Atherosclerosis is an inflammatory disease of the arteries(Reference Libby1) that is the primary cause of CVD and stroke, the leading causes of morbidity and mortality in Western societies. Many years of research have established the multifactorial nature of atherosclerosis. In developed countries, unhealthy diets represent one of the major modifiable risk factors. Among dietary factors that may influence development of CVD, there is considerable epidemiological support for a protective effect of fruit and vegetables(Reference Ness and Powles2). Indeed, the WHO has estimated that low fruit and vegetable intake contributes to 31 % of the risk for CHD and 11 % of ischaemic stroke in developed countries(3).
Fruit and vegetable consumption may lower risk of CVD by several mechanisms. Elevated serum concentrations of total and LDL-cholesterol have long been recognized as risk factors(4). Isolated viscous dietary fibres such as pectins and mucilages, present in fruits and vegetables, have a well-established hypocholesterolaemic effect(Reference Brown, Rosner, Willett and Sacks5). Further, pectin-rich foods such as a mixture of vegetables or apples(Reference Stasse-Wolthuis, Albers, van Jeveren, Wil de Jong, Hautvast, Hermus, Katan, Brydon and Eastwood6), carrots(Reference Robertson, Brydon, Tadesse, Wenham, Walls and Eastwood7) and guava fruit(Reference Singh, Rastogi, Singh, Ghosh, Gupta and Niaz8) have demonstrated cholesterol lowering in man. Considerable evidence indicates an association between elevated oxidative stress and the development of atherosclerosis(Reference Singh and Jialal9). Many fruits and vegetables are concentrated sources of antioxidants due to the presence of a variety of secondary plant metabolites(Reference Halvorsen, Carlsen, Phillips, Bohn, Holte, Jacobs and Blomhoff10) that may act to reduce oxidative stress. For example, pomegranate juice, a rich source of antioxidants, increased plasma antioxidant activity and reduced plasma lipid peroxides in man(Reference Aviram, Dornfeld, Rosenblat, Volkova, Kaplan, Coleman, Hayek, Presser and Fuhrman11) and reduced atherosclerotic lesion size in apoE-deficient mice(Reference Aviram, Dornfeld, Rosenblat, Volkova, Kaplan, Coleman, Hayek, Presser and Fuhrman11, Reference Kaplan, Hayek, Raz, Coleman, Dornfeld, Vaya and Aviram12). Inflammation also plays a prominent role in both the initiation and progression of atherosclerosis(Reference Libby13). Serum C-reactive protein, a non-specific systemic marker of inflammation, has been shown to be moderately predictive of CHD(Reference Wilson, Ryan and Boyle14). The serum concentration of C-reactive protein is positively associated with a Western pattern diet(Reference Fung, Rimm, Spiegelman, Rifai, Tofler, Willett and Hu15), and, in elderly subjects, a significant inverse association between frequency of intake of fruits and vegetables and serum C-reactive protein has been found(Reference Gao, Bermudez and Tucker16). Increasing fruit and vegetable consumption in healthy men for 4 weeks significantly reduced C-reactive protein(Reference Watzl, Kulling, Moseneder, Barth and Bub17). Thus, fruits and vegetables may be effective in reducing the risk of atherosclerosis by reducing serum cholesterol, oxidative stress and inflammation.
Dried plums, also known as prunes, are cultivar fruits of Prunus domestica L. native to the Caucasus region in Western Asia. Dried plums are a good source of the cholesterol-lowering fibre pectin, and have been shown to lower serum cholesterol in both rats and man. Tinker et al. (Reference Tinker, Schneeman, Davis, Gallaher and Waggoner18) found that mildly hypercholesterolaemic men experienced a reduction in both total and LDL-cholesterol after consuming 100 g dried plums/d. Tinker et al. (Reference Tinker, Davis and Schneeman19) further demonstrated that dried plum fibre significantly reduced plasma and liver cholesterol in cholesterol-fed rats. Dried plums are also a rich source of antioxidants(Reference Halvorsen, Carlsen, Phillips, Bohn, Holte, Jacobs and Blomhoff10, Reference Miller, Rigelhof, Marquart, Prakash and Kanter20), with an antioxidant content similar to blueberries and similar to or much greater than raisins, depending on the method of assay. Dried plum extracts and prune juice extracts contain significant amounts of antioxidant phenolic compounds that have been found to inhibit LDL oxidation in vitro (Reference Donovan, Meyer and Waterhouse21). Neochlorogenic acid and chlorogenic acid, the two major polyphenols in dried plums, have been shown to function as potent superoxide scavengers(Reference Donovan, Meyer and Waterhouse21, Reference Nakatani, Kayano, Kikuzaki, Sumino, Katagiri and Mitani22). Thus, dried plum consumption may have utility in reducing the development of atherosclerosis.
The apoE-deficient mouse is a useful animal model for studying the development of atherosclerosis directly(Reference Zhang, Reddick, Piedrahita and Maeda23). The animals develop severe hypercholesterolaemia and develop atherosclerotic lesions similar in appearance and distribution to those observed in man consuming a Western-type diet(Reference Plump and Breslow24). In addition, the histological appearance of the atherosclerotic lesions in apoE-deficient mice is similar to those observed in man. Proteomic and metabolomic analyses of atherosclerotic lesions in apoE-deficient mice indicate increased oxidative stress and inflammatory changes as lesions become more severe(Reference Mayr, Chung and Mayr25). Thus, the apoE-deficient mouse appears to be an excellent model to investigate the effect of dietary components on atherosclerotic lesion development.
The objectives of the current study were to determine whether a diet containing dried plums, in the form of a powder, could alter the progression of atherosclerosis in the apoE-deficient mouse, influence blood lipid levels, and alter markers of oxidative stress and inflammation.
Methods and materials
Animals and diets
Male C57BL/6J apoE-deficient mice (5 weeks old; Jackson Laboratory, Bar Harbor, ME, USA) with an initial body weight of between 14 and 17 g were divided into four dietary groups of twelve animals each. Three to four animals were housed per box in a temperature-controlled animal facility with a daily photoperiod of 12 h of light. The experimental protocol for animal use was approved by the University of Minnesota Animal Care Committee. Mice were fed one of the four experimental diets modified from the AIN-93G rodent diet(Reference Reeves, Nielsen and Fahey26). The composition of the diets is shown in Table 1. Dried plums were incorporated into the basal diet as a commercially available powder (Low Moisture Prune Powder, Sunsweet Growers, Yuba City, CA, USA). According to the manufacturer, the powder is produced by further drying of dried plums at 93–148°C for 10–20 min followed by grinding. The macronutrient composition of the dried plum powder, as provided by the manufacturer, was as follows (g/100 g): protein, 3; fat, 0·5; total carbohydrate, 89·0; total dietary fibre, 9·0; total sugars, 62·0 (glucose, 15·0; fructose, 46·1; sucrose, 0·6). Based on the composition of the dried plum powder, all diets were matched for moisture, protein, carbohydrate, fat, total dietary fibre and total energy. All diets contained 52 % energy from digestible carbohydrate, 17 % from protein and 31 % from fat. One basal diet (B+C) and the low (Lo DP, 4·75 %) and high (Hi DP, 9·5 %) concentration dried plum diets contained 0·15 % cholesterol. The second basal diet (B − C), which was cholesterol-free, served as a negative control.
Table 1 Composition of diets (g/kg)
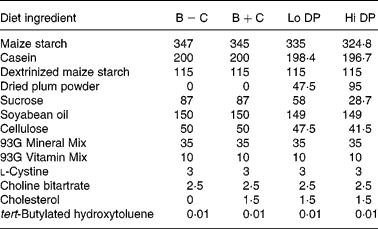
B − C, basal group with no added cholesterol; B+C, basal group with added cholesterol; Hi DP, 9·5 % dried plum powder with added cholesterol group; Lo DP, 4·75 % dried plum powder with added cholesterol group.
The diets were fed ad libitum for 20 weeks. Animal body weights were measured at the beginning of the study, after 15 weeks and at the end of the study. A 24 h urine collection, pooled by box, was taken during week 17.
Characterization of dried plum powder
Phenolic compounds in dried plum powder were extracted as previously described(Reference Kim, Chun, Kim, Moon and Lee27). An aliquot of the extract was diluted with an equal volume of distilled deionized water, the mixture centrifuged at 4°C at 12 000 g for 20 min and the supernatant stored at − 20°C until analysis of total phenolic compounds. Another aliquot of extract solution was evaporated with nitrogen gas, reconstituted with deionized water, and used for analysis of neochlorogenic, chlorogenic, cryptochlorogenic and caffeic acids by HPLC, as described later.
Total phenolics in the dried plum powder extract were measured using a spectrophotometric method(Reference Kim, Chun, Kim, Moon and Lee27). The absorbance was read at 750 nm and total phenolics quantitated using a standard curve of gallic acid. Total phenolics in the dried plum powder were expressed as mg gallic acid equivalents per 100 g dried plum powder.
The extract solution was partially purified and HPLC analysis of neochlorogenic, chlorogenic, cryptochlorogenic and caffeic acids conducted as described(Reference Takeoka and Dao28). The solvent system used for HPLC was a linear gradient of 2–40 % methanol in 0·07 m-KH2PO4 (pH 2·5) over 40 min. The flow rate was set at 1 ml/min and detection was monitored at 325 nm.
For determination of the oxygen radical absorbance capacity (ORAC) of dried plum powder, 2 g dried plum powder was combined with 20 ml methanol and mixed for 1·5 h. The methanol extracts were filtered, the filtrates evaporated with nitrogen gas and 20 ml dimethyl sulphoxide were added to each residue. An aliquot of 10 μl was used for analysis. The ORAC value of dried plum powder was determined by a modification of the method of Cao et al. (Reference Cao, Alessio and Cutler29). Trolox (9 μm), a water-soluble vitamin E analogue, was used as a reference. The ORAC value was expressed as Trolox equivalents/mg dried plum powder.
Lipids
Blood was collected from non-fasted animals at weeks 4 and 15 by tail-nick. At the end of the feeding trial, animals were fasted for 6–8 h, anaesthetized with Isoflurane (USP, Phoenix Pharmaceutical Inc.) and blood was collected by cardiac puncture through the right ventricle, the serum was obtained and stored at − 70°C. Serum cholesterol and TAG concentrations were determined enzymatically using commercial kits (Sigma Diagnostics Catalog #352-100 and TRO100, respectively; St. Louis, MO, USA). Liver lipids were extracted from a sample of perfused tissue using chloroform–methanol(Reference Folch, Lees and Sloane Stanley30). Chloroform–methanol was evaporated under nitrogen gas and total liver cholesterol was determined by enzymatic assay after samples were solubilized in Triton X-100–acetone.
Arterial preparation for visualization of atherosclerotic lesions
The arterial system was perfused through the left ventricle (exiting via the right atria) with 3 ml PBS, followed by 3 ml 10 % formalin in PBS at a flow of approximately 1 ml/min.
The arterial tree was dissected with the aid of a stereo microscope and stored in 70 % ethanol. Atherosclerotic lesions were visualized by staining with 0·2 % Oil Red O in 78 % methanol and 22 % 1 m-NaOH for 50 min. The arterial tree was then pinned on to black wax, water added to the pan to prevent drying, and the tree photographed using a digital camera with a polarizing filter. Areas of the arterial tree and aortic arch and the atherosclerotic lesion area within the tree and arch were calculated using computer-assisted morphometry (Image Pro Plus; Media Cybernetics, Silver Spring, MD, USA) by an investigator blinded to the treatment groups. Lesion areas of the aortic arch or arterial tree were expressed as a percentage of the respective areas.
Urinary thiobarbituric acid-reactive substances
Total body oxidative stress was estimated by measure of urinary thiobarbituric acid-reactive substances (TBARS) following the method of Lee et al. (Reference Lee, Shoeman and Csallany31). Each TBARS measurement was based on a pooled collection from animals housed together by box.
Serum amyloid P-component
Inflammation was estimated by measurement of serum amyloid P-component (SAP) concentration by ELISA as previously described(Reference Burlingame, Volzer, Harris and Du Clos32).
Statistical analysis
Data were examined by one-way ANOVA using Statistical Analysis Systems statistical software package version 9.1 (SAS Institute, Cary, NC, USA). Values for SAP were analysed as ranks, due to a lack of equal variance among the groups for this variable. Differences among group means were inspected using Duncan's multiple comparison test. A probability of P < 0·05 was used as the critical level of significance. Pearson correlation analysis was used to determine associations between measurements, except for SAP, in which case Spearman correlations were calculated.
Results
Characterization of dried plum powder
The dried plum powder was found to contain by HPLC analysis (mg/kg): 83·1, neochlorogenic acid; 40·0, chlorogenic acid; 108·1, cryptochlorogenic acid; 7·5, caffeic acid. The total phenolics concentration, determined spectrophotometrically, was 13·8 g/kg. The ORAC value of the dried plum powder was 4·22 Trolox equivalents/mg.
Body weights
There were no significant differences in either initial (14·3–16·4 g) or final body weights (26·0–28·2 g) among the dietary groups.
Blood lipids
After 4 weeks of feeding, plasma cholesterol concentration in the B+C group was more than double that of the B − C group (Table 2). Neither diet containing dried plum powder affected plasma cholesterol at 4 weeks; however, there was a trend towards lower plasma cholesterol concentration at 15 weeks in the Hi DP group. No significant differences were found in fasting serum cholesterol at 20 weeks with the addition of dried plum powder at either level. Final serum cholesterol concentrations were greater in the mice of all cholesterol-containing diets (12·9–14·6 mmol/l) compared to those fed the cholesterol-free diet (10·1 mmol/l). Overall, fasting serum cholesterol values were significantly lower than levels from fed animals, regardless of diet group.
Table 2 Effect of dried plum diets on plasma and serum lipid levels in the apoE mouse*
(Mean values with their standard errors)
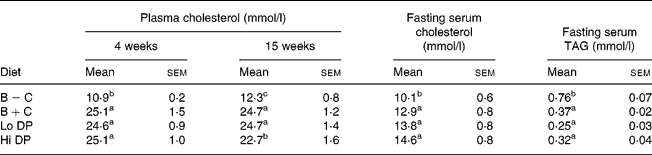
B − C, basal group with no added cholesterol; B+C, basal group with added cholesterol; Hi DP, 9·5 % dried plum powder with added cholesterol group; Lo DP, 4·75 % dried plum powder with added cholesterol group.
a,b,cMean values within a column with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see the methods and materials section and Table 1.
Fasting final serum TAG concentrations were significantly greater in mice fed the B − C diet (0·76 mmol/l) compared to the B+C diet (0·37 mmol/l). There were no significant differences in serum TAG levels between the dried plum-containing diets and the B+C group.
Atherosclerotic lesions
Lesion area in the aortic arch and the entire arterial tree is shown quantitatively in Table 3, and representative images of the arterial trees are shown in Fig. 1. As can be seen, mice fed the cholesterol-free B − C diet had a small lesion area. However, addition of 0·15 % cholesterol to the diet (B+C group) dramatically increased lesion area in the arterial tree. Cholesterol addition to the diet resulted in a significant increase in lesion area from 1·85 to 19·6 % in the aortic arch and from 1·2 to 8 % in the arterial tree. The addition of 4·75 % dried plums, an amount approximately equivalent to consuming ten to twelve dried plums per day in man, resulted in a significant reduction (P < 0·05) of atherosclerotic lesion occurrence in both the aortic arch and arterial tree. Mice fed twice this amount, 9·5 % dried plum, showed a trend towards a reduction in lesion area in both the aortic arch and the entire arterial tree, but the differences did not achieve statistical significance.
Table 3 Atherosclerotic lesion area in the aortic arch and arterial trees of apoE-deficient mice fed dried plum diets for 20 weeks*
(Mean values with their standard errors)
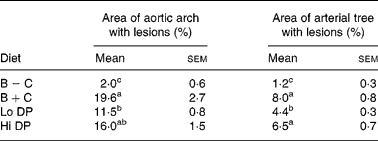
B − C, basal group with no added cholesterol; B+C, basal group with added cholesterol; Hi DP, 9·5 % dried plum powder with added cholesterol group; Lo DP, 4·75 % dried plum powder with added cholesterol group.
a,b,cMean values within a column with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see the methods and materials section and Table 1.

Fig. 1 Atherosclerotic lesions in the arterial trees of apoE-deficient mice. (A), Basal diet with no added cholesterol; (B), basal diet with added cholesterol; (C), basal diet with added cholesterol and 4·75 % dried plum powder.
Oxidative stress and inflammation
The 24 h urinary excretion of TBARS did not differ among the groups. However, there was a trend for a difference between animals in the B+C diet compared to the B − C diet (P = 0·058 by Student's t test) (Table 4).
Table 4 Effect of dried plum diets on markers of oxidative stress and inflammation*
(Mean values with their standard errors)
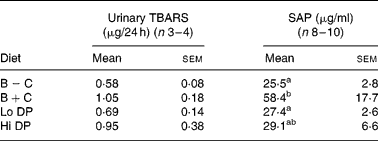
B − C, basal group with no added cholesterol; B+C, basal group with added cholesterol; Hi DP, 9·5 % dried plum powder with added cholesterol group; Lo DP, 4·75 % dried plum powder with added cholesterol group; SAP, serum amyloid P-component; TBARS, thiobarbituric acid-reactive substances.
a,bMean values within a column with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see the methods and materials section and Table 1.
SAP is the primary acute-phase protein in the mouse, and represents a systemic marker of inflammation. Although the concentration of SAP was numerically lower in both dried plum diets and the B − C group relative to the B+C group, only the B − C and Lo DP groups were significantly different from the B+C group (P = 0·031). Although there was no significant correlation between SAP and lesions in the entire arterial tree (r 0·22, r 0·19), the correlation between SAP and lesions in the aortic arch approached statistical significance (r 0·31, P = 0·065). Further, there was no significant correlation between the group means of the 24 h urinary excretion of TBARS and SAP (r 0·768, P = 0·23). However, there was a high correlation between the group means for 24 h urinary excretion of TBARS and lesion area of the aortic arch (r 0·945, P = 0·055) or the total arterial tree (r 0·965, P = 0·035).
Discussion
In the present study we have used an animal model of atherosclerosis development, the apoE-deficient mouse, to examine directly the effect of a fruit, dried plums, on development of the disease. The present findings indicate that dried plums, fed in the form of a dried powder, significantly reduced the area of atherosclerotic lesion in the entire arterial tree as well as the aortic arch, when incorporated into the diet at 4·75 %. At 9·5 % of the diet, there was a trend for reduction in lesion area in the aortic arch. It is not clear why the higher concentration of dried plum powder was less effective than the lower concentration at reducing atherosclerotic lesion area. The drying used to create the powder would likely have created some Maillard reaction products. It may be that these products, at the concentration found in the 9·5 % dried plum powder diet, attenuated the effect of dried plums on reducing lesion area. However, this is speculative, since there appear to be no studies of the effects of Maillard reaction products on atherosclerosis.
Although there are numerous studies of the effects of fruits and vegetables on serum cholesterol, few studies exist on the effect of fruits or vegetables on atherosclerosis itself. A mixture of green vegetables, fed at 30 % of the diet to LDL receptor − / − mice for 16 weeks, resulted in a significant decrease in aortic cholesteryl ester(Reference Adams, Golden, Chen, Register and Gugger33). In contrast to the present study, in which the Lo DP group showed reduced concentrations of the acute-phase protein SAP, no reduction was seen in the acute-phase protein serum amyloid A. Pomegranate juice given in the drinking water to apoE-deficient mice has been shown to reduce aortic lesion size(Reference Aviram, Dornfeld, Rosenblat, Volkova, Kaplan, Coleman, Hayek, Presser and Fuhrman11, Reference Kaplan, Hayek, Raz, Coleman, Dornfeld, Vaya and Aviram12). Dealcoholized red wine, also administered in the drinking water, reduced both aortic arch lesion area and wall thickness(Reference Stocker and O'Halloran34). However, neither red wine nor red wine powder altered plaque area in the aortic bulb or the brachiocephalic trunk in apoE-deficient mice(Reference Bentzon, Skovenborg, Hansen, Moller, de Gaulejac, Proch and Falk35). No vegetables appear to have been examined for their ability to retard development of atherosclerosis in apoE-deficient mice. However, large decreases in aortic arch lesion area (44 %) were found in apoE-deficient mice administered ginger extracts in their drinking water(Reference Fuhrman, Rosenblat, Hayek, Coleman and Aviram36). Thus, the present study using dried plums, fed in the form of a powder, appears to be the first examining the effect of a fruit in a relatively intact form (i.e. not a juice or extract) on the development of atherosclerosis.
In the present study, apoE-deficient mice fed a diet containing 0·15 % cholesterol experienced a large and significant increase in serum cholesterol compared to those fed the cholesterol-free diet, by 4 weeks of feeding. This elevation in serum cholesterol persisted through to the end of the experiment, although in the final blood sample, collected as a fasting sample, the difference relative to mice fed the cholesterol-free diet was not as large as in unfasted animals (weeks 4 and 15). It is well established that the apoE-deficient mouse shows an elevation in serum cholesterol when fed an atherogenic diet, e.g. a diet high in saturated fat and cholesterol(Reference Quarfordt, Oswald, Landis, Xu, Zhang and Maeda37). However, studies that have examined the effect of only cholesterol, without the addition of other atherogenic agents such as saturated fats or bile acids, are few. ApoE-deficient mice fed a chow diet containing 2 % cholesterol also showed elevated serum cholesterol compared to those fed a cholesterol-free chow diet(Reference Amigo, Quinones, Mardones, Zanlungo, Miquel, Nervi and Rigotti38), whereas apoE-deficient mice fed 0·02 % cholesterol did not(Reference Ando, Tomoyori and Imaizumi39). It appears that a modest dietary cholesterol concentration of 0·15 % is sufficient to elevate serum cholesterol in this animal model.
Interestingly, a lack of change in total plasma cholesterol concentrations did not seem to preclude a reduction in development of atherosclerosis. The dried plum diets had no influence on plasma cholesterol, except for a modest but statistically significant decrease in the Hi DP group at 15 weeks relative to the B+C group. Although apoE-deficient mice fed black rice pigment exhibited both a lower total serum cholesterol and reduced plaque area in the aortic sinus(Reference Xia, Ling, Ma, Kitts and Zawistowski40), other studies have not found an association between plasma cholesterol and lesion area(Reference Calleja, Paris and Paul41, Reference Moghadasian, McManus, Godin, Rodrigues and Frohlich42). This inconsistent association clearly suggests that other factors play a major role in determining lesion development.
ApoE-deficient mice are well known to exhibit elevated serum TAG concentrations, due to accumulation of VLDL and chylomicron remnants, as apoE is necessary for efficient catabolism of lipoprotein remnants via the LDL receptor(Reference Zhang, Reddick, Piedrahita and Maeda23). The addition of cholesterol to the diet reduced fasting serum TAG concentration by half, with no further significant reduction by the dried plum diets. This dramatic effect of dietary cholesterol on serum TAG concentration in apoE-deficient mice does not appear to have been previously documented. There does not seem to be an obvious explanation for this unexpected result.
It is now acknowledged that atherosclerosis is an inflammatory condition and that the degree of inflammation, as measured by the acute-phase protein C-reactive protein, is positively associated with the incidence of coronary events(Reference Danesh, Wheeler, Hirschfield, Eda, Eiriksdottir, Rumley, Lowe, Pepys and Gudnason43). Dried plums contain small amounts of flavonoids, predominately rutin(Reference Donovan, Meyer and Waterhouse21). Flavonoids have been shown to affect the immune response and the generation of inflammatory processes. In the present study, the major acute-phase protein of mice, SAP, was measured as an indicator of inflammation(Reference Pepys, Baltz, Gomer, Davies and Doenhoff44). Mice from both dried plum diets had SAP concentrations numerically lower than the B+C diet and approximately equal to the B − C diet, only the B − C and Lo DP groups were statistically significantly different from the B+C group. There was a strong trend for a correlation between aortic lesion area and SAP concentration, suggesting a connection between inflammation and lesion area in this model of atherosclerosis.
The role of oxidative events in the development of atherosclerosis has been intensively studied but remains unclear. Considerable evidence has accumulated demonstrating the oxidative modification of LDL particles, which are suggested to lead to endothelial injury and foam cell formation. However, the general lack of effect of dietary antioxidants on CVD in human trials and the dissociation of the development of atherosclerosis from lipoprotein lipid oxidation in animal models(Reference Stocker and O'Halloran34, Reference Witting, Pettersson, Letters and Stocker45) raises questions about the role of dietary antioxidants in reducing atherosclerosis development. Although we found no statistically significant differences among the groups in a well-accepted marker of oxidative stress, urinary TBARS excretion, likely due to the small sample size, it is interesting that a high correlation was found between the group means of urinary TBARS and lesion areas. Dried plums are concentrated sources of chlorogenic and neochlorogenic acids, which are potent antioxidants in vitro (Reference Donovan, Meyer and Waterhouse21). However, chlorogenic acid is poorly absorbed within the small intestine, and largely passes into the large intestine where it is metabolized by gut microflora. This is consistent with the recent finding that neither dried plums or dried plum juice consumption raised plasma ORAC in man(Reference Prior, Gu, Wu, Jacob, Sotoudeh, Kader and Cook46). In the present study, the dried plum powder contained < 5 % neochlorogenic acid and < 10 % chlorogenic acid of that reported for dried plums, depending on variety and drying conditions of the dried plums(Reference Piga, Del Caro and Corda47). Given the low concentration of neochlorogenic and chlorogenic acid in the dried plum powder and their demonstrated poor intestinal absorption, it seems unlikely that the dried plum diets in this experiment produced a significant biological antioxidant effect.
The present results, which demonstrate a reduction in atherosclerosis by dried plum powder, reinforce the idea that consumption of fruits in general and dried plums in particular may be useful for reducing the risk of heart disease and stroke. This benefit may be mediated by reducing inflammatory events within the arterial wall. Further studies examining the anti-inflammatory effects of fruits seem warranted.
Acknowledgements
D. D. G. serves on the Nutrition Advisory Panel for the California Dried Plum Board, for which he receives an annual honorarium. This work was sponsored by the California Dried Plum Board and the Minnesota Agricultural Experiment Station. C. M. G. conducted all the experimental work. D. D. G. designed the experiment and wrote the manuscript.







