factors at baseline differed between (a) participants taking medication with less or greater than 14 days of antipsychotic medication prior to baseline and (b) participants who were taking medication and those who were unmedicated prior to baseline.
To account for multiple testing a stricter alpha of 0.01 was prespecified as the significance level.Reference Lang and Secic26 Tests with a P-value between 0.01 and 0.05 were discussed as a trend and conclusions should be treated as explorative. Sensitivity analyses were carried out for all the analyses using complete cases from the full data-set, and also using a reduced data-set (with only participants with <5 variables of interest missing) prior to using multiple imputation to check the extent to which the original multiple imputation was plausible.
Results
We screened 11 705 people for eligibility; 971 were eligible of whom 321 (33.1%) consented to participate (263 declined, 50 transferred, 337 uncontactable). Twenty-eight were excluded after consent when further information became available, leaving 293 eligible people, (mean age 30.6 years (s.d. = 10.5)) in the study at baseline, 46.4% of whom were of White ethnicity; 36.9% Black and 7.8% Asian. Sixteen withdrew at baseline, and 251 completed baseline measures with 26 remaining in the study but missing or declining data collection at that time point. A further 10 participants withdrew during the study, 1 died and 140 dropped out (51 uncontactable, 73 declined, 11 missed and 5 in prison), with 125 completing 12-month follow-up assessments. Demographic and clinical characteristics are reported in supplementary Table 1 available at https://doi.org/10.1192/bjp.2019.159.
Twelve-month follow-up was completed for 125 patients with additional selected anthropometric and blood measures taken from clinical records where available and required. There were no differences in baseline demographics or cardiometabolic measures between those who had 12-month follow-up assessments (n = 125) and those who did not (n = 168) (supplementary Table 4).
Cardiometabolic and lifestyle factors in the year following onset of psychosis
Rates of obesity, hypertriglyceridaemia, diabetes, raised CRP and low HDL cholesterol all rose over the first year of illness, and the proportion of people with HbA1c in the glucose dysregulation range almost doubled (Tables 1 and 2, supplementary Tables 2 and 7 and Fig. 1) Rates of tobacco smoking were high (Tables 2 and 3); smokers consumed a median of ten cigarettes per day, with little change over time. Combining both self-report and urinary drug screen data, 49.5% (102/206) of participants were current users of cannabis at baseline, and 23/183 (12.6%) reported current usage of ‘other recreational drugs’. The corresponding figures at 12 months were 38.1% (40/105) and 12/102 (11.8%), respectively. From baseline to 12 months, 12 (11.3%) participants initiated cannabis use while 16 (15.5%) stopped using cannabis.
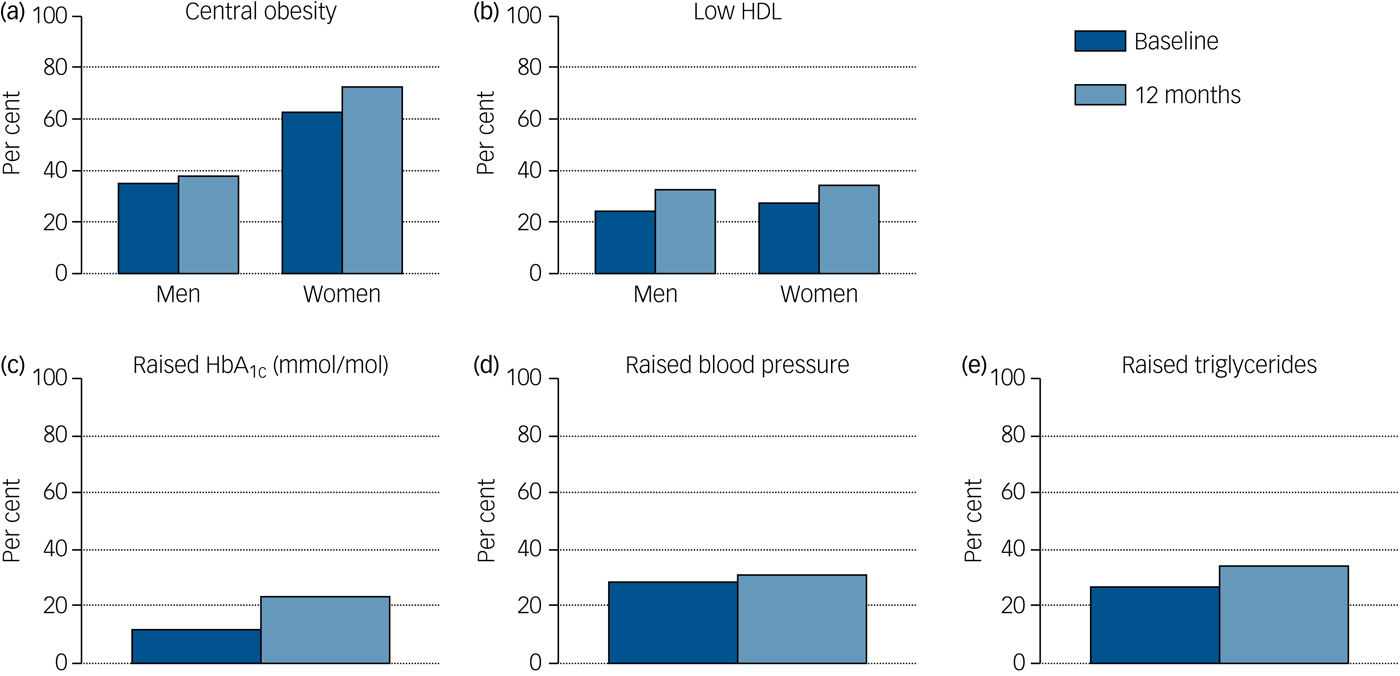
Fig. 1 Rates of identified cardiometabolic risk at each time point.
Table 1 Descriptives at each time point
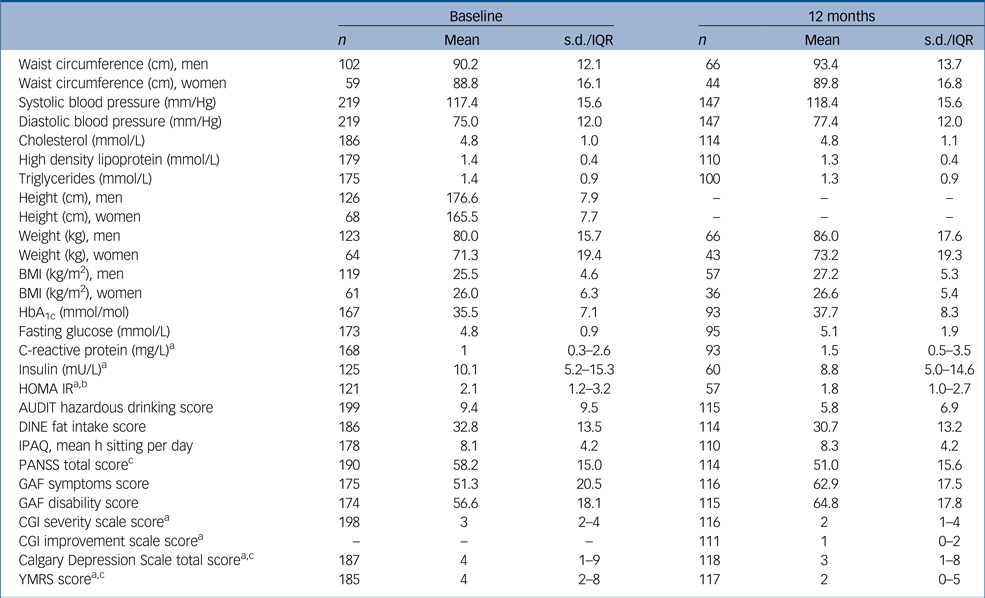
s.d., standard deviation; IQR, interquartile range; BMI, body mass index; HbA1c, haemoglobin A1c; HOMA IR, homeostasis model assessment – insulin resistance; AUDIT, Alcohol Use Disorders Identification Test; DINE, Dietary Instrument for Nutrition Education; IPAQ, International Physical Activity Questionnaire; PANSS, Positive and Negative Symptom Scale; GAF, Global Assessment of Functioning; CGI, Clinical Global Impression Scale; YMRS, Young Mania Rating Scale.
a. Medians and upper/lower quartiles presented instead of means standard deviations because of skewed data.
b. Values of fasting glucose over 11 mmol/L were excluded from the calculation of HOMA IR.
c. PANSS, Calgary and YMRS totals were calculated even where individual items were missing (although PANSS was considered missing if any of the three subscale totals, positive symptoms, negative symptoms and general psychopathology were entirely missing).
Table 2 Rates of identified cardiometabolic and lifestyle risk factors at each time point
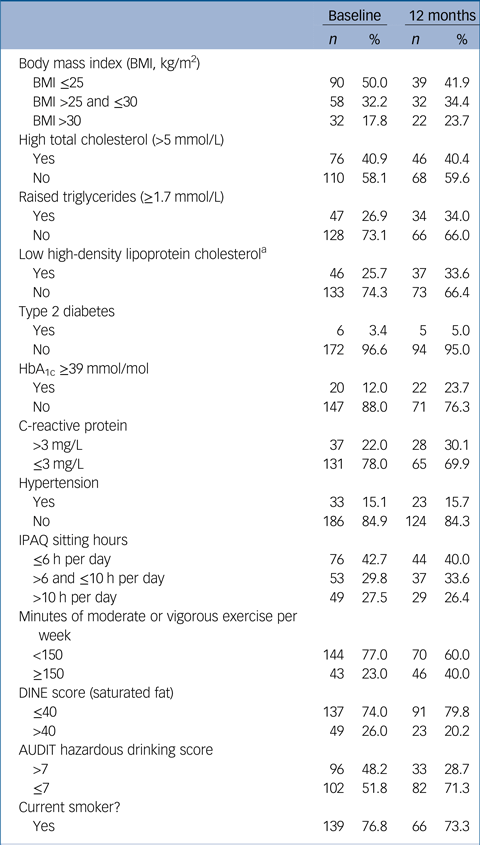
HbA1c, haemoglobin A1c; IPAQ, International Physical Activity Questionnaire; DINE, Dietary Instrument for Nutrition Education; AUDIT, Alcohol Use Disorders Identification Test.
a. Men: <1.03 mmol/L; women: <1.29 mmol/L.
Table 3 Effects of gender and ethnicity

s.d., standard deviation; HbA1c, haemoglobin A1c.
Data were available on antipsychotic prescriptions prior to baseline for 242 (82.6%) participants. In total 22% (54/242) were antipsychotic-free before baseline, and a further 65 (26.9%) had received antipsychotics for less than 2 weeks. The median duration of antipsychotic treatment for those prescribed antipsychotics pre-baseline was 21 days (interquartile range (IQR)=9.0–55.5 days) (mean 43.3 days (s.d.) = 53.3), with most (94.7%; n = 178/188) prescribed second-generation antipsychotics. Overall, 128 people were prescribed olanzapine across the year, 102 as their first antipsychotic. Of those on olanzapine at baseline (n = 89; median dose 10 mg (IQR = 10–75; range 2.5–25)), 71% (52/73) remained on olanzapine at follow-up.
At follow-up, 66 people were prescribed olanzapine (we had dose data of 45 people), at a median dose of 12.5 mg (n = 45, IQR = 10–17.5; range 2.5–30). All bar 15 had been prescribed antipsychotic medication over the course of the study, for a median of 378 (IQR = 302–434) days (mean of 354.6 (s.d. = 138.9) days), with those receiving olanzapine prescribed it for a median of 236 (IQR = 61–388) days (mean of 239.6 (s.d. = 169.8) days). No patients were prescribed clozapine. We did not have data on somatic medications prescribed over the course of the follow-up.
Participants taking medication prior to baseline had higher baseline average waist circumferences (+7.7 cm, 95% CI 2.0–13.4; P = 0.009) compared with those unmedicated. Those who had received over 2 weeks antipsychotic medication prior to baseline had higher total cholesterol (+0.5 mmol/L, 95% CI 0.1–0.8; P = 0.007) than those prescribed antipsychotics for less than 2 weeks (excluding those prescribed no antipsychotic) (supplementary Table 3).
At baseline, 57% consumed a carbonated drink daily, half (50%) drinking more than one 500 mL bottle per day and 21.4% of the total sample drank diet carbonated drinks daily. Three-quarters (75.7%) consumed tea on a daily basis, most (90.8%) drinking more than one cup, with 44% of the sample adding 2 teaspoons or more of sugar to each cup of tea. Salt was added to food during cooking by 66.5% of participants. A total of 78.5% consumed take-away meals, three-quarters of whom (75.8%) did so more than once weekly.
Relationship between lifestyle/medication and cardiometabolic outcomes at baseline and 12-month follow-up.
Baseline DINE fat scores, sedentary behaviour, AUDIT scores and pre-baseline olanzapine use were not significantly associated with any of the baseline cardiometabolic outcomes either unadjusted or after adjusting for potential confounders (supplementary Table 6). Nor did the linear regression models show any association between baseline lifestyle factors and change in any of the cardiometabolic outcomes over 12 months either unadjusted or after adjusting for potential confounders (Table 4). Nor was there evidence of a difference in change in cardiometabolic outcomes by 12 months in those participants prescribed olanzapine between baseline and 12 months and those not prescribed olanzapine (unadjusted and adjusted) (Table 4).
Table 4 Association between lifestyle factors at baseline and 12-month change in cardiometabolic risk factors (adjusted for age, gender, ethnicity and pre-baseline number of days on antipsychotic medication)
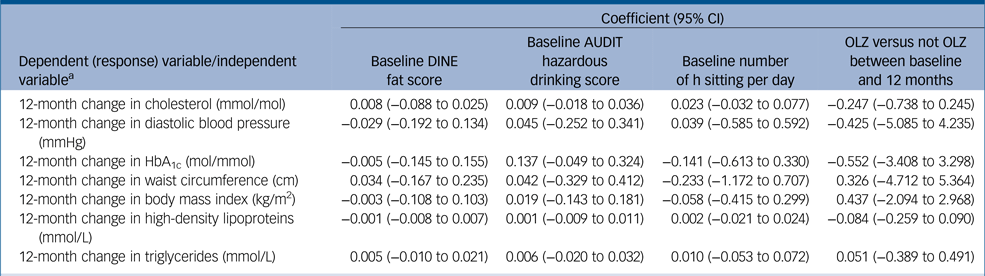
CI, confidence interval; DINE, Dietary Instrument for Nutrition Education; AUDIT, Alcohol Use Disorders Identification Tes; OLZ, olanzapine; HbA1c, haemoglobin A1c.
a. Separate models used for each association.
Sensitivity analyses using complete cases only gave similar results with no evidence of any associations. Sensitivity analyses using a reduced data-set prior to imputing also gave similar results.
Effects of gender and ethnicity
Rates of central obesity at baseline were higher in women (62.7%, n = 37/59) than in men (35.3%, n = 36/102), (χ2 = 11.34, P = 0.001), with a trend towards women from Black and minority ethnic (BME) groups having larger waists than their White counterparts (t = 2.37, P = 0.02), this gap increasing over time in the paired sample subgroup (supplementary Table 5). White men overall gained a mean of 4.9 cm in waist circumference over the year, whereas men from BME groups gained 1.6 cm (Table 3), with similar findings in the paired sample subgroup (supplementary Table 5). There was no relationship between ethnicity and BMI. There was a trend towards lower total cholesterol at 12 months (P = 0.04) in BME patients.
Although HbA1c levels at baseline were comparable, BME patients exhibited a significant increase in median HbA1c levels over 12 months (P<0.01), rising by 3.3 mmol/mol. In contrast, no change was evident in White patients (Table 3). Where only complete paired data-sets were included in the analysis, median HbA1c rose in White patients by 1.1 mm/mol and by 3.3 mmol/mol in participants from BME groups (supplementary Table 5). HbA1c levels above 39 mmol/mol were seen in 10% (4/40) of White participants and 34% (18/53) of those from BME groups at 12 months. There was no significant difference in ethnicity between those prescribed olanzapine and those not prescribed it (χ2(3) = –3.10, P = 0.376).
Discussion
This comprehensive large-scale cohort study of patients with FEP prospectively considers the relationship between lifestyle factors, medication and cardiometabolic trajectories. Weight gain is the most obvious problem for many patients both cosmetically and for their health – here 17.8% were obese at onset, rising to 23.7% within 1 year. A case has recently been made for obesity to be recognised as a chronic diseaseReference Bray, Kim and Wilding27 to allow the preventative strategies needed at a population level; in psychosis, the rapidity of weight gain over the first year offers an unrivalled opportunity for prevention, with diet, exercise and medication being key targets. We noted high consumption of saturated fat, carbonated, high-sugar drinks, sweetened beverages and added salt. Calorie intake was further augmented by alcohol use (Table 2). Although neither fat intake nor alcohol use in the early weeks of psychosis predicted longer-term outcomes in our population, the Keeping the Body in Mind programme has successfully worked with dieticians and exercise physiologists to prevent weight gain using lifestyle interventions for all early in the disease process.Reference Curtis, Watkins, Rosenbaum, Teasdale, Kalucy and Samaras28
We also found early emergence of glucose dysregulation; by 1 year twice as many people had HbA1c levels at or above the American Diabetes Association glucose dysregulation range (Tables 1 and 2). Diabetes is highly predictive of cardiometabolic disease and can have an especially significant impact on health in people with psychosis, given the other practical challenges they may face.
There was a remarkably high baseline prevalence of tobacco smoking of 76.8%. Smoking confers an almost fivefold risk of mortality in people with schizophrenia.Reference Dickerson, Stallings, Origoni, Schroeder, Khushalani and Yolken29 Further, nicotine dependence after psychosis onset predicts both poor medication adherence and non-remission of psychosis.Reference Colizzi, Carra, Fraietta, Lally, Quattrone and Bonaccorso30 It is therefore worrisome that we saw little reduction in smoking rates over time. Since this work was completed, many UK mental health hospitals have become smoke-free environmentsReference Robson, Spaducci, McNeill, Stewart, Craig and Yates31 and although a recent trial has demonstrated that smoking cessation can be achieved in the community, work remains to be done to sustain quitting.Reference Gilbody, Peckham, Bailey, Arundel, Heron and Crosland32 Rates of current cannabis use and lifetime use of other substances were high, in keeping with previous work.Reference Di Forti, Morgan, Dazzan, Pariante, Mondelli and Marques22
People with psychosis engage in low levels of physical activity.Reference Stubbs, Williams, Gaughran and Craig33 Although physical activity at baseline did not predict cardiometabolic outcome in our study, by 12 months 40% of the participants were engaging in the 150 min of physical activity per week recommended by the WHO (2014).34 Worryingly however, sedentary behaviour changed little and CRP levels increased over time. Sedentary behaviour is associated with inflammation in psychosis,Reference Stubbs, Gardner-Sood, Smith, Ismail, Greenwood and Farmer35 and inflammation is in turn linked to cardiometabolic disease.Reference Vepsäläinen, Soinio, Marniemi, Lehto, Juutilainen and Laakso36 Both exercise and sedentary behaviour are important targets for health promotion with studies underway to examine ways of changing patterns of behaviour in clinical settings.Reference Williams, Stubbs, Gaughran and Craig37
Our regression analyses revealed no relationship between cardiometabolic outcomes and lifestyle, nor any effect of dibenzodiazepine medication, despite the high risk of weight gain demonstrated with olanzapine in other settings.Reference Fleischhacker, Siu, Bodén, Pappadopulos, Karayal and Kahn3 A high proportion were prescribed olanzapine as a first antipsychotic, a practice now not recommended.Reference Buchanan, Kreyenbuhl, Kelly, Noel, Boggs and Fischer38 Of note, no participants were prescribed clozapine in the first year of their illness, in keeping with the reported mean lag time to starting clozapine of 47.7 months (s.d. = 49.7).Reference Howes, Vergunst, Gee, McGuire, Kapur and Taylor39 The omission of clozapine is noteworthy as 23% of people presenting with their first episode of psychosis are treatment resistant from illness onset.Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha and Kolliakou40
Our sensitivity analyses illustrated the rapidity of cardiometabolic change in early psychosis; those exposed to antipsychotic medication for more than 14 days before baseline had higher cholesterol levels than those on antipsychotics for less than a fortnight, and those exposed to any antipsychotic medication had a larger waist circumference at baseline than did individuals who were antipsychotic naive.
Effects of ethnicity and gender
Men of White ethnicity appeared to have a particular vulnerability to emergence of central obesity, increasing their mean waist size by 4.9 cm, whereas men of other ethnicities gained a more modest 1.6 cm. These changes are clinically significant and the variation between ethnic groups was in keeping with findings in established psychosis.Reference Gardner-Sood, Lally, Smith, Atakan, Ismail and Greenwood41 Central obesity is a better predictor of CVD risk than is general obesity,Reference Goh, Dhaliwal, Welborn, Lee and Della42 so this may relate to the lower hazard ratios seen for all-cause mortality in Black African, Black Caribbean and South Asian patients with severe mental illness compared with their White British counterparts.Reference Das-Munshi, Chang, Dutta, Morgan, Nazroo and Stewart43
Women were at risk of central obesity throughout, with 72.7% in that category by 1 year, again consistent with the local figure of 95% seen in women with established psychosis.Reference Gardner-Sood, Lally, Smith, Atakan, Ismail and Greenwood41 Central adiposity in women is predictive of all-cause mortality in White, but not Black women.Reference Stevens, Keil, Rust, Tyroler, Davis and Gazes44
Minority ethnic groups appeared vulnerable to emergent glucose dysregulation, with highly clinically relevant increases in median HbA1c of 3.3 mmol/mol (Table 3 and supplementary Table 5), but no change seen in White patients. Within the non-diabetic range, this reflects a highly clinically significant shift in glycaemia in the BME group, with one-third exceeding the American Diabetes Association HbA1c threshold for pre-diabetes15 (>39 mmol/mol (5.7%)), although it is useful to note that some would not have been identified as at risk by current National Institute for Health and Care Excellence guidelines.45 This change in glucose regulation in a relatively young population over just 1 year highlights the risk of accelerated emergence of diabetes in people of ethnicities other than White, and is in keeping with a recent large cross-sectional analysis of London primary careReference Das-Munshi, Ashworth, Dewey, Gaughran, Hull and Morgan46 data that showed a three- to tenfold relative risk of type 2 diabetes in young people with severe mental illness.
Strengths and limitations
This is to our knowledge the most comprehensive study to date examining the effects of lifestyle choice on emergent cardiometabolic risk. In conducting this study, we recruited an incident sample of people presenting to both in-patient and community services for the first time with psychosis and we followed them up for 1 year, thereby capturing the period with the greatest change in physical health. The diverse nature of the population studied allows exploration of the effect of ethnicity on emergence of cardiometabolic risk. The use of multiple imputation on a reduced data-set allowed confidence in the results while retaining generalisability.Reference Stuart, Azur, Frangakis and Leaf47
The study must be interpreted taking into account a number of methodological aspects. Over half our patients required acute in-patient care at the time of recruitment and many had received treatment for some weeks before being well enough to consent to inclusion. To minimise this effect and minimise missing data, we therefore sought permission to use clinical data, where available and comparable, as well as adjusting for the number of days prescribed antipsychotics before baseline in our analyses. Also, in follow-up studies there is a risk that attrition may not be random. Therefore, considerable efforts were made to minimise loss to follow-up with contact made with 81% (217/268) of the then eligible participants at 12 months, although 73 participants declined 12-month follow-up. There was recompense for time but no financial incentivisation to remain in the study. Analysis showed no differences in baseline demographic or clinical attributes between completers and non-completers. The supplementary tables include a descriptive table of complete paired measures. We also note that although 89 people were prescribed olanzapine at baseline, some did not sustain that prescription throughout the study period, possibly because of emergent metabolic effects. Finally, the number of statistical tests carried out was reasonably large and although we took a more conservative threshold of statistical significance, we cannot completely discount the possibility of type I errors.
In conclusion, our results identify that cardiometabolic risk factors are already pronounced in those presenting to FEP services and worsen over the first year under standard care. No baseline behaviour predicted risk of worsening cardiometabolic parameters, but a greater degree of emergent glucose dysregulation was observed in those from BME groups.
Funding
This paper summarises independent research funded by the National Institute for Health Research (NIHR) under its IMPACT Programme (Grant Reference Number RP-PG-0606-1049) in collaboration with Genetics and Psychosis Project funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. B.S. and F.G. are, in part, supported by the Maudsley Charity and the NIHR Collaboration for Leadership in Applied Health Research and Care South London (NIHR CLAHRC South London) at King's College Hospital NHS Foundation Trust. B.S. is supported by a Clinical Lectureship (ICA-CL-2017-03-001) jointly funded by Health Education England (HEE) and the NIHR. D.S. was part-funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgements
Members of the IMPACT team are: Maurice Arbuthnott, Bee Harries, Philippa Lowe, Susan Moore, Conan O'Brien, Ali Featherman, Catherine Fung, Margaret Heslin, Keji Dalemo, Stella Anakwe-Umeh, Gill Todd, Manyara Mushore, Diana Orr, Ruth Ohlsen, Evangelos Papanastasiou, Mudasir Firdosi, Hannah Sallis, Irene Sambath, Guilia Di Clemente, Josefine Breedvelt, Hugh Williams, Candice Joseph, Jonas Eberhard and Marco Colizzi.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.159.








eLetters
No eLetters have been published for this article.